Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6717
Peer-review started: March 2, 2021
First decision: April 4, 2021
Revised: April 6, 2021
Accepted: May 15, 2021
Article in press: May 15, 2021
Published online: August 16, 2021
Processing time: 156 Days and 9.9 Hours
Respiratory infections in children are common pediatric diseases caused by pathogens that invade the respiratory system. Children are considerably susceptible to Mycoplasma pneumoniae infection. There has been widespread clinical attention on treatment strategies for this disease.
To analyze the clinical efficacy of different antibiotics in treating pediatric respiratory mycoplasma infections.
We included 106 children with a confirmed diagnosis of respiratory mycoplasma infection who were admitted to our hospital from April 2017 to July 2019 and grouped them using a random number table. Among them, 53 children each received clarithromycin or erythromycin. The clinical efficacy of both drugs was evaluated and compared. We performed the multiplex polymerase chain reaction (MP-PCR) test and determined the MP-PCR negative rate in children after the end of the treatment course. We compared the incidence of toxic and side effects, including nausea, diarrhea, and abdominal pain; further, we recorded the length of hospitalization, antipyretic time, and drug costs. Additionally, we evaluated and compared the compliance of the children during treatment.
The erythromycin group showed a significantly higher total effective rate of clinical treatment than the clarithromycin group. MP-PCR test results showed that the clarithromycin group had a significantly higher MP-PCR negative rate than the erythromycin group. Moreover, children in the clarithromycin group had shorter fever time, shorter hospital stays, and lower drug costs than those in the erythromycin group. The clarithromycin group had a significantly higher overall drug adherence rate than the erythromycin group. The incidence of toxic and side effects was significantly lower in the clarithromycin group than in the erythromycin group (P < 0.05).
Our findings indicate that clarithromycin has various advantages over erythromycin, including higher application safety, stronger mycoplasma clearance, and higher medication compliance in children; therefore, it can be actively promoted.
Core tip: This study aimed to explore the efficacy of different antibiotics for treating respiratory mycoplasma infection in children. Compared with erythromycin, clarithromycin showed numerous advantages, including high safety, a strong mycoplasma clearance rate, and high drug compliance.
- Citation: Zhang MY, Zhao Y, Liu JF, Liu GP, Zhang RY, Wang LM. Efficacy of different antibiotics in treatment of children with respiratory mycoplasma infection. World J Clin Cases 2021; 9(23): 6717-6724
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6717.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6717
Respiratory infections in children are common pediatric diseases caused by pathogens that invade the respiratory system. Given that children have worse lung and immune function than adults, they are more susceptible to infection with Mycoplasma pneumoniae, are more susceptible to disease after infection, have difficulty in breathing, and experience respiratory failure or critical illness[1]. Epidemiological studies have suggested that respiratory infections are among the main mortality causes in children in China[2]. Pediatric mycoplasma infections are common pediatric respiratory infections observed in clinical practice[3], with their proportion among non-bacterial pneumonia cases reaching > 30%[4]. There has been widespread clinical attention on the treatment of these infections.
Currently, erythromycin is the most commonly used drug for treating pediatric mycoplasma infection and efficiently kills mycoplasma[5]; however, it has numerous disadvantages regarding gastrointestinal adverse reactions[6-8]. An additional disadvantage is that its main administration route is intravenous. During drug administration, children are prone to resist treatment given their young age and a strong sense of fear; consequently, medication compliance is low[9,10].
With the development of modern medicine, clarithromycin has been gradually applied to treat mycoplasma infection-related diseases[11]. Moreover, studies have reported that it is better and safer for clinical treatment than erythromycin[9,10,12,13]. We aimed to perform a comprehensive review to investigate the clinical utility of clarithromycin for treating pediatric respiratory mycoplasma infection.
From April 2017 to July 2019, we included 106 children with mycoplasma respiratory infections. All children met the diagnostic criteria for mycoplasma respiratory infection based on the Chinese Expert Consensus for Laboratory Diagnosis of Mycoplasma pneumoniae respiratory infections.
The inclusion criteria were as follows: (1) Aged 3–12 years; (2) Having positive multiplex polymerase chain reaction (MP-PCR) test results; (3) Presenting with fever, cough, and other symptoms; (4) Their guardians understanding the treatment plan after voluntary enrollment; (5) No previous history of drug allergy; and (6) Having normal intellectual development as well as no typical mental retardation or mental developmental disability manifestations.
The exclusion criteria were as follows: (1) Having conditions that simultaneously affect the lungs, heart, kidneys, and other organs or sexually transmitted diseases; (2) Experiencing meningitis and sepsis; (3) Having incomplete information for checking the basic information; (4) Failing to complete the treatment course, being transferred from the hospital, or requesting to withdraw from the study; and (5) Not meeting any of the aforementioned inclusion criteria.
Based on a random number table, 106 children were assigned to either a clarithromycin (n = 53) or an erythromycin group (n = 53). There was no significant between-group difference in the baseline data (P > 0.05). Table 1 presents details regarding the data and corresponding test values. This study was approved by the ethics committee of our hospital after comprehensive assessment, including drug safety and research significance evaluation.
| Baseline data | Clarithromycin group (n = 53) | Erythromycin group (n = 53) | χ2/t | P value |
| Gender | 0.641 | 0.423 | ||
| Male | 28 (52.83) | 29 (54.72) | ||
| Female | 25 (47.17) | 24 (45.28) | ||
| School | ||||
| Kindergarten | 11 (20.75) | 10 (18.87) | 0.111 | 0.739 |
| Primary school | 38 (71.70) | 37 (69.81) | 0.086 | 0.769 |
| Junior high school | 4 (7.55) | 6 (11.32) | 0.832 | 0.362 |
| Age | 6.74 (1.25) | 6.83 (1.22) | 1.050 | 0.862 |
After admission, all the children were allowed to rest on a bed, provided with water and electrolyte balance correction solutions, administered with oxygen as appropriate to maintain the airway open, and provided with ice/wet towels to cool down.
Erythromycin group: Children in this group were treated using erythromycin, which was provided by Northeast Pharmaceutical Group Shenyang First Pharmaceutical Co., Ltd. (National Drug Standard H21022427; intravenous drip, 10-15 mg/kg per time, 2 times/day, and continuous administration for 14 d).
Clarithromycin group: Children in this group were treated using clarithromycin, which was provided by Baiyunshan Pharmaceutical General Factory of Guangzhou Baiyunshan Pharmaceutical Group Co., Ltd. (National Medicine Standard H20063961; 5–10 mg/kg per time, 2 times per day, and continuous administration for 14 d).
After 14 d of treatment, we obtained a deep throat swab; the PCR test was performed to record the results.
Efficacy was classified as follows: (1) Significant effect: After treatment completion, MP-PCR test results turned negative with the disappearance of clinical symptoms, including cough and fever; (2) Effective: After treatment, MP-PCR test results were positive but with significant improvement of serum mycoplasma antibody and immunoglobulin M levels and X-ray examination results as well as cough and other symptoms; and (3) Invalid: MP-PCR test results were positive without improvement or with worsening of clinical symptoms and various test results. The total clinical effectiveness rate was calculated as (total number of cases-invalid cases)/total number of cases × 100%.
The medication status of children was obtained using the Morisky medication compliance questionnaire. For children who were young and could not accept the questionnaire, it was completed by the parents. Based on the grading results, 8 points were considered as full compliance, 6 points ≤ score < 8 points as compliance, and < 6 points as non-compliance. The medication compliance rate was calculated as (number of complete compliance cases + number of compliance cases)/total number of cases × 100%.
Moreover, we recorded the antipyretic time, length of hospital stay, and medication costs and calculated the incidence of nausea and diarrhea during the treatment of children.
Statistical analyses were performed using SPSS 23.0 software. Measurement data are presented as the mean ± SD measurement and were compared using t test. Count data are presented as percentages and were compared using χ2 test. Statistical significance was set at P < 0.05
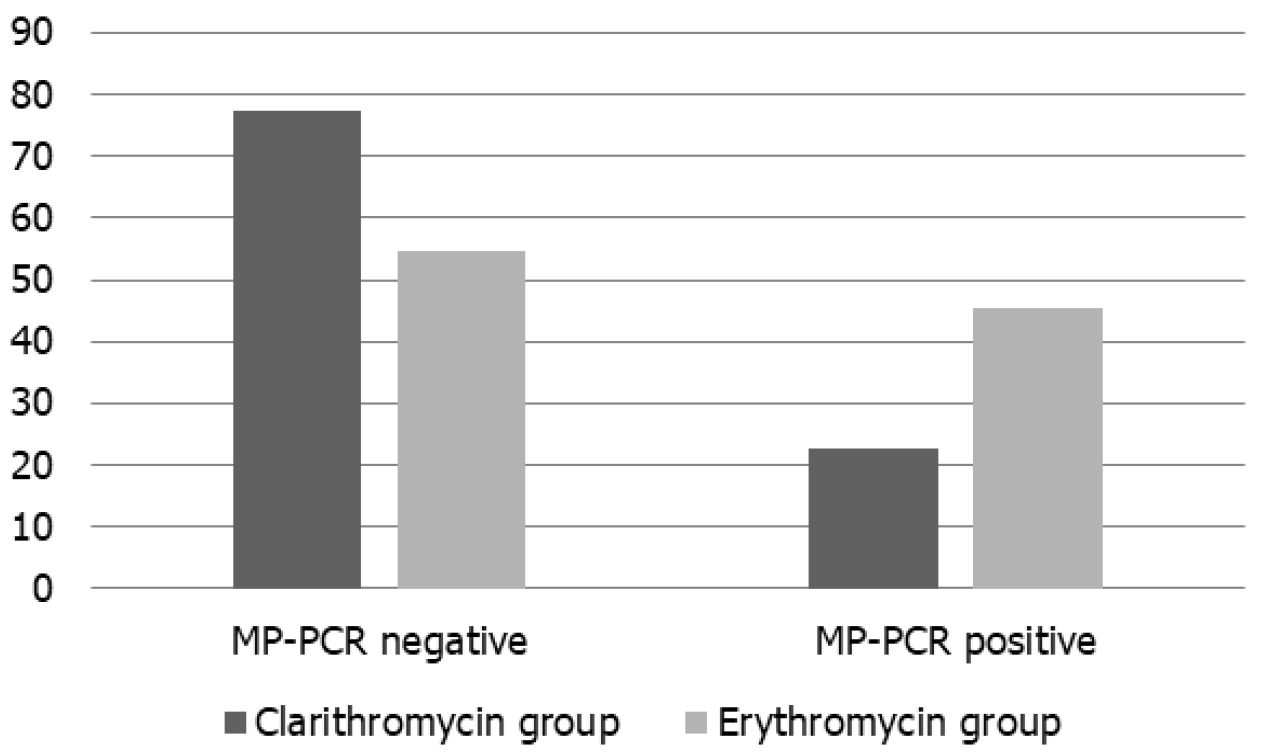
As shown in Figure 1, the clarithromycin group showed a significantly higher MP-PCR negative rate than the erythromycin group (χ2 = 11.427, P = 0.001).
As shown in Table 2, the clarithromycin group showed a significantly higher total clinical effective rate than the erythromycin group (P < 0.05).
| Group | Number of cases | Invalid | Effective | Marked effect | Total clinical effectiveness |
| Clarithromycin group | 53 | 2 (3.77) | 19 (35.85) | 32 (60.38) | 51 (96.23) |
| Erythromycin group | 53 | 13 (24.53) | 22 (41.51) | 18 (33.96) | 40 (75.47) |
| χ2 | - | 17.739 | 0.675 | 14.005 | 17.739 |
| P value | - | 0.001 | 0.411 | 0.001 | 0.001 |
The clarithromycin group showed a significantly lower hospitalization time, antipyretic time, and drug cost than the erythromycin group (P < 0.05; Table 3).
| Clarithromycin group (n = 53) | Erythromycin group (n = 53) | t | P value | |
| Hospital stays (d) | 15.14 ± 2.01) | 20.63 ± 3.15 | 10.696 | 0.001 |
| Drug cost (Yuan) | 1654.63 ± 100.21 | 2152.14 ± 163.48 | 18.889 | 0.001 |
| Antipyretic time (d) | 2.63 ± 0.57 | 4.06 ± 0.75 | 11.051 | 0.001 |
As shown in Table 4, the clarithromycin group showed a significantly lower incidence of toxic and side effects than the erythromycin group (P < 0.05).
| Group | Number of cases | Stomach ache | Diarrhea | Nausea | Other | Incidence of side effects |
| Clarithromycin group | 53 | 2 (3.77) | 1 (1.89) | 2 (3.77) | 3 (5.66) | 8 (15.09) |
| Erythromycin group | 53 | 8 (15.09) | 7 (13.21) | 10 (18.87) | 11 (20.75) | 36 (67.92) |
| χ2 | - | 7.502 | 9.179 | 11.357 | 9.934 | 57.479 |
| P value | - | 0.006 | 0.002 | 0.001 | 0.002 | 0.001 |
As shown in Table 5, the clarithromycin group showed a significantly higher compliance rate than the erythromycin group (P < 0.05).
| Group | Number of cases | Full compliance | Obey | Disobey | Medication compliance |
| Clarithromycin group | 53 | 40 (81.13) | 12 (22.64) | 1 (1.89) | 52 (98.11) |
| Erythromycin group | 53 | 18 (33.96) | 20 (37.74) | 15 (28.30) | 38 (71.70) |
| χ2 | - | 45.537 | 5.409 | 27.211 | 27.211 |
| P value | - | 0.001 | 0.020 | 0.001 | 0.001 |
We evaluated the efficacy of erythromycin and clarithromycin for treating respiratory mycoplasma infection in children. Our findings indicated that clarithromycin had various advantages over erythromycin, including higher application safety, stronger mycoplasma clearance, and higher medication compliance.
Human immunity gradually increases with age until the age of 35 years, peaking at the age of 22–35 years, and subsequently decreasing with age. Given the immature body development in children, they have relatively low immune function and are vulnerable to pathogen infection. Therefore, respiratory tract infections are more common in children than in adults. Studies have shown that respiratory infections caused by the invasion of various pathogens are among the main childhood mortality causes in China.
Mycoplasma belongs to a class of pathogens that infect viruses and bacteria. Epidemiological studies have shown that with the modernization process in China, there has been an increase in the urban population, population density, and risk of multiple pathogen infections; accordingly, there has been an annual increase in the rate of mycoplasma-caused respiratory infections in children. Children with respiratory mycoplasma infections present with fever, headache, and cough. Failure to administer timely interventions can result in critical illnesses, including respiratory failure, which can seriously endanger the physical and mental health of children.
Currently, the main treatment for mycoplasma respiratory infections is antibiotic treatment, which involves drug interventions for suppressing and killing pathogens in children. For many years, a treatment regimen based on macrolide antibiotics has been used in clinical practice[14-16]. Macrolide antibiotics strongly affect protein synthesis in pathogens by blocking 50 S ribose endopeptide acyltransferase activity in the pathogens, which quickly kills the pathogens. Therefore, significant therapeutic effects can be achieved in bacterial infection treatment[17].
Erythromycin and clarithromycin are both macrolide antibiotics, with the former being a first-generation macrolide antibiotic. Erythromycin has been used for many years in clinical practice and has been widely applied to treat respiratory mycoplasma infection in children. However, it has high toxicity and side effects, and causes poor metabolic capacity in children, which makes them prone to various gastrointestinal reactions after drug administration. Clarithromycin is a 14-ring semisynthetic derivative of macrolides that is similar to erythromycin and has good oral absorption. The peak blood concentration occurs within 2 h of clarithromycin administration; moreover, it has a higher drug bioavailability than erythromycin. The peak blood concentration of clarithromycin can be more than twice that of erythromycin. Further, the half-life of clarithromycin is approximately 4–5 times that of erythromycin, with reduced gastrointestinal tract irritation and few toxic side effects[18-20].
In our study, the clarithromycin group had a significantly higher negative rate of MP-PCR and overall clinical effectiveness, as well as a significantly lower incidence of side effects (P < 0.05) than the erythromycin group. This indicates that clarithromycin has better clinical application than erythromycin. Moreover, compared with erythromycin, clarithromycin was less toxic, had fewer side effects, and had better safety. The clarithromycin group had a significantly shorter hospitalization length and antipyretic time than the erythromycin group (P < 0.05), which indicates that clarithromycin allows faster symptom relief and a better pathogen inhibitory effect than erythromycin. In addition, the clarithromycin group showed a significantly lower drug cost (P < 0.05), indicating that clarithromycin has higher bioavailability, and thus requires a relatively lower dosage with a concomitant reduction in the drug cost than erythromycin. Additionally, clarithromycin has great economic benefits.
In addition, the clarithromycin group showed a significantly higher compliance rate than the erythromycin group (P < 0.05). Generally, rational cognition in children is incomplete; accordingly, children are prone to fear intravenous injections in the clinical treatment process and are likely to show resistance. Clarithromycin is generally orally administered, which effectively solves the aforementioned problem. Children may have low compliance, including resistance and crying, during intravenous administration. Studies have reported the clinical efficacy of erythromycin and clarithromycin in children with respiratory mycoplasma infections. These previous findings have indicated that clarithromycin has a higher total effective rate and a lower incidence of adverse reactions than erythromycin, which is consistent with our findings and further confirms that clarithromycin has more application advantages than erythromycin.
In summary, different antibiotics for treating clinical mycoplasma respiratory infections in children have different treatment outcomes. Clarithromycin is superior to erythromycin with respect to its application, safety, and economic benefits; accordingly, it can be preferentially selected over erythromycin.
Children are more susceptible to infection with Mycoplasma pneumoniae. At the same time, they are more susceptible to disease after infection and have difficulty breathing and can experience respiratory failure or critical illness. The treatment of this disease has received widespread clinical attention.
To search drugs that can replace erythromycin in the treatment of respiratory tract infections in children.
This study aimed to analyze the clinical efficacy of different antibiotics in the treatment of pediatric respiratory mycoplasma infection.
One hundred and six children diagnosed with respiratory mycoplasma infection were included in this study. The clinical efficacy was evaluated and compared between groups. The compliance of children during treatment was evaluated and compared between groups.
The total effective rate of clinical treatment of children in the clarithromycin group was significantly higher than that in the erythromycin group. The incidence of toxic and side effects in the clarithromycin group was significantly lower than that in the erythromycin group, and the above data comparisons were statistically significant.
Clarithromycin has a variety of advantages over erythromycin, such as higher application safety, stronger mycoplasma clearance, and higher medication compliance in children, and can be actively promoted.
Clarithromycin is superior to erythromycin in terms of application effect, safety, and economic benefits and can be preferentially selected.
Manuscript source: Unsolicited manuscript
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Printz C S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Mathur S, Fuchs A, Bielicki J, Van Den Anker J, Sharland M. Antibiotic use for community-acquired pneumonia in neonates and children: WHO evidence review. Paediatr Int Child Health. 2018;38:S66-S75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Brown N, Kukka AJ, Mårtensson A. Efficacy of zinc as adjunctive pneumonia treatment in children aged 2 to 60 months in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Paediatr Open. 2020;4:e000662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Tagliabue C, Techasaensiri C, Torres JP, Katz K, Meek C, Kannan TR, Coalson JJ, Esposito S, Principi N, Leff R, Baseman JB, Hardy RD. Efficacy of increasing dosages of clarithromycin for treatment of experimental Mycoplasma pneumoniae pneumonia. J Antimicrob Chemother. 2011;66:2323-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Gendrel D, Raymond J, Moulin F, Iniguez JL, Ravilly S, Habib F, Lebon P, Kalifa G. Etiology and response to antibiotic therapy of community-acquired pneumonia in French children. Eur J Clin Microbiol Infect Dis. 1997;16:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Ruhrmann H, Blenk H. [Erythromycin vs amoxicillin for the treatment of pneumonia in children (author's transl)]. Infection. 1982;10 Suppl 2:S86-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Nascimento-Carvalho CM, Souza-Marques HH. [Recommendation of the Brazilian Society of Pediatrics for antibiotic therapy in children and adolescents with community-acquired pneumonia]. Rev Panam Salud Publica. 2004;15:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Han R, Yu Q, Zhang G, Li B, Han S, Li G. Comparison of azithromycin and erythromycin in the treatment of mycoplasma pneumonia in children. Pak J Med Sci. 2020;36:156-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Vasilos LV, Rumel' NB, Shchuka SS. [Chemotherapeutic effectiveness of erythromycin, rifampicin and tetracyclines in chlamydiosis and mycoplasmosis in children]. Antibiot Khimioter. 1995;40:40-42. [PubMed] |
| 9. | Lee PI, Wu MH, Huang LM, Chen JM, Lee CY. An open, randomized, comparative study of clarithromycin and erythromycin in the treatment of children with community-acquired pneumonia. J Microbiol Immunol Infect. 2008;41:54-61. [PubMed] |
| 10. | Alvarez-Elcoro S, Enzler MJ. The macrolides: erythromycin, clarithromycin, and azithromycin. Mayo Clin Proc. 1999;74:613-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 212] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Chien SM, Pichotta P, Siepman N, Chan CK. Treatment of community-acquired pneumonia. A multicenter, double-blind, randomized study comparing clarithromycin with erythromycin. Canada-Sweden Clarithromycin-Pneumonia Study Group. Chest. 1993;103:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Schönwald S, Gunjaca M, Kolacny-Babić L, Car V, Gosev M. Comparison of azithromycin and erythromycin in the treatment of atypical pneumonias. J Antimicrob Chemother. 1990;25 Suppl A:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Langtry HD, Brogden RN. Clarithromycin. A review of its efficacy in the treatment of respiratory tract infections in immunocompetent patients. Drugs. 1997;53:973-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 15. | Fujii R, Meguro H, Arimasu O, Hiruma F, Sugamata K, Sugie N, Higa A, Shinozaki T, Abe T, Sunagawa K. [Bacteriological, pharmacokinetic and clinical studies on clarithromycin in the pediatric field. Pediatric Study Group of Clarithromycin]. Jpn J Antibiot. 1989;42:512-541. [PubMed] |
| 16. | Ogura H, Kubota H, Nomura I, Tomoda T, Araki K, Ogura Y, Kurashige T. [Clinical efficacy of clarithromycin in the field of pediatrics]. Jpn J Antibiot. 1989;42:401-410. [PubMed] |
| 17. | Rogozinski LE, Alverson BK, Biondi EA. Diagnosis and treatment of Mycoplasma pneumoniae in children. Minerva Pediatr. 2017;69:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Watanabe K, Otabe K, Shimizu N, Komori K, Mizuno M, Katano H, Koga H, Sekiya I. High-sensitivity virus and mycoplasma screening test reveals high prevalence of parvovirus B19 infection in human synovial tissues and bone marrow. Stem Cell Res Ther. 2018;9:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Nishimura T, Tabuki K, Aoki S, Takagi M. [Laboratory and clinical studies of clarithromycin in pediatric fields]. Jpn J Antibiot. 1989;42:353-369. [PubMed] |
| 20. | Ito S, Mayumi M, Mikawa H. [Clinical evaluation of clarithromycin in pediatric patients]. Jpn J Antibiot. 1989;42:343-351. [PubMed] |