Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6734
Peer-review started: March 15, 2021
First decision: April 6, 2021
Revised: April 11, 2021
Accepted: June 25, 2021
Article in press: June 25, 2021
Published online: August 16, 2021
Processing time: 143 Days and 10.5 Hours
The incidence and mortality rates of hepatocellular carcinoma (HCC) are increasing in the United States. However, the increases in different racial and socioeconomic groups have not been homogeneous. Access to healthcare based on socioeconomic status and cost of living index (COLI), especially in HCC management, is under characterized.
The aim was to investigate the relationship between the COLI and tumor characteristics, treatment modalities, and survival of HCC patients in the United States.
A retrospective study of the Surveillance, Epidemiology, and End Results (SEER) database was conducted to identify patients with HCC between 2007 and 2015 using site code C22.0 and the International Classification of Disease for Oncology, 3rd edition (ICD-O-3) codes 8170-8173, and 8175. Cases of fibrolamellar HCC were excluded. Variables collected included demographics, COLI, insurance status, marital status, stage, treatment, tumor size, and survival data. Interquartile ranges for COLI were obtained. Based on the COLI, the study population was separated into four groups: COLI ≤ 901, 902-1044, 1045-1169, ≥ 1070. The χ2 test was used to compare categorical variables, and the Kruskal-Wallis test was used to compare continuous variables without normal distributions. Survival was estimated by the Kaplan-Meier method. We defined P < 0.05 as statistically significant.
We identified 47,894 patients with HCC. Patients from the highest COLI areas were older (63 vs 61 years of age), more likely to be married (52.8% vs 48.0%), female (23.7% vs 21.1%), and of Asian and Pacific Islander descent (32.7% vs 4.8%). The patients were more likely to have stage I disease (34.2% vs 32.6%), tumor size ≤ 30 mm (27.1% vs 23.1%), received locoregional therapy (11.5% vs 6.1%), and undergone surgical resection (10.7% vs 7.0%) when compared with the lowest quartile. The majority of patients with higher COLIs resided in California, Connecticut, Hawaii, and New Jersey. Patients with lower COLIs were more likely to be uninsured (5.7% vs 3.4%), have stage IV disease (15.2% vs 13%), and have received a liver transplant (6.6% vs 4.4%) compared with patients from with the highest COLI. Median survival increased with COLI from 8 (95%CI: 7-8), to 10 (10-11), 11 (11-12), and 14 (14-15) mo (P < 0.001) among patients with COLIs of ≤ 901, 902-1044, 1045-1169, ≥ 1070, respectively. After stratifying by year, a survival trend was present: 2007-2009, 2010-2012, and 2013-2015.
Our study suggested that there were racial and socioeconomic disparities in HCC. Patients from lower COLI groups presented with more advanced disease, and increasing COLI was associated with improved median survival. Future studies should examine this further and explore ways to mitigate the differences.
Core Tip: This was a retrospective study to evaluate the relationship between the cost of living index (COLI) of patients with hepatocellular carcinoma (HCC) and treatment options, tumor characteristics, and median overall survival. Patients from lower COLIs were more likely to be uninsured (5.7% vs 3.4%), had more stage IV disease (15.2% vs 13%), and required more liver transplants (6.6% vs 4.4%) compared with those having the highest COLI. Median survival individuals with HCC from the highest COLI areas was significantly longer compared with the lowest COLI (14 mo vs 8 mo), suggesting that socioeconomic and racial disparities may contribute to survival for HCC.
- Citation: Sempokuya T, Patel KP, Azawi M, Ma J, Wong LL. Increased morbidity and mortality of hepatocellular carcinoma patients in lower cost of living areas. World J Clin Cases 2021; 9(23): 6734-6746
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6734.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6734
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide[1]. In 2018, there were more than 841,000 new primary liver cancer cases and 782,000 deaths globally, most of which were HCC[2]. Chronic hepatitis B (HBV) or hepatitis C (HCV) virus infection, heavy alcohol consumption, diabetes, and nonalcoholic fatty liver disease (NAFLD) are the most important risk factors for developing HCC[3]. Notably, nonalcoholic steatohepatitis (NASH) has become the fastest growing cause of HCC-related liver transplants in the United States[3].
Nearly 80% of all HCC cases are attributable to HBV or HCV infections, and can develop subsequent to infection without any evidence of cirrhosis[4]. Men of Asian and East African descent have historically experienced the highest age-adjusted incidence, of HCC attributed to active chronic HBV infection[2]. With the reduction of aflatoxin exposure, increased in HBV vaccination coverage, and subsequent generations of United States-born Asians having lower HBV infection rates, the incidence of infection among the Asian demographic has improved[4]. HCC age-adjusted incidence rates have recently shifted, with higher rates occurring in Hispanic individuals[5,6]. Compared with foreign-born Hispanics, United States-born Hispanics were previously noted to have a higher incidence of HCC[3].
When considering socioeconomic status, Yu et al[7] noted that black patients in their large tertiary transplant institution were much less likely (OR = 0.03, 95%CI: 0.00–0.37) than white patients to receive a liver transplant, which may be related to Blacks and Hispanics being more likely to be diagnosed with advanced-stage disease, having higher initial Child-Pugh scores and AFP levels, and coming from lower median-income households. In support, a study by Shah et al[8] suggested that therapy for HCC had historically been underutilized; their no-treatment groups included more individuals with a lower socioeconomic status, of black heritage, and evaluated at small and medium-sized hospitals. Despite recent increases in the incidence and mortality rates of HCC, the rise is not homogenous across various racial and socioeconomic groups. Furthermore, it is unclear how socioeconomic status, access to medical care, and underlying racial disparities currently impact the outcome of HCC. Therefore, we aimed to characterize the relationship between the cost of living index (COLI), sociodemographic factors, tumor characteristics, treatment modalities, and overall survival in HCC patients using Surveillance, Epidemiology, and End Results (SEER) data from 2007 to 2015.
Population data from the SEER database published by the National Cancer Institute was obtained through the Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat/) version <8.3.6>[9]. SEER Registries include population-based data that report cancer incidence, characteristics, treatment and, mortality in selected states of the United States since 1973. Approximately 34.6% of all cancer cases in the United States population are included[10]. The SEER data analyzed in this study was obtained in 18 states and regions available to conduct survival analysis, including the Alaska Native Tumor Registry, California (San Francisco-Oakland, San Jose-Monterey, Los Angeles, Greater California), Connecticut, Georgia (Atlanta, Greater Georgia, Rural Georgia), Hawaii, Iowa, Kentucky, Louisiana, Michigan (Detroit), New Jersey, New Mexico, Utah and Washington (Seattle-Puget Sound) More details are available at https://seer.cancer.gov/registries/terms.html. This study was conducted after complying with the SEER Research Data Use Agreement. As we utilized a publicly available, de-identified database, approval from an institutional review board was not required to conduct this study.
We collected data on patients diagnosed with HCC between 2007 and 2015 by using site code C22.0, the International Classification of Disease for Oncology, third edition (ICD-O-3) codes 8170-8173, and 8175. As fibrolamellar HCC has a distinct phenotype, it was excluded. Variables of interest that were collected included age at the time of diagnosis, year of diagnosis, sex, race (White, Blacks, Hispanic, Asian or Pacific Islander (API), or others/unknown), marital status, COLI, insurance status, stage of disease by the American Joint Committee on Cancer (AJCC) Staging Manual, sixth edition[11], modality and frequency of treatment, tumor size, and survival data. The SEER database calculates COLI using a family budget analysis done by the Economic Policy Institute (https://www.epi.org/resources/budget/). The COLI was based on a family of two parents and one child living in a county and with an essential family expenditures including housing, food, childcare, transportation, health care, and taxes (https://seer.cancer.gov/seerstat/variables/countyattribs/static.html#col). The United States population-weighted mean cost of living is valued at 1,000. The COLI is the ratio of the local cost of living and the mean cost. Values greater than 1,000 suggest higher than the mean cost of living in the area.
Statistical analysis was performed with R version 3.4.1 (The R Foundation for Statistical Computing, Vienna, Austria), EZR version 1.36 (Division of Hematology, Saitama Medical Center, Jichi Medical University, Japan)[12], and SAS version 9.4 (SAS Institute Inc., Cary, NC, United States). Interquartile ranges for COLI were calculated, and based on that number, we stratified the study population into four groups: COLI ≤ 901, 902-1044, 1045-1169, ≥ 1070. The χ2 test was used to compare categorical variables, and the Kruskal-Wallis test was used to compare continuous variables without normal distributions. Survival was estimated by the Kaplan-Meier method. Univariate and multivariate Cox regression analysis was used to compare socioeconomic variables. P < 0.05 was considered significant. The statistical methods of this study were reviewed by Jihyun Ma from the Department of Biostatistics, College of Public Health, University of Nebraska Medical Center.
We identified 47,894 patients with a diagnosis of HCC. Table 1 shows the characteristics of the individuals included in this study. There were 13,515 patients in the COLI ≤ 901 group, 11,379 in the COLI 902-1044 group, 12,167 in the COLI 1045-1169 group, and 10,833 in the COLI ≥ 1070 group. The median age at the time of diagnosis was 63 years and was higher in areas with a higher COLI. All COLI groups had a male predominance. Patients living in the higher COLI areas were more often married, older, and insured when compared with the lowest COLI group. There was also a lower proportion of Black and Hispanic individuals and a higher proportion of API individuals living in higher COLI areas. The majority of the study patients with higher COLIs resided in California, Connecticut, Hawaii, and New Jersey.
| COLI group | Total | < 901 | 902-1044 | 1048-1169 | 1170+ | P value | |
| Number | 47894 | 13515 | 11379 | 12167 | 10833 | ||
| Median age [IQR] | 62.00 [56.00, 70.00] | 61.00 [55.00, 69.00] | 61.00 [56.00, 68.00] | 62.00 [56.00, 71.00] | 63.00 [57.00, 72.00] | < 0.001 | |
| Survival mo [IQR] | 10.00 [2.00, 27.00] | 8.00 [2.00, 23.00] | 10.00 [2.00, 27.00] | 10.00 [2.00, 28.00] | 13.00 [3.00, 31.00] | < 0.001 | |
| Male sex (%) | 37202 (77.7) | 10661 (78.9) | 8921 (78.4) | 9357 (76.9) | 8263 (76.3) | < 0.001 | |
| Race (%) | White | 23220 (48.5) | 8062 (59.7) | 6108 (53.7) | 4864 (40.0) | 4186 (38.6) | < 0.001 |
| Black | 6537 (13.6) | 2094 (15.5) | 2231 (19.6) | 1197 (9.8) | 1015 (9.4) | ||
| Hispanic | 9695 (20.2) | 2497 (18.5) | 1504 (13.2) | 3708 (30.5) | 1986 (18.3) | ||
| API | 7723 (16.1) | 652 (4.8) | 1231 (10.8) | 2294 (18.9) | 3546 (32.7) | ||
| Other/Unkn | 719 (1.5) | 210 (1.6) | 305 (2.7) | 104 (0.9) | 100 (0.9) | ||
| Marital status (%) | Divorced | 6125 (12.8) | 1978 (14.6) | 1673 (14.7) | 1466 (12.0) | 1008 (9.3) | < 0.001 |
| Married | 23338 (48.7) | 6490 (48.0) | 5237 (46.0) | 5888 (48.4) | 5723 (52.8) | ||
| Separated | 900 (1.9) | 241 (1.8) | 242 (2.1) | 248 (2.0) | 169 (1.6) | ||
| Single | 10474 (21.9) | 2938 (21.7) | 2477 (21.8) | 2778 (22.8) | 2281 (21.1) | ||
| Unknown | 2545 (5.3) | 585 (4.3) | 710 (6.2) | 611 (5.0) | 639 (5.9) | ||
| Unmarried | 120 (0.3) | 33 (0.2) | 37 (0.3) | 28 (0.2) | 22 (0.2) | ||
| Widowed | 4392 (9.2) | 1250 (9.2) | 1003 (8.8) | 1148 (9.4) | 991 (9.1) | ||
| Insurance status (%) | Any Medicaid | 11851 (24.7) | 3346 (24.8) | 2591 (22.8) | 3415 (28.1) | 2499 (23.1) | < 0.001 |
| Unknown | 1864 (3.9) | 536 (4.0) | 450 (4.0) | 456 (3.7) | 422 (3.9) | ||
| Insured | 24225 (50.6) | 6531 (48.3) | 5811 (51.1) | 5970 (49.1) | 5913 (54.6) | ||
| Insured/Unspecified | 7796 (16.3) | 2337 (17.3) | 2053 (18.0) | 1776 (14.6) | 1630 (15.0) | ||
| Uninsured | 2158 (4.5) | 765 (5.7) | 474 (4.2) | 550 (4.5) | 369 (3.4) | ||
| State (%) | Alaska | 60 (0.1) | 0 | 60 (0.5) | 0 | 0 | < 0.001 |
| California | 23501 (49.1) | 4266 (31.6) | 3005 (26.4) | 9219 (75.8) | 7011 (64.7) | ||
| Connecticut | 1880 (3.9) | 0 | 307 (2.7) | 1573 (12.9) | 0 | ||
| Georgia | 4124 (8.6) | 1767 (13.1) | 2357 (20.7) | 0 | 0 | ||
| Hawaii | 1054 (2.2) | 0 | 0 (0.0) | 293 (2.4) | 761 (7.0) | ||
| Iowa | 1103 (2.3) | 1003 (7.4) | 100 (0.9) | 0 (0.0) | 0 | ||
| Kentucky | 2047 (4.3) | 2047 (15.1) | 0 | 0 | 0 | ||
| Louisiana | 2740 (5.7) | 2740 (20.3) | 0 | 0 | 0 | ||
| Michigan | 2129 (4.4) | 0 (0.0) | 2129 (18.7) | 0 | 0 | ||
| New Jersey | 4131 (8.6) | 0 | 0 | 1082 (8.9) | 3049 (28.1) | ||
| New Mexico | 1335 (2.8) | 636 (4.7) | 687 (6.0) | 0 | 12 (0.1) | ||
| Utah | 772 (1.6) | 770 (5.7) | 2 (0.0) | 0 | 0 | ||
| Washington | 3018 (6.3) | 286 (2.1) | 2732 (24.0) | 0 | 0 | ||
| Year (%) | 2007-2009 | 3599 (26.6) | 3164 (27.8) | 3646 (30.0) | 3242 (29.9) | < 0.001 | |
| 13651 (28.5) | |||||||
| 2010-2012 | 16115 (33.6) | 4413 (32.7) | 3868 (34.0) | 4139 (34.0) | 3695 (34.1) | ||
| 2013-2015 | 18128 (37.9) | 5503 (40.7) | 4347 (38.2) | 4382 (36.0) | 3896 (36.0) |
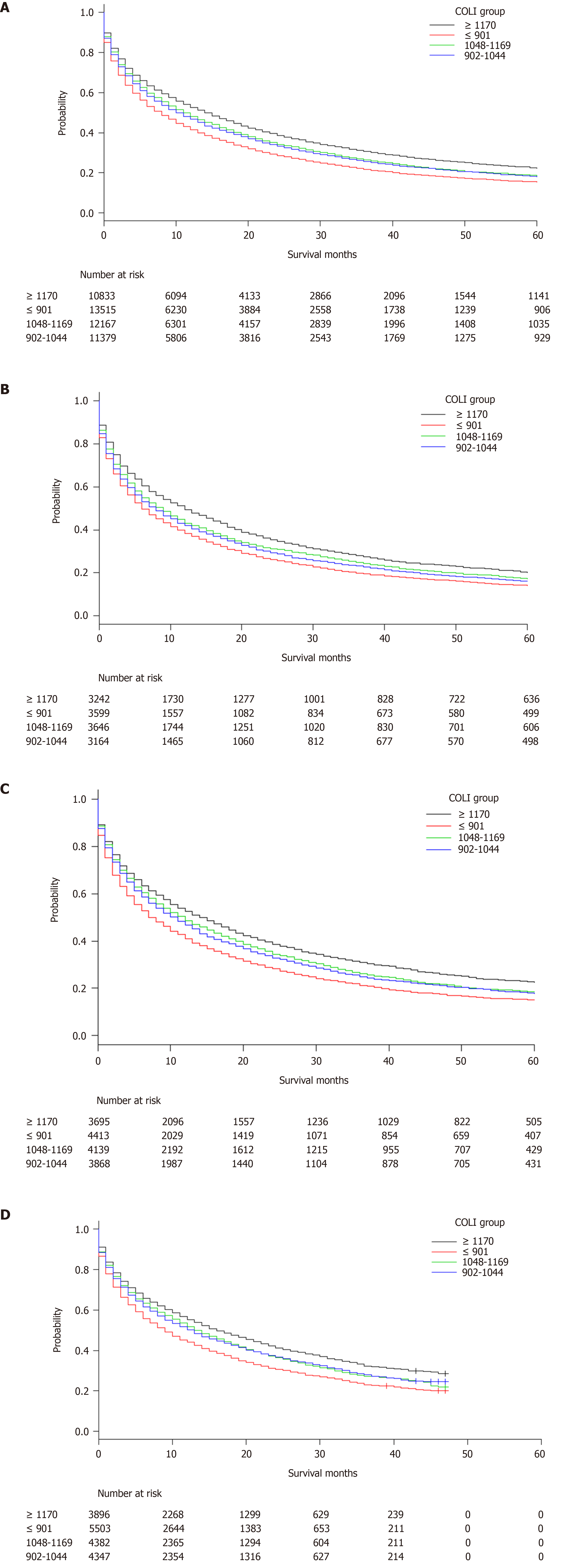
Table 2 summarizes the characteristics of HCC in the study patients. Those with the highest COLI were found to more often have stage I disease (34.2% vs 32.6%), tumor size ≤ 30 mm (27.1% vs 23.1%), have received locoregional therapy (11.5% vs 6.1%), and have been treated by surgical resection (10.7% vs 7.0%) compared with the those with the lowest COLI. Patients from the lowest COLI group more often had stage IV disease (15.2% vs 13%) and had received liver transplants (6.6% vs 4.4%). The Kaplan-Meier survival analysis (Figure 1A) demonstrated that median survival increased with increasing COLI from 8 (95%CI: 7-8), to 10 (95%CI: 10-11), to 11 (95%CI: 11-12), and to 14 (95%CI: 14-15) mo (P < 0.001). The trend in median survival remained after stratification by year (2007-2009, 2010-2012, and 2013-2015), as shown in Table 3 and Figure 1B-D.
| COLI group | ≤ 901 | 902-1044 | 1048-1169 | ≥ 1170 | P value | |
| Stage (%) | I | 4403 (32.6) | 3764 (33.1) | 3977 (32.7) | 3704 (34.2) | < 0.001 |
| II | 2201 (16.3) | 2057 (18.1) | 2006 (16.5) | 1988 (18.4) | ||
| III | 2804 (20.7) | 2380 (20.9) | 2350 (19.3) | 2100 (19.4) | ||
| IV | 2055 (15.2) | 1582 (13.9) | 1674 (13.8) | 1410 (13.0) | ||
| UNK | 2052 (15.2) | 1596 (14.0) | 2160 (17.8) | 1631 (15.1) | ||
| Tumor size (%) | ≤ 20 | 1335 (9.9) | 1379 (12.1) | 1378 (11.3) | 1338 (12.4) | < 0.001 |
| 21-30 | 1786 (13.2) | 1835 (16.1) | 1776 (14.6) | 1598 (14.8) | ||
| 31-50 | 2701 (20.0) | 2353 (20.7) | 2522 (20.7) | 2136 (19.7) | ||
| 51 | 4780 (35.4) | 3830 (33.7) | 4164 (34.2) | 3664 (33.8) | ||
| UNK | 2913 (21.6) | 1982 (17.4) | 2327 (19.1) | 2097 (19.4) | ||
| ≤ 20 mm (%) | 1335 (9.9) | 1379 (12.1) | 1378 (11.3) | 1338 (12.4) | < 0.001 | |
| ≤ 30.mm (%) | 3121 (23.1) | 3214 (28.2) | 3154 (25.9) | 2936 (27.1) | < 0.001 | |
| ≤ 50.mm (%) | 5822 (43.1) | 5567 (48.9) | 5676 (46.7) | 5072 (46.8) | < 0.001 | |
| LRT (%) | 819 (6.1) | 1268 (11.1) | 1286 (10.6) | 1244 (11.5) | < 0.001 | |
| Resection (%) | 949 (7.0) | 695 (6.1) | 1000 (8.2) | 1154 (10.7) | < 0.001 | |
| Transplant (%) | 889 (6.6) | 637 (5.6) | 507 (4.2) | 481 (4.4) | < 0.001 |
| COLI group | Survival in mo (95%CI) | P value | |||
| 2007-2015 | 2007-2009 | 2010-2012 | 2013-2015 | ||
| ≤ 901 | 8 (7-8) | 6 (6-7) | 7 (7-8) | 9 (8-10) | < 0.001 |
| 902-1044 | 10 (10-11) | 8 (7-9) | 11 (10-11) | 13 (12-13) | |
| 1048-1169 | 11 (11-12) | 9 (8-10) | 12 (11-13) | 13 (13-14) | |
| ≥ 1170 | 14 (14-15) | 12 (11-13) | 14 (13-16) | 16 (15-18) | |
Table 4 summarizes the results of univariate and multivariate Cox regression analysis of various socioeconomic risk factors and patient backgrounds. The majority of factors (COLI, age, sex, race, insurance status, stage, tumor size, locoregional therapy (LRT), resection, transplant, and year) had a significant hazard ratios (HRs). Univariate analysis found no significant differences for COLI 1048-1169 vs 902-1044, Hispanic vs White, uninsured vs unknown, and stage III vs unknown. Multivariate analysis found no significant differences for API vs other/unknown, black vs white, married (including common law) vs unknown, separated/divorced vs widowed, and single/unmarried or domestic partner vs widowed. Notably, after multivariate analysis, the HR for COLI 1048-1169 vs 902-1044 became significant. Variables associated with marital factors were no longer significant after multivariate analysis. The highest HRs were for LRT, resection, and transplant.
| Univariate | Multivariate | |||
| HR | 95%CI | HR | 95%CI | |
| COLI ≤ 901- vs 1048-1169 | 1.162 | 1.131-1.195 | 1.141 | 1.109-1.174 |
| COLI ≤ 901- vs 1170 ≤ | 1.300 | 1.263-1.338 | 1.218 | 1.181-1.255 |
| COLI ≤ 901- vs 902-1044 | 1.130 | 1.099-1.163 | 1.067 | 1.037-1.097 |
| COLI 1048-1169 vs 1170 ≤ | 1.118 | 1.085-1.001 | 1.067 | 1.035-1.100 |
| COLI 1048-1169 vs 902-1044 | 0.972 | 0.944-1.001 | 0.934 | 0.907-0.963 |
| COLI 1170 ≤ vs 902-1044 | 0.869 | 0.843-0.896 | 0.876 | 0.849-0.904 |
| Age | 1.014 | 1.013-1.015 | 1.012 | 1.011-1.013 |
| Sex female vs male | 0.903 | 0.881-0.926 | 0.925 | 0.901-0.950 |
| API vs Black | 0.710 | 0.684-0.737 | 0.817 | 0.785-0.851 |
| API vs Hispanic | 0.809 | 0.781-0.838 | 0.874 | 0.842-0.906 |
| API vs Other/unknown | 0.895 | 0.818-0.980 | 1.000 | 0.913-1.096 |
| API vs White | 0.813 | 0.788-0.838 | 0.839 | 0.813-0.867 |
| Black vs Hispanic | 1.139 | 1.100-1.180 | 1.069 | 1.031-1.108 |
| Black vs Other/unknown | 1.261 | 1.152-1.380 | 1.223 | 1.118-1.339 |
| Black vs White | 1.145 | 1.110-1.180 | 1.027 | 0.996-1.060 |
| Hispanic vs Other/unknown | 1.107 | 1.012-1.210 | 1.145 | 1.047-1.252 |
| Hispanic vs White | 1.005 | 0.978-1.032 | 0.961 | 0.935-0.988 |
| Other/unknown vs White | 0.908 | 0.832-0.991 | 0.839 | 0.769-0.916 |
| Married (including common law) vs Separated/Divorced | 0.839 | 0.814-0.865 | 0.909 | 0.881-0.937 |
| Married (including common law) vs Single/Unmarried or domestic partner | 0.799 | 0.779-0.820 | 0.876 | 0.852-0.901 |
| Married (including common law) vs Unknown | 0.916 | 0.873-0.961 | 1.037 | 0.988-1.089 |
| Married (including common law) vs Widowed | 0.708 | 0.683-0.733 | 0.881 | 0.847-0.915 |
| Separated/divorced vs Single/unmarried or domestic partner | 0.953 | 0.921-0.985 | 0.964 | 0.932- 0.998 |
| Separated/divorced vs Unknown | 1.092 | 1.036-1.150 | 1.142 | 1.083-1.204 |
| Separated/divorced vs Widowed | 0.843 | 0.809-0.879 | 0.969 | 0.927-1.013 |
| Single/unmarried or domestic partner vs Unknown | 1.146 | 1.090-1.205 | 1.184 | 1.125-1.247 |
| Single/Unmarried or domestic partner vs Widowed | 0.885 | 0.852-0.920 | 1.005 | 0.963-1.049 |
| Unknown vs Widowed | 0.773 | 0.731-0.817 | 0.849 | 0.801-0.899 |
| Any Medicaid vs Insured | 1.211 | 1.182-1.240 | 1.141 | 1.113-1.170 |
| Any Medicaid vs Uninsured | 0.705 | 0.670-0.741 | 0.830 | 0.789-0.874 |
| Any Medicaid vs Unknown | 0.678 | 0.644-0.715 | 0.904 | 0.856-0.956 |
| Insured vs Uninsured | 0.582 | 0.555-0.611 | 0.728 | 0.693-0.764 |
| Insured vs Unknown | 0.560 | 0.533-0.589 | 0.792 | 0.751-0.836 |
| Uninsured vs Unknown | 0.962 | 0.900-1.029 | 1.089 | 1.016-1.168 |
| Stage I vs II | 0.938 | 0.907-0.970 | 0.833 | 0.805-0.862 |
| Stage I vs III | 0.373 | 0.363-0.385 | 0.570 | 0.552-0.590 |
| Stage I vs IV | 0.221 | 0.214-0.229 | 0.372 | 0.359-0.386 |
| Stage vs Unknown | 0.370 | 0.359-0.382 | 0.656 | 0.632-0.681 |
| Stage II vs III | 0.398 | 0.385-0.412 | 0.685 | 0.658-0.713 |
| Stage II vs IV | 0.236 | 0.227-0.245 | 0.447 | 0.429-0.466 |
| Stage II vs Unknown | 0.395 | 0.381-0.409 | 0.788 | 0.754-0.823 |
| Stage III vs IV | 0.593 | 0.574-0.613 | 0.653 | 0.631-0.675 |
| Stage III vs Unknown | 0.992 | 0.961-1.024 | 1.151 | 1.108-1.196 |
| Stage IV vs Unknown | 1.673 | 1.6161.732 | 1.763 | 1.699-1.830 |
| 20 vs 21-30 | 0.796 | 0.758-0.835 | 0.835 | 0.796-0.876 |
| 20 vs 31-50 | 0.564 | 0.540-0.590 | 0.672 | 0.643-0.703 |
| 20 vs 50+ | 0.308 | 0.296-0.321 | 0.506 | 0.484-0.529 |
| 20 vs Unknown | 0.235 | 0.225-0245 | 0.454 | 0.432-0.476 |
| 21-30 vs 31-50 | 0.710 | 0.683-0.737 | 0.805 | 0.774-0.837 |
| 21-30 vs 50+ | 0.387 | 0.374-0.401 | 0.606 | 0.582-0.630 |
| 21-30 vs Unknown | 0.295 | 0.284-0.306 | 0.543 | 0.520-0.568 |
| 31-50 vs 50+ | 0.546 | 0.530-0.562 | 0.753 | 0.728- 0.778 |
| 31-50 vs Unknown | 0.416 | 0.403-0.430 | 0.675 | 0.650-0.701 |
| 50+ vs Unknown | 0.763 | 0.742-0.783 | 0.897 | 0.869-0.926 |
| LRT | 2.184 | 2.099-2.273 | 1.931 | 1.852-2.012 |
| Resection | 2.670 | 2.548-2.799 | 2.986 | 2.845-3.135 |
| Transplant | 6.311 | 5.837-6.822 | 5.352 | 4.941-5.796 |
| 2007-2009 vs 2010-2012 | 1.071 | 1.044-1.098 | 1.074 | 1.048-1.102 |
| 2007-2009 vs 2013-2015 | 1.153 | 1.123-1.184 | 1.143 | 1.113-1.174 |
| 2010-2012 vs 2013-2015 | 1.077 | 1.050-1.104 | 1.064 | 1.037-1.091 |
In general, HCC has a poor prognosis, with an overall median survival of 6-16 mo, depending on the extent of disease[13]. Our study found that median survival was significantly longer in individuals from the highest COLI than in those with the lowest COLI (14 mo vs 8 mo), suggesting that socioeconomic disparities contributed to survival in HCC. The differences may have resulted from disparities in accessing healthcare for early detection and treatment options available at different disease stages. Individuals from the highest COLI were more likely to receive locoregional (11.5% vs 6.1%) and surgical resection (10.7% vs 7.0%), whereas those from the lowest COLI more often required liver transplantation (6.6% vs 4.4%). The differences may have resulted from the stage or extent of the disease at the time of diagnosis. Compared with the highest COLI, individuals from the lowest COLI were more likely to present with stage IV disease (15.2% vs 13.0%) and less likely to have smaller tumors (23.1% vs 27.1%), suggesting that tumor characteristics, treatment modalities, and overall survival vary by sociodemographic group.
Chronic HBV and HCV infection are predominant risk factors for the development of HCC in various ethnic groups[3,14]. Globally, the disease burden of HCC was the highest in sub-Saharan African and East Asia due to HBV[14]. High rates of HCC have also been noted in APIs, but vaccination programs and healthcare access may have shifted the trend[15]. Our study suggests that more APIs live in the highest COLI areas. A relatively higher HBV burden among API individuals may contribute to more readily available definitive treatments by hepatic resection or locoregional therapy, thus prolonging survival. In the United States, cases of HCC in Hispanics have now surpassed those in APIs, likely related to heavy alcohol consumption, obesity, diabetes, and NAFLD[3,6,14]. Such NAFLD and NASH-related HCC cases may present at advanced stages because of a lack of universal surveillance strategies, particularly in this population. Rich et al[16] suggested that Black and Hispanic patients were historically less likely to be diagnosed with early-stage HCC when compared with their white counterparts. Additionally, African American and Hispanic patients have previously been shown to undergo transplants at rates lower than Whites, and they were less likely than Whites and APIs to undergo ablation or hepatectomy despite disproportionately higher rates of HCC[17].
Our study demonstrated the challenges and ramifications of socioeconomic status on treatment and outcome in HCC, with individuals in the lowest COLI having a worse disease course and overall prognosis. Previous studies regarding the outcomes of non-metastatic HCC suggested a worse liver cancer-specific survival in patients treated with ablation or surgery with low socioeconomic backgrounds[18]. In general, low-income individuals are more likely to be uninsured and have less access to medical care, including routine screening, diagnosis, and treatment[6,19]. As reported by low-income families, the significant barriers to healthcare access have reinforced a growing concern of lack of insurance coverage, access to appropriate services, and ultimately facing unaffordable costs[20]. Although patients in a lower COLI area have insurance, they may be subject to high copays and deductibles, which make it difficult to afford coverage. Given an increased financial burden of health care, these individuals may opt to forgo routine office visits and screening.
Our study supported a higher rate of uninsured individuals coming from the lower COLI area. While the expansion of Medicaid has improved healthcare access, it has had little effect on cancer screening[21]. Shah et al[8] reported that less than 30% of Medicare patients with HCC underwent therapy, with nearly half of those undergoing noncurative transarterial chemoembolization (TACE) as their treatment option. Furthermore, Medicaid recipients had higher Child-Pugh scores, HCC beyond Milan criteria, and higher AJCC staging[7]. As such, minorities and various socioeconomic groups face serious barriers in disease surveillance and treatment for HCC based on their insurance status and the quality of insurance alone.
As mentioned, our findings suggest that there is improved survival in those living in the higher COLI areas. In contrast, those living in rural areas with lower COLI may be significantly disadvantaged by long travel distances and transportation obstacles to receive the same specialized care. Although one-fifth of the United States population resides in rural regions, only one-tenth of practicing physicians service those areas[22]. As a result, those individuals likely face more socioeconomic obstacles because of the distance to specialists and complex treatments. Optimal treatment of HCC requires regular surveillance and prompt referral to a multidisciplinary team for management. Early tumor detection and evaluation by gastroenterology care were independently associated with receiving curative treatment, ultimately contributing to improved outcomes[23]. Patients with lower socioeconomic status and without private insurance were historically less likely to receive any surgical options; additionally, the same issue occurred if patients were evaluated at public, rural, and nonteaching hospitals[17]. Unfortunately, the lack of preventative care and surveillance will continue to contribute to the late presentation of disease and ultimately poor outcomes of HCC.
Despite demonstrating that individuals from lower COLIs have lower mean survival and more advanced stages of the disease, there are limitations to our study. The survival differences among individuals from various COLIs should be interpreted with caution, as lead-time bias may play a role. Also, cancer registries that participate in the SEER database capture data from 18 states across the United States. Although there is a large sample size, generalizability of the SEER database may be limited by geographical variation and missing data from the remaining 32 states. Assignment of COLI is based on living location, which may include homeless individuals or transient workers living in the area at the time of diagnosis, thus may not necessarily reflect their financial status. Besides, the SEER database has no information on underlying liver disease etiology, comorbidities, laboratory data, whether HCC surveillance was performed, type of locoregional therapy, disease recurrence, or subsequent therapies. Finally, the SEER database is an administrative database and is limited by missing data, variety on a coder-to-coder basis, and the tumor registry staff required to maintain the data.
In summary, our study suggested that there are racial and socioeconomic disparities associated with the diagnosis and treatment of HCC. Patients who live in areas with a high COLI are more likely to have earlier detection of malignancy and often experience better outcomes than those in lower COLI areas. As a COLI may not be the most accurate way to delineate differences in society, additional studies are needed to identify and address disparities in the care provided to all individuals with HCC.
The incidence and mortality rates of hepatocellular carcinoma (HCC) are increasing in the United States. However, the increases in different racial and socioeconomic groups have not been homogeneous.
Access to healthcare based on socioeconomic status and cost of living index (COLI), especially in HCC management, is under characterized. Therefore, a study to characterize disparity in HCC care is needed.
To investigate the relationship between the COLI and tumor characteristics, treatment modalities, and survival of HCC patients in the United States.
A retrospective study of the Surveillance, Epidemiology, and End Results (SEER) database was conducted to identify patients with HCC between 2007 and 2015. Interquartile ranges for COLI were obtained and were used to separate the study population into four groups: COLI ≤ 901, 902-1044, 1045-1169, ≥ 1070. The χ2 test was used to compare categorical variables, and the Kruskal-Wallis test was utilized to compare continuous variables without normal distributions. Survival analysis was done by the Kaplan-Meier method.
We identified 47,894 patients with HCC. Patients from the highest COLI areas were more likely to have stage I disease (34.2% vs 32.6%), tumor size ≤ 30 mm (27.1% vs 23.1%), have received locoregional therapy (11.5% vs 6.1%), and undergone surgical resection (10.7% vs 7.0%) compared with the lowest quartile. Patients from lower COLI were more likely to be uninsured (5.7% vs 3.4%), have stage IV disease (15.2% vs 13%), and have received a liver transplant (6.6% vs 4.4%) compared with patients from the highest COLI. The median survival increased with increasing COLI; from 8 (95%CI: 7-8), to 10 (95%CI: 10-11), 11 (95%CI: 11-12), and 14 (95%CI: 14-15) mo (P < 0.001) in the COLI ≤ 901, 902-1044, 1045-1169, ≥ 1070 groups, respectively.
Our study suggested that there were racial and socioeconomic disparities in HCC. Patients from lower COLIs presented with more advanced disease, and increasing COLI was associated with improved median survival.
Future studies should examine this further and explore ways to mitigate the differences.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Gastroenterological Association; American Society for Gastrointestinal Endoscopy; and American Association for the Study of Liver Diseases.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li CP S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Li JH
| 1. | Waller LP, Deshpande V, Pyrsopoulos N. Hepatocellular carcinoma: A comprehensive review. World J Hepatol. 2015;7:2648-2663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55666] [Article Influence: 7952.3] [Reference Citation Analysis (132)] |
| 3. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1210] [Article Influence: 201.7] [Reference Citation Analysis (1)] |
| 4. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP Jr. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 805] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 6. | White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152:812-820.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 331] [Article Influence: 41.4] [Reference Citation Analysis (1)] |
| 7. | Yu JC, Neugut AI, Wang S, Jacobson JS, Ferrante L, Khungar V, Lim E, Hershman DL, Brown RS Jr, Siegel AB. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer. 2010;116:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Shah SA, Smith JK, Li Y, Ng SC, Carroll JE, Tseng JF. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer. 2011;117:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER Research Data, 18 Registries, Nov 2019 Sub (2000-2017) - Linked To County Attributes - Time Dependent (1990-2017) Income/Rurality, 1969-2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission. |
| 10. | National Cancer Institute. About the SEER Registries [Internet]. Surveillance, Epidemiol. End Results Program. Available from: https://seer.cancer.gov/registries/. |
| 11. | American Joint Committee on Cancer; Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6th ed. New York: Springer, 2002. [DOI] [Full Text] |
| 12. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13160] [Article Influence: 1096.7] [Reference Citation Analysis (0)] |
| 13. | Greten TF, Papendorf F, Bleck JS, Kirchhoff T, Wohlberedt T, Kubicka S, Klempnauer J, Galanski M, Manns MP. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 878] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 15. | Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 362] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 16. | Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, Yopp AC, Singal AG. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2019;17:551-559.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 17. | Zak Y, Rhoads KF, Visser BC. Predictors of surgical intervention for hepatocellular carcinoma: race, socioeconomic status, and hospital type. Arch Surg. 2011;146:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Abdel-Rahman O. Treatment choices and outcomes of non-metastatic hepatocellular carcinoma patients in relationship to neighborhood socioeconomic status: a population-based study. Int J Clin Oncol. 2020;25:861-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Dickman SL, Himmelstein DU, Woolhandler S. Inequality and the health-care system in the USA. Lancet. 2017;389:1431-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 394] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 20. | Devoe JE, Baez A, Angier H, Krois L, Edlund C, Carney PA. Insurance + access not equal to health care: typology of barriers to health care access for low-income families. Ann Fam Med. 2007;5:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Tummalapalli SL, Keyhani S. Changes in Preventative Health Care After Medicaid Expansion. Med Care. 2020;58:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of Rural Cancer Care in the United States. Oncology (Williston Park). 2015;29:633-640. [PubMed] |
| 23. | Scaglione S, Adams W, Caines A, Devlin P, Mittal S, Singal AG, Parikh ND. Association Between Race/Ethnicity and Insurance Status with Outcomes in Patients with Hepatocellular Carcinoma. Dig Dis Sci. 2020;65:1669-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |