Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6566
Peer-review started: April 12, 2021
First decision: April 23, 2021
Revised: May 1, 2021
Accepted: May 15, 2021
Article in press: May 15, 2021
Published online: August 6, 2021
Processing time: 106 Days and 20.5 Hours
Primary non-dural central nervous system mucosa-associated lymphoid tissue (MALT) lymphoma is a rare indolent B-cell lymphoma, with only a few reported cases worldwide.
A 33-year-old man presented with a 5-mo history of left blepharoptosis and a 4-mo history of right limb numbness and weakness. Magnetic resonance imaging showed a significantly enhanced mass in the left midbrain. Subsequent positron emission tomography revealed that the lesion had increased glucose uptake. A stereotactic robotic biopsy supported a diagnosis of MALT lymphoma. Then he was treated with radiation therapy (30Gy/15F), which resulted in complete remission. We also review the literature on brain parenchymal-based MALT lymphoma, including the clinical presentation, treatment options, and outcomes.
Although there is no consensus on the optimal treatment for this rare disease, patients can respond well when treated with radiotherapy alone.
Core Tip: Primary central nervous system mucosa-associated lymphoid tissue (MALT) lymphoma is a rare disease, especially in the brain parenchyma. A clear diagnosis is important because it can be cured. This report presents the treatment of MALT lymphoma developing in the midbrain. The patient received local radiotherapy and was in complete remission without apparent adverse effects.
- Citation: Zhao YR, Hu RH, Wu R, Xu JK. Primary mucosa-associated lymphoid tissue lymphoma in the midbrain: A case report. World J Clin Cases 2021; 9(22): 6566-6574
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6566.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6566
Primary central nervous system lymphoma (PCNSL) is an extranodal non-Hodgkin lymphoma (NHL). Approximately 90% of PCNSL cases are diffuse large B-cell lymphomas, defined as aggressive neoplasms[1]. The incidence of primary central nervous system (CNS) indolent lymphoma is much lower, and marginal zone lymphoma (MZL) is comparatively the most common type. Mucosa-associated lymphoid tissue (MALT) lymphoma, also known as extranodal MZL, is one subtype of MZL. It is a B-cell lymphoma originating from mucosal-associated lymphoid tissue, originally described as a low-grade lymphoma in the gastrointestinal tract by Isaacson and Wright[2]. The stomach is the most common primary site of MALT lymphoma; the salivary glands, thyroid, ocular adnexa, lungs, and breasts are other common sites[3]. Primary CNS MALT lymphoma is rare. Most previous case reports and case series have reported primary CNS MALT lymphoma arising in the dura mimicking meningioma or subdural haematoma[4-6]. Rare cases involving the brain parenchyma have been reported, and some patients are clinically misdiagnosed with glioma[7,8]. There are also case reports that describe spinal or both brain and spinal involvement[9,10].
Herein, we present a case of primary CNS MALT lymphoma occurring in the midbrain. To the best of our knowledge, this is the first report of midbrain MALT lymphoma. We also present a review of MALT lymphoma arising in the brain parenchyma, including the clinical presentation, treatment options, and outcomes.
A 33-year-old human immunodeficiency virus-negative man visited our hospital in April 2020 with left blepharoptosis and right limb numbness and weakness.
The patient’s symptoms started 5 mo ago with left blepharoptosis and were not taken seriously. Four months earlier, the patient began to experience right limb numbness and weakness.
The patient had a 1-year history of non-insulin-dependent type 2 diabetes mellitus and tuberculosis (TB). TB lesions were confined to the lung. Computed tomography (CT) of the chest showed multiple nodular infiltrations on both sides of the lung, and the main lesion was located in the right upper lobe. He was receiving anti-TB treatment with rifampicin, isoniazid, ethambutol, and moxifloxacin.
There was no special history or personal history. The patient had no known family history of cancer.
Neurologic examination revealed right-sided limb numbness at the distal end, the muscle strength of the right limb was weakened (grade 4), and the superficial sensation in the right limb was hypoesthesia, without any other pathological signs.
Laboratory evaluations revealed that the level of C-reactive protein was 21.6 mg/L, the erythrocyte sedimentation rate was 33 mm/h, the blood glucose level was 6.8 mmol/L, and the T-SPOT-TB test was positive.
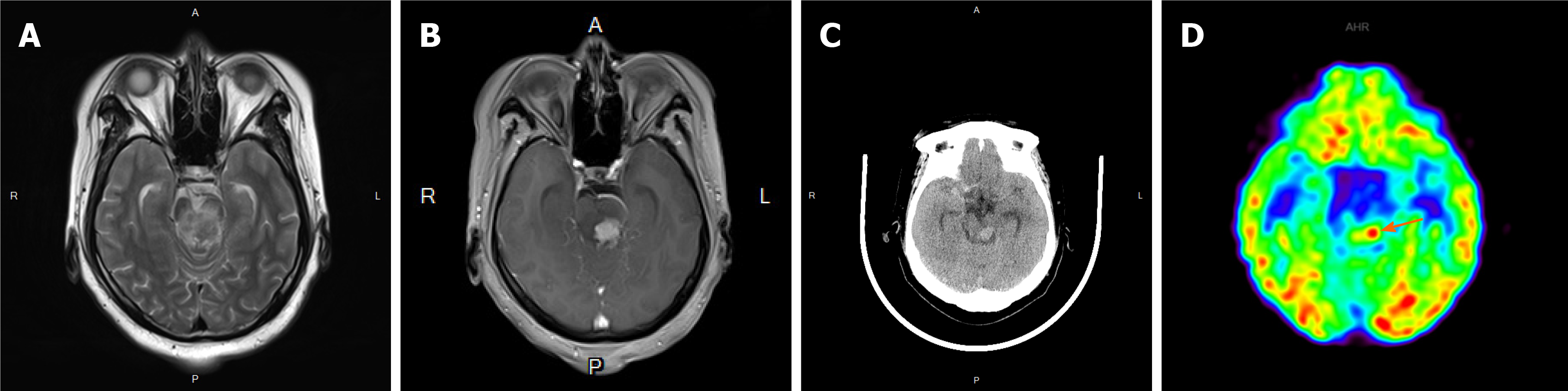
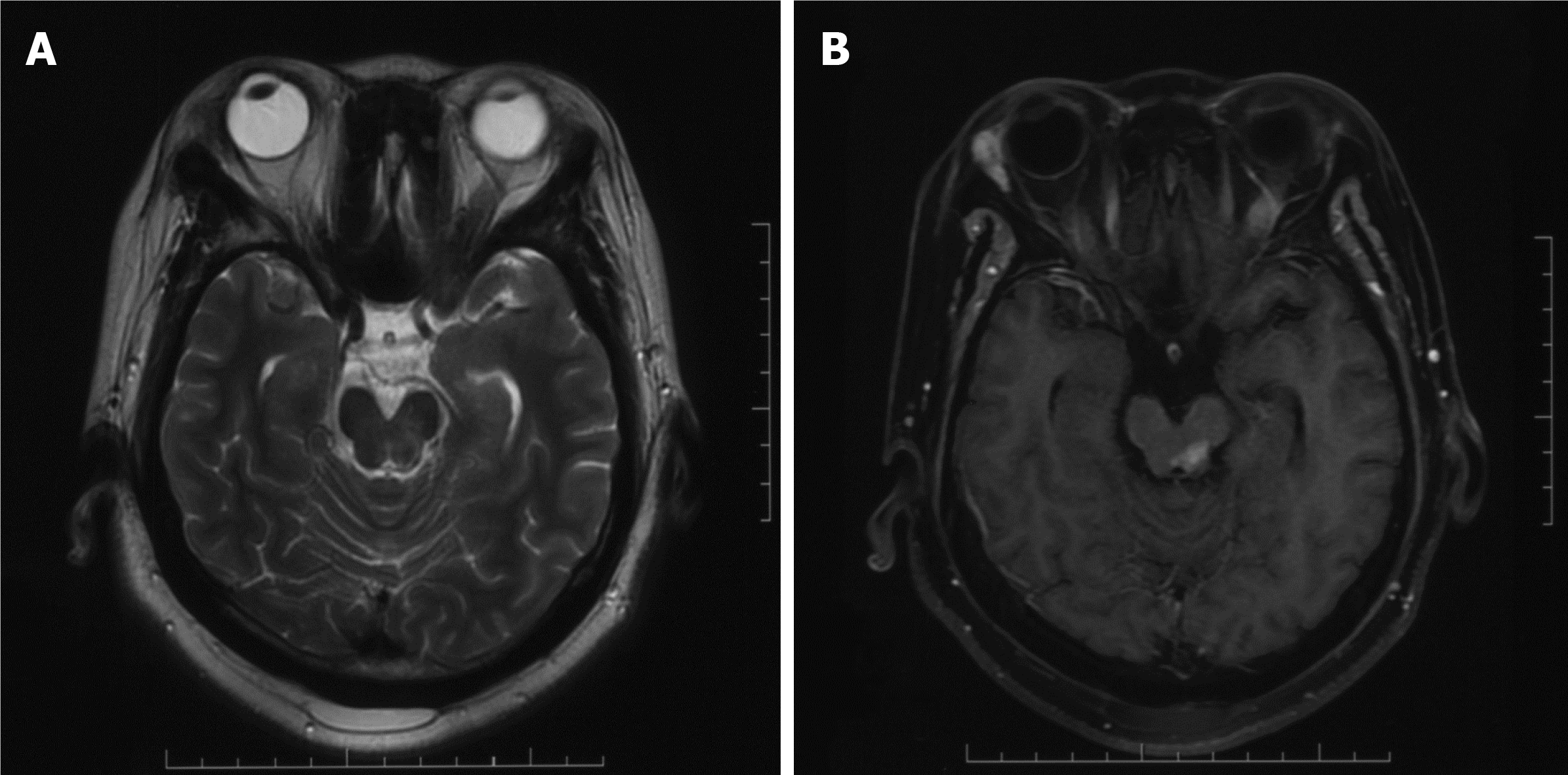
A CT scan, as well as a magnetic resonance imaging (MRI) scan, revealed a significantly enhanced mass of 1.9 cm × 1.8 cm in size in the left midbrain (Figure 1A-C). Flaky edema could be seen around the lesion, and no signal abnormalities were noted elsewhere in the brain. Due to the relatively homogeneous enhancement of the lesion, the clinical impression was that the lesion most likely represented a lymphoma. Fluorodeoxyglucose positron emission tomography (PET) showed that the maximum standardized uptake volume (SUV) was 7.48, which matched with an enhanced lesion of the brain (Figure 1D). At the same time, a lesion in the right third fore rib was identified, and the maximum SUV was 5.70.
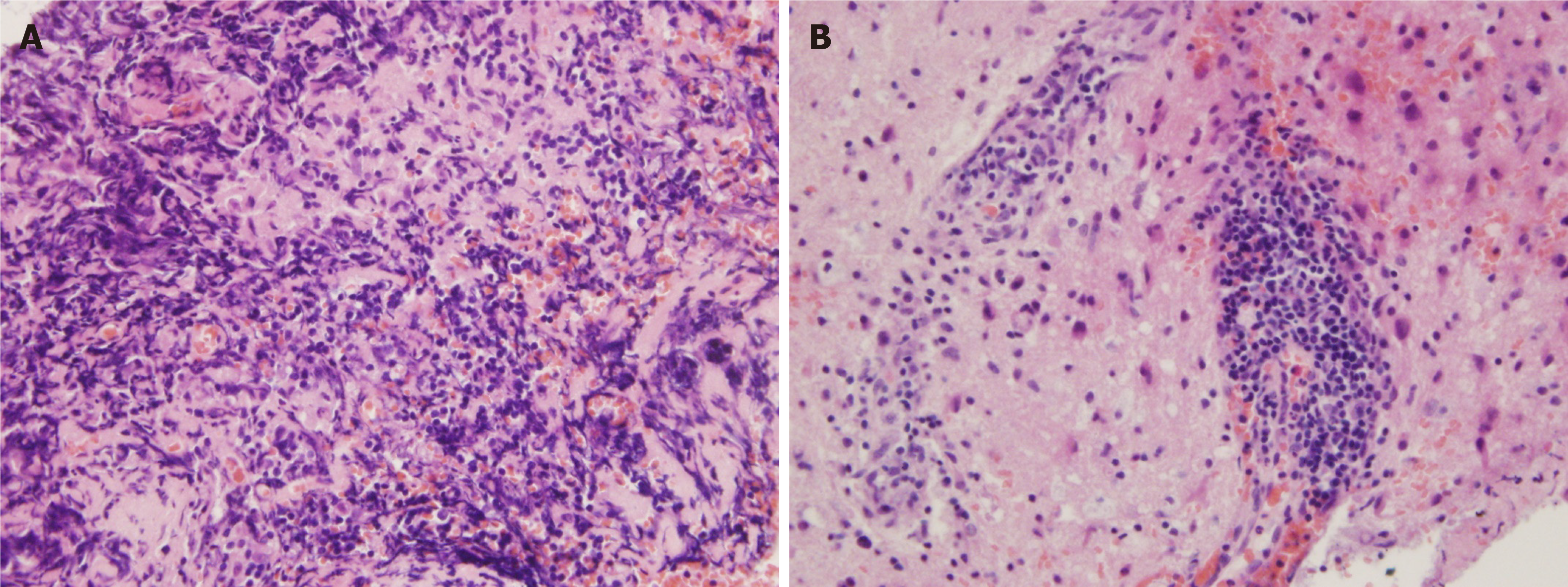
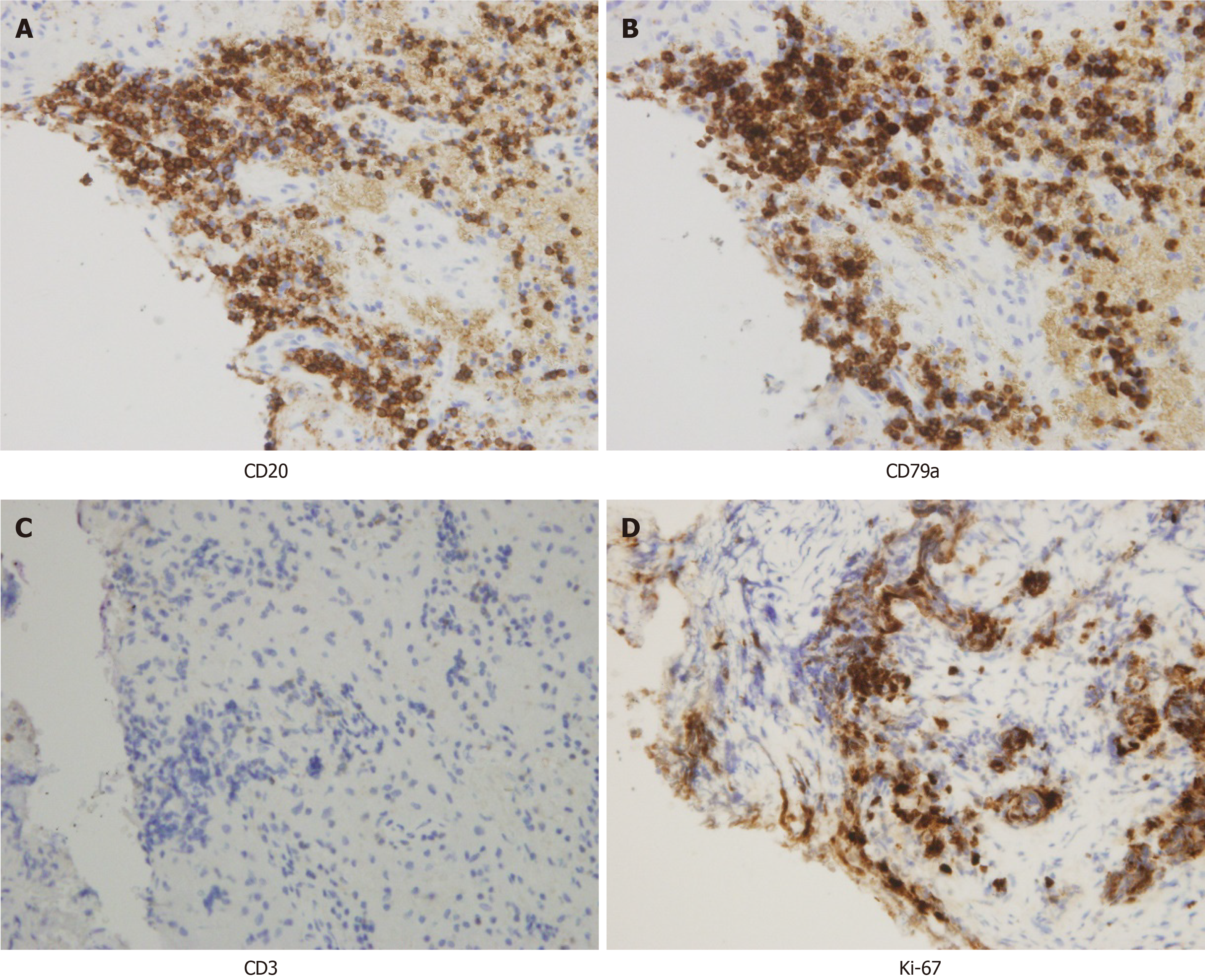
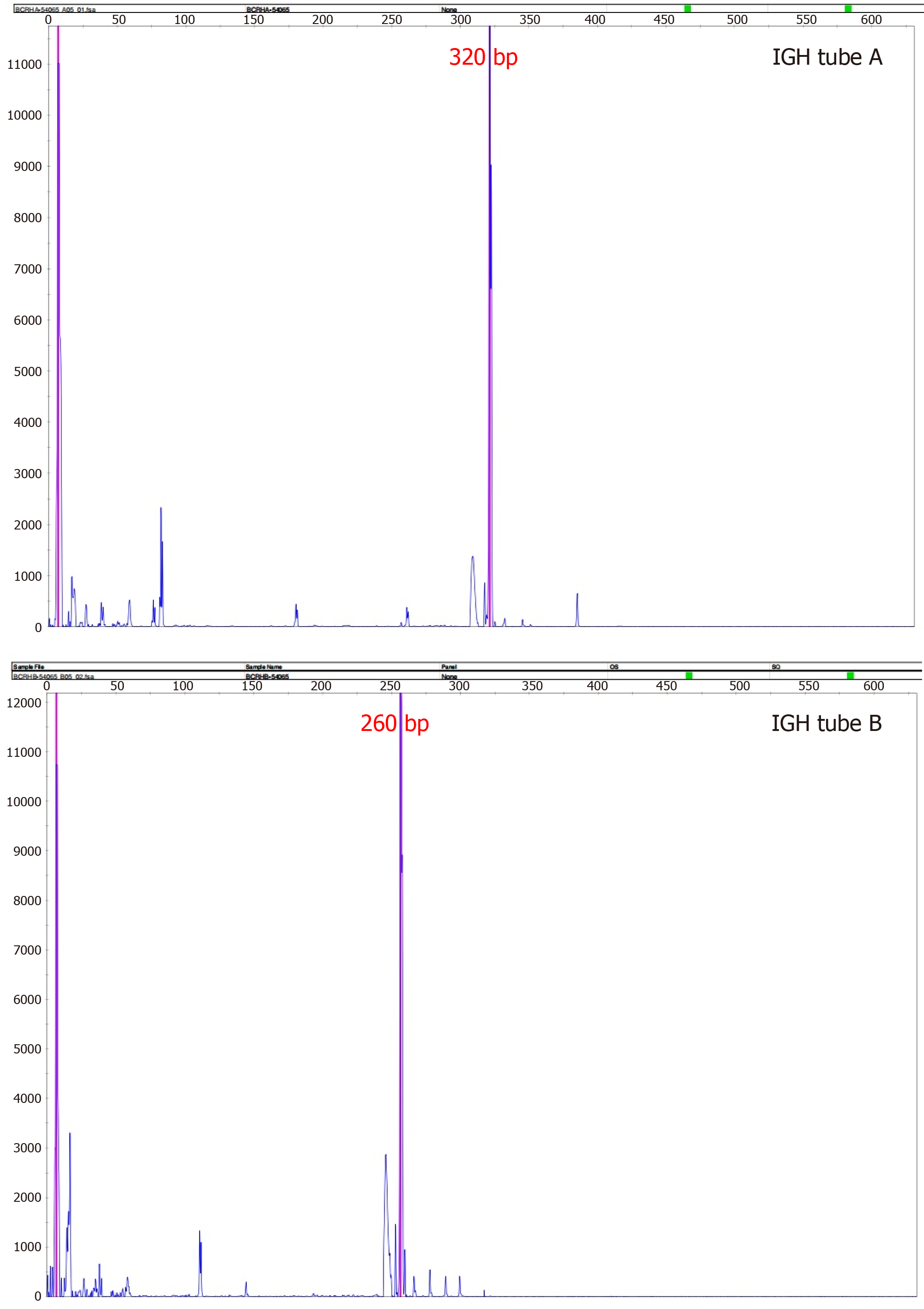
A stereotactic robotic biopsy of the brain was performed by the left frontal-lateral paraventricular approach on June 4, 2020. The patient was in a supine position under general anesthesia. The biopsy needle was implanted into the center of the lesion according to the preoperative plan, and the pathological tissue was cut out. The histopathological evaluation of the midbrain lesion supported a diagnosis of indolent B-cell lymphoma. The morphology indicated infiltration of low-grade B-cell lymphoma with a perivascular growth pattern (Figure 2). Immunohistochemical detection showed CD20+, CD79a+, and CD38+/- results but negativity for CD3 and CD5. The Ki-67 proliferation rate was 10%-20% (Figure 3), and the other results were LCA (+), CD138 (-), CD21 (-), CD68 (scattered +), PAX-5 (+), and TdT (-). Polymerase chain reaction (PCR) analysis detected clonal rearrangement of the immunoglobulin heavy chain gene (IgH) (Figure 4). DNA sequencing indicted no mutations in the B-cell lymphoma genes, including Bcl-2.
Routine biochemical examination of cerebrospinal fluid (CSF) from lumbar puncture showed a cell count of 132 × 106/L, leucocyte count of 32 × 106/L, glucose level of 3.94 mmol/L, protein level of 54 mg/dL, and chlorine level of 124 mmol/L. The pathology of CSF was scattered lymphocytes, erythrocytes, and mononuclear cells. Bone marrow aspiration and biopsy with flow cytometry were normal, and ophthalmologic evaluations revealed no abnormalities. However, the rapid urease test for Helicobacter pylori was positive. Then, a rib lesion biopsy was performed on August 10, 2020.
The final pathological result was MALT lymphoma. The pathology of the rib was callus formation.
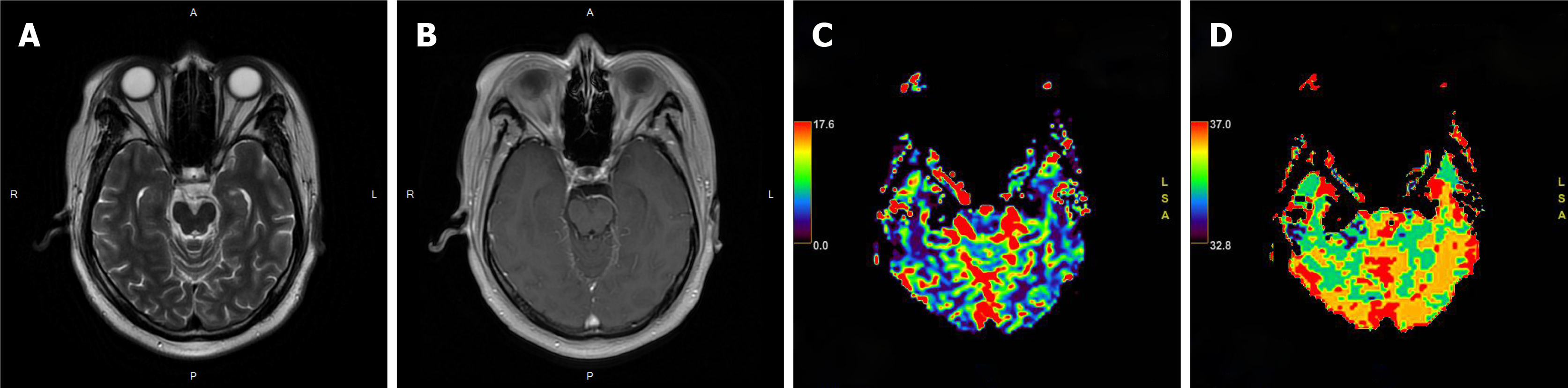
The patient received local external beam radiotherapy without chemotherapy, and target delineation was based on the fusion image obtained from simulated CT and MRI. The gross target volume (GTV) was defined on MRI and PET, excluding the edema zone. The planning GTV (PGTV) was the GTV plus 3 mm of setup margin. Initially, we intended to administer a radiotherapy dose of 24 Gy, but re-examination by MRI showed residual lesion during the treatment course after 20 Gy was administered (Figure 5). We added 6 Gy to the total dose of 30 Gy. Radiotherapy was administered in the period from September 7, 2020 to September 25, 2020.
One month after radiotherapy, follow-up MRI showed no abnormal enhancement, and perfusion-weighted imaging showed no hyperperfusion (Figure 6). After 6 mo of follow-up, the patient's clinical symptoms significantly improved. The patient’s muscle strength recovered to grade 5-, and the superficial sensation was normal. He could walk normally, but he could not hold heavy things in his right hand and sometimes felt numbness in the right limb at the distal end. The follow-up data showed no recurrence.
MZL is an NHL arising from post-germinal center marginal zone B cells. According to the 2016 World Health Organization classification, MZL is subdivided into three types: Extranodal MZL or MALT lymphoma, nodal MZL, and splenic MZL[11]. MALT lymphoma is the most typical type, but primary CNS MALT lymphoma is an extremely rare entity, especially in the brain parenchyma. Initial studies showed that the most common location was the dura[12]. Only seven cases with brain parenchyma involvement have been reported, including our patient. The site of origin was the midbrain in our patient. The lesion location and clinical characteristics of the other six patients are shown in Table 1. Clinical symptoms are not specific, depending on the site of the lesion.
| Ref. | Age (yr) | Sex | Location | Presentation | Treatment | Outcome |
| Tu et al[13] | 66 | M | R, frontal | Seizures | Radiation (WBRT, dose NA) | CR |
| Park et al[8] | 18 | M | L, basal ganglia | Right-sided central facial nerve palsy, right-sided weakness, dizziness, dysarthria | Radiation (CTV = GTV + 15 mm, PTV = CTV + 5 mm; 30.6 Gy/17F) | CR |
| Papanicolau-Sengos et al[14] | 70 | M | L, posteriorputamen | Right extremity numbness, dysarthria, blurry vision | Chemotherapy (dexamethasone, temozolamide, rituximab) | SD |
| Schiefer et al[15] | 39 | F | R, frontal | Seizures | Chemotherapy (intrathecal: Methotrexate, cytarabin, dexamethasone; Intravenous: High-dose methotrexate) | SD |
| Aqil et al[7] | 48 | M | L, frontal | Seizures, memory loss | Radiation (WBRT, 24 Gy; GTV boosted 6 Gy) | CR |
| Ueba et al[10] | 53 | M | R, temporal; L, occipital; spinal cord | Recent memory disturbance, gait disturbance, urinary incontinence | Chemotherapy (high-dose methotrexate, cytoarabine) | PR |
The CNS has no mucosa or MALT tissue, and dural-based MALT lymphoma can be explained by the embryological analogy that meningothelial cells of the arachnoid membrane could be analogous to epithelial cells, where MALT lymphomas arise[13-16]. However, non-dural-based MALT lymphoma is questionably explained by this theory. It is currently believed that the etiology of MALT lymphoma is related to chronic immune stimulation caused by infection or inflammation. For instance, gastric MALT lymphoma is associated with Helicobacter pylori, Sjögren syndrome, or Hashimoto thyroiditis and carries a significant risk for the development of MZL[17]. Interestingly, our patient had a 1-year history of TB and received standardized anti-TB treatment. After admission, the Helicobacter pylori examination was positive, and the patient also underwent Helicobacter pylori eradication therapy. The pathogenesis may be explained by the inflammation-based theory. However, we have no direct evidence that primary CNS MALT lymphoma is associated with Mycobacterium tuberculosis or Helicobacter pylori infection.
The diagnosis of MALT lymphoma should be confirmed by histopathological and immunohistochemical features. Differential diagnoses include lymphoplasmacytic lymphoma (LPL) and follicular lymphoma. The immunohistochemistry results of follicular lymphoma usually indicate positivity for CD10 and Bcl-2[18]. LPL and MALT lymphoma have similar morphological and immunohistochemical profiles, but relative to MALT lymphoma, LPL typically involves the bone marrow and is associated with Waldenstrom’s macroglobulinemia[19]. Our patient’s immunohistochemical findings indicated CD20+ and CD79a+ results, the Ki-67 index was 10%-20%, and there was no bone marrow involvement and no clinical history of Waldenstrom’s macroglobulinemia. At the same time, clonal rearrangement of IgH was detected by PCR. According to these findings, the diagnosis was most consistent with MALT lymphoma.
There is no standard treatment for CNS MALT lymphoma. The treatment modalities reported in the existing literature include surgery, radiotherapy, and chemotherapy. As shown in Table 1, among patients with lesions arising from the brain parenchyma, three of the six patients received chemotherapy: Two patients had stable disease, one showed tumor remission, and the other three received radiotherapy and had a complete response. In other words, radiotherapy may provide superior outcomes in parenchymal-based cases[20].
MALT lymphoma tends to be indolent and radiosensitive. In 2011, a randomized phase III trial reported that there was no difference in clinical efficacy between radiotherapy doses of 24 Gy and 40-45 Gy for indolent NHL[21]. Currently, reduced-dose (24-30 Gy) radiotherapy is preferred for indolent lymphoma. Unlike high-grade CNS lymphoma, the role of intrathecal chemotherapy or systemic chemotherapy in low-grade CNS lymphoma currently remains unclear[22]. Because of the particularity of the lesion location, the lesion in our patient could not be totally resected by surgery and he achieved complete remission by radiotherapy alone. Involved-site radiation therapy is an effective initial treatment for extranodal MZL[23]. The radiation field in our case included only the primary lesion demonstrated on MRI and PET, not as reported in the prior literature[7,8,13]. Reexamination during treatment showed residual disease, so we believe that 1 mo after the end of radiotherapy might be the best timing to evaluate the effect.
Considering the low biological and clinical aggressiveness of MALT lymphoma, it is curable in cases of localized disease. The data showed that there was no recurrence during the follow-up of up to 22 mo in primary left basal ganglia MALT lymphoma with radiation therapy[8]. The follow-up of our patient was short (6 mo), and we will continue to pay attention to any changes in the patient's condition.
In conclusion, primary non-dural CNS MALT lymphoma is a rare disease. The exact mechanism is still unclear. Diagnosis is based on morphological and immunohistochemical findings. It is radiosensitive and can be cured with radiotherapy. Chemotherapy alone cannot achieve good treatment outcomes. Due to the small number of cases, it is difficult to draw conclusions regarding the use of radiotherapy as the primary treatment for brain parenchymal-based MALT lymphoma. More clinical data are needed to confirm this opinion.
We acknowledge Wang YM, a neurosurgeon of Xuanwu Hospital Capital Medical University, for his special contribution to this case. We acknowledge the work of colleagues in the Pathology and Radiology Department in offering the original images and data related to this article.
Manuscript source: Unsolicited manuscript
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fagioli F, Li C, Ono T, S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Li JH
| 1. | Batchelor TT. Primary central nervous system lymphoma: A curable disease. Hematol Oncol. 2019;37 Suppl 1:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Khalil MO, Morton LM, Devesa SS, Check DP, Curtis RE, Weisenburger DD, Dores GM. Incidence of marginal zone lymphoma in the United States, 2001-2009 with a focus on primary anatomic site. Br J Haematol. 2014;165:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Razaq W, Goel A, Amin A, Grossbard ML. Primary central nervous system mucosa-associated lymphoid tissue lymphoma: case report and literature review. Clin Lymphoma Myeloma. 2009;9:E5-E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Jesionek-Kupnicka D, Smolewski P, Kupnicki P, Pluciennik E, Zawlik I, Papierz W, Kordek R. Primary extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type in the central nervous system (MZL CNS) presented as traumatic subdural hematoma and subarachnoid bleeding - case report. Clin Neuropathol. 2013;32:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Choi JY, Chung JH, Park YJ, Jung GY, Yoon TW, Kim YJ, Lim Tk, Kim BS, Nam SH. Extranodal Marginal Zone B-Cell Lymphoma of Mucosa-Associated Tissue Type Involving the Dura. Cancer Res Treat. 2016;48:859-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Aqil B, Rouah E, Verstovsek G. Primary CNS marginal zone lymphoma: a case report and review of the literature. Open J Pathol. 2013;3:55-59. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Park I, Huh J, Kim JH, Lee SW, Ryu MH, Kang YK. Primary central nervous system marginal zone B-cell lymphoma of the Basal Ganglia mimicking low-grade glioma: a case report and review of the literature. Clin Lymphoma Myeloma. 2008;8:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Ahmadi SA, Frank S, Hänggi D, Eicker SO. Primary spinal marginal zone lymphoma: case report and review of the literature. Neurosurgery. 2012;71:E495-508; discussion E508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Ueba T, Okawa M, Abe H, Inoue T, Takano K, Hayashi H, Nabeshima K, Oshima K. Central nervous system marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type involving the brain and spinal cord parenchyma. Neuropathology. 2013;33:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5407] [Article Influence: 600.8] [Reference Citation Analysis (0)] |
| 12. | Sunderland AJ, Steiner RE, Al Zahrani M, Pinnix CC, Dabaja BS, Gunther JR, Nastoupil LJ, Jerkeman M, Joske D, Cull G, El-Galaly T, Villa D, Cheah CY. An international multicenter retrospective analysis of patients with extranodal marginal zone lymphoma and histologically confirmed central nervous system and dural involvement. Cancer Med. 2020;9:663-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Tu PH, Giannini C, Judkins AR, Schwalb JM, Burack R, O'Neill BP, Yachnis AT, Burger PC, Scheithauer BW, Perry A. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low-grade CNS lymphoma that mimics meningioma. J Clin Oncol. 2005;23:5718-5727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Papanicolau-Sengos A, Wang-Rodriguez J, Wang HY, Lee RR, Wong A, Hansen LA, Mahooti S, Rashidi HH. Rare case of a primary non-dural central nervous system low grade B-cell lymphoma and literature review. Int J Clin Exp Pathol. 2012;5:89-95. [PubMed] |
| 15. | Schiefer AI, Vastagh I, Molnar MJ, Bereczki D, Varallyay G, Deak B, Csomor J, Turanyi E, Kovacs GG, Müllauer L. Extranodal marginal zone lymphoma of the CNS arising after a long-standing history of atypical white matter disease. Leuk Res. 2012;36:e155-e157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Kumar S, Kumar D, Kaldjian EP, Bauserman S, Raffeld M, Jaffe ES. Primary low-grade B-cell lymphoma of the dura: a mucosa associated lymphoid tissue-type lymphoma. Am J Surg Pathol. 1997;21:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Zucca E, Bertoni F, Vannata B, Cavalli F. Emerging role of infectious etiologies in the pathogenesis of marginal zone B-cell lymphomas. Clin Cancer Res. 2014;20:5207-5216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020;95:316-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 19. | Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR, Dimopoulos MA. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 682] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 20. | Nomani L, Cotta CV, Hsi ED, Ferry JA, Cook JR. Extranodal Marginal Zone Lymphoma of the Central Nervous System Includes Parenchymal-Based Cases With Characteristic Features. Am J Clin Pathol. 2020;154:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Lowry L, Smith P, Qian W, Falk S, Benstead K, Illidge T, Linch D, Robinson M, Jack A, Hoskin P. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol. 2011;100:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 22. | Ayanambakkam A, Ibrahimi S, Bilal K, Cherry MA. Extranodal Marginal Zone Lymphoma of the Central Nervous System. Clin Lymphoma Myeloma Leuk. 2018;18:34-37.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Teckie S, Qi S, Chelius M, Lovie S, Hsu M, Noy A, Portlock C, Yahalom J. Long-term outcome of 487 patients with early-stage extra-nodal marginal zone lymphoma. Ann Oncol. 2017;28:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |