Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6557
Peer-review started: April 12, 2021
First decision: April 23, 2021
Revised: May 1, 2021
Accepted: May 15, 2021
Article in press: May 15, 2021
Published online: August 6, 2021
Processing time: 106 Days and 22.7 Hours
Reports of necrotizing enterocolitis (NEC) caused by umbilical arterial catheter (UAC)-associated abdominal aortic embolism in neonates are rare. Herein, we report the case of an extremely low birth weight (ELBW) infant with NEC caused by UAC-associated abdominal aortic embolism.
A female infant, aged 21 min and weighing 830 g at 28+6 wk of gestational age, was referred to our hospital because of premature birth and shallow breathing. The patient was diagnosed with ELBW, neonatal respiratory distress syndrome, neonatal intrauterine infection, and neonatal asphyxia. Umbilical arterial and venous catheters were inserted on the day after birth and were removed 9 d later, according to the doctor’s plan. Within 48 h after extubation, the patient’s manifestations included poor responsiveness, heart rate range of 175-185/min, and currant jelly stool. Therefore, we considered a diagnosis of NEC. To determine the cause, we used B-mode ultrasound, which revealed a partial abdominal aortic embolism (2 cm × 0.3 cm) and abdominal effusion. The patient was treated with nil per os, gastrointestinal decompression, anti-infective therapy, blood transfusion, and low-molecular-weight heparin sodium q12h for anticoagulant therapy (from May 20 to June 1, the dosage of low-molecular-weight heparin sodium was adjusted according to the anti-Xa activity during treatment). On the 67th day after admission, the patient fully recovered and was discharged.
The abdominal aortic thrombosis in this patient was considered to be catheter related, which requires immediate treatment once diagnosed. The choice of treatment should be determined according to the location of the thrombus and the patient’s condition.
Core Tip: We report a rare case of an extremely low birth weight infant with necrotizing enterocolitis (NEC) caused by umbilical arterial catheter-associated abdominal aortic embolism. Umbilical arterial and venous catheters were inserted on the day after birth and were removed 9 d later. Within 48 h after extubation, the patient’s manifestations were considered consistent with NEC. Color Doppler ultrasound showed partial thrombosis of the abdominal aorta (2 cm × 0.3 cm). She was treated with nil per os, gastrointestinal decompression, blood transfusion, anti-infective therapy, and low-molecular-weight heparin sodium q12h for anticoagulant therapy. On the 67th day after admission, the patient fully recovered and was discharged.
- Citation: Huang X, Hu YL, Zhao Y, Chen Q, Li YX. Neonatal necrotizing enterocolitis caused by umbilical arterial catheter-associated abdominal aortic embolism: A case report. World J Clin Cases 2021; 9(22): 6557-6565
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6557.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6557
Neonatal necrotizing enterocolitis (NEC) is a common gastrointestinal emergency characterized by abdominal distension, vomiting, diarrhea, hematochezia, and even shock and multiple organ failure as the main clinical manifestations and pneumatosis intestinalis upon abdominal X-ray examination[1]. The incidence of NEC is negatively correlated with birth weight and gestational age, where a younger gestational age and lower birth weight correlate with a higher incidence of NEC. The incidence in infants with very low birth weight is 7%-10%, and the mortality rate is as high as 23%-30%[1,2]. The causes of the disease are mostly related to premature delivery, infection, and improper feeding. The reported hypoxia/ischemia-related injuries mainly include birth asphyxia, but NEC caused by abdominal aortic embolism due to an umbilical vascular catheter has rarely been reported.
Herein, we present the case of an extremely low birth weight (ELBW) infant who was admitted to the neonatal intensive care unit (NICU) in Chengdu, Sichuan Province, China. During hospitalization, an umbilical arterial catheter (UAC) was inserted for intravenous medication and parenteral nutrition administration. At 48 h after extubation, color Doppler ultrasound showed partial thrombosis of the abdominal aorta. The history of the case, family history, imaging, laboratory, and surgical examinations, and treatment options of the infant are reported below.
Shallow breathing was observed for 21 min after premature delivery.
A female infant, aged 21 min and weighing 830 g at 28+6 wk of gestational age, was admitted to our hospital on May 9, 2020, because of premature birth and shallow breathing. She received endotracheal intubation in the delivery room after resuscitation, and a pulmonary surfactant was instilled through the endotracheal tube. When her condition was stable, she was transferred to the NICU with T-piece ventilation. After admission, the doctor assessed that the patient's breathing condition was improved, and therefore, the endotracheal tube was removed, and she was treated with nasal continuous positive airway pressure, vitamin K1 to prevent bleeding, cefoperazone sodium/sulbactam sodium combined with ampicillin for anti-infection, caffeine citrate to stimulate breathing, minimal enteral feeding, intravenous fluid infusion, and warming.
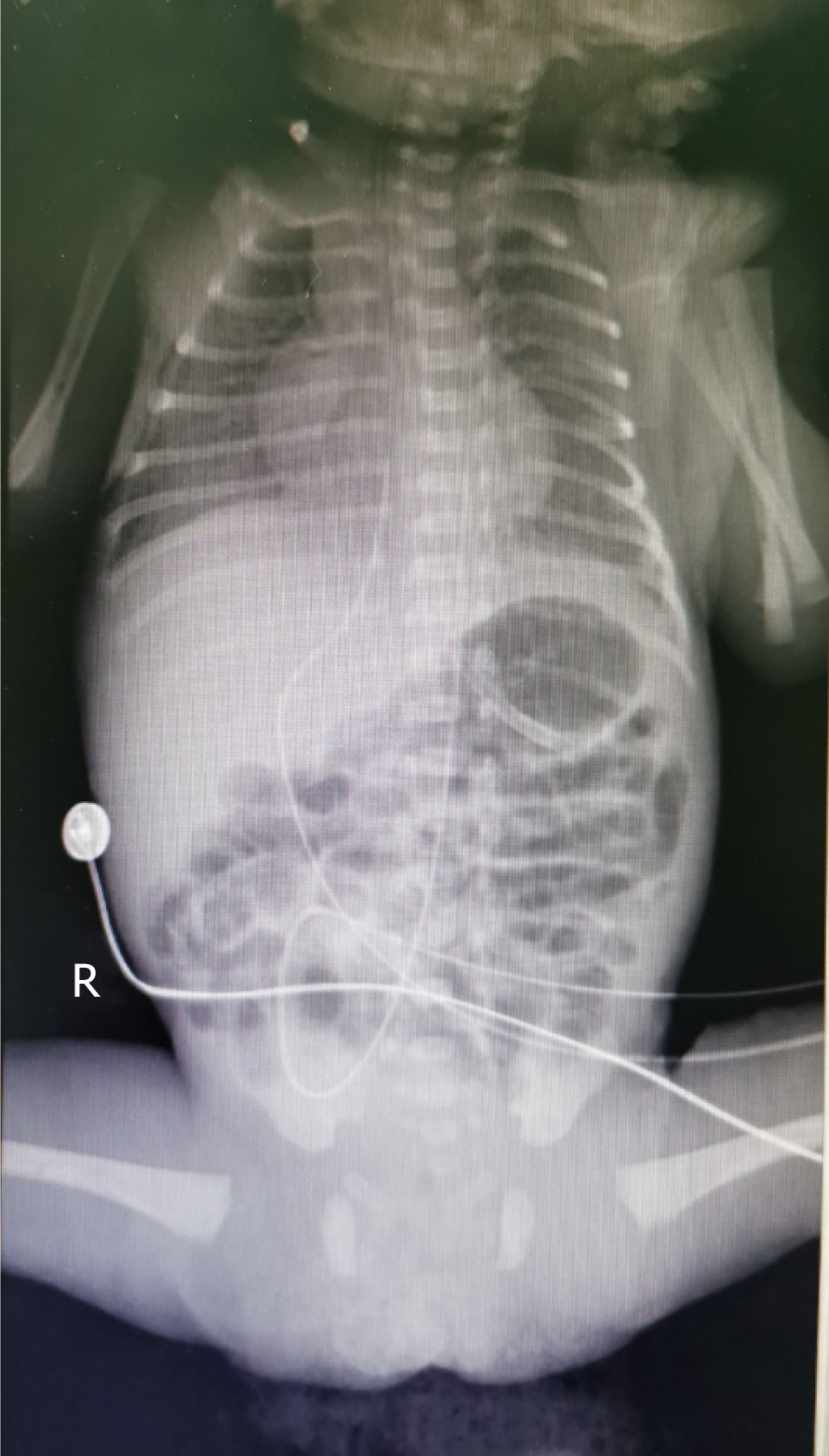
After obtaining signed informed consent from the patient’s parents, a doctor and nurses qualified for umbilical arterial and venous catheterization inserted a 3.5 Fr UAC/umbilical venous catheter (UVC) (3.5 Fr, single lumen polyurethane umbilical catheter, Utah Medical Products, Inc., West Midvale, UT, United States) on the day of admission (Figure 1). The UAC was used for continuous invasive arterial blood pressure monitoring, and the UVC was used for intravenous medication and parenteral nutrition administration. UAC patency was maintained by flushing with 0.5 IU/mL heparin solution q6h, in addition to continuous infusion of 0.5 IU/mL heparin solution.
On May 18, i.e., day 9 of catheterization, according to the doctor’s plan, the UAC and UVC were removed, and a peripherally inserted central catheter (PICC; 1.9 Fr, single lumen Vascu-PICC, medCOMP, Inc., Harleysville, PA, United States) was inserted (cannulation via an upper limb).
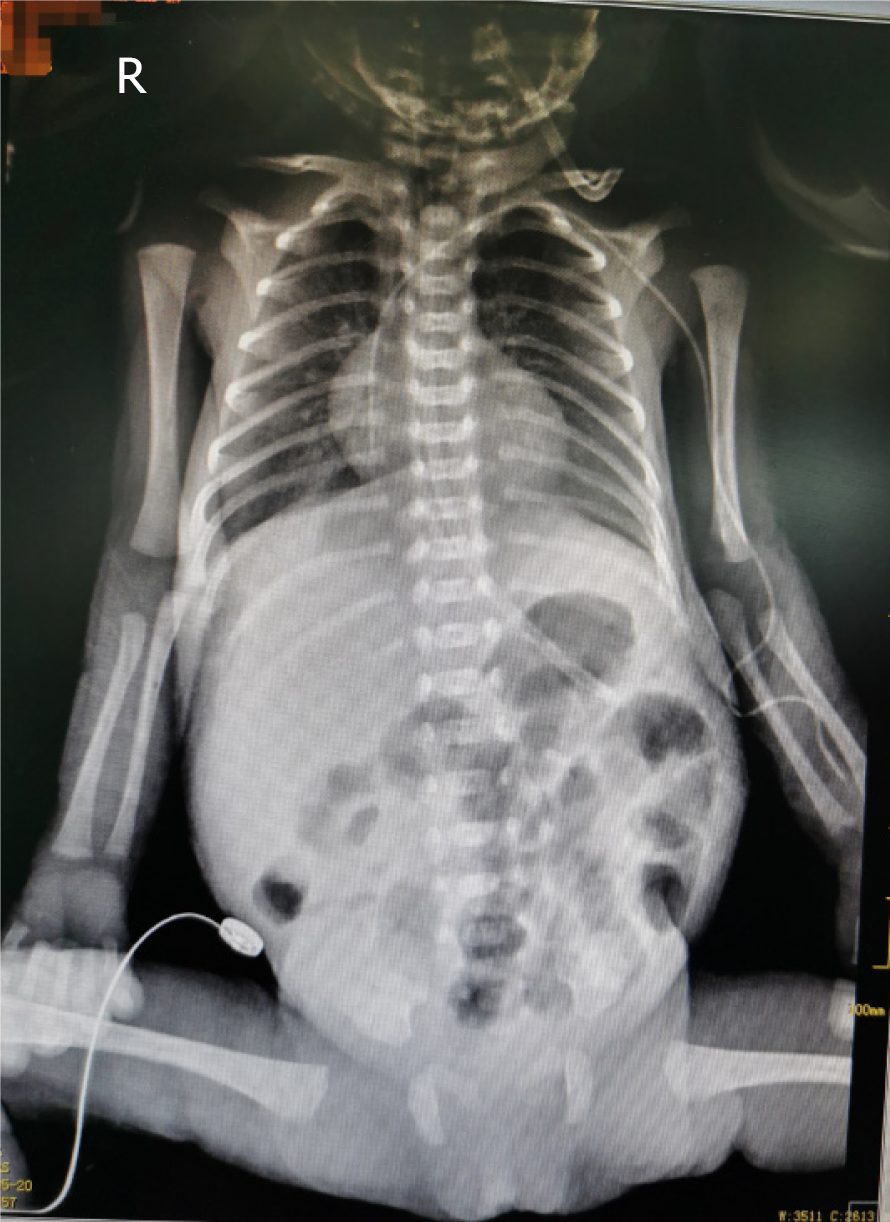
On May 20, i.e., 2 d after removal of the UAC/UVC, the patient’s manifestations included poor responsiveness, a heart rate range of 175-185/min, and a total of 24 g of currant jelly stool. Therefore, we considered a diagnosis of NEC. Abdominal X-ray showed inflation of the small intestine but no obvious dilatation of the intestinal lumen or effusion (Figure 2). Color Doppler ultrasound showed partial thrombosis of the abdominal aorta (2 cm × 0.3 cm) and abdominal effusion. On May 21, abdominal X-ray showed that the range of intestinal inflation increased over previous measurements (Figure 3). Additional diagnosis included abdominal aortic thrombosis and NEC (stage IIA; the diagnostic criteria for NEC in this patient were based on the modified Bell's staging criteria, combined with her clinical manifestations). The patient was treated with nil per os, gastrointestinal decompression, anti-infective therapy, blood transfusion, and low-molecular-weight heparin sodium q12h for anticoagulant therapy. On the 67th day after admission, the patient fully recovered and was discharged.
On May 19, i.e., 1 d after removal of the UAC/UVC, the patient’s manifestations included significant abdominal distension, a visible bowel pattern, and weakened bowel sounds, and her feeding was paused once.
The female infant was delivered by cesarean section at 28+6 wk of gestational age to a gravida 3 para 1 mother. The pregnancy was complicated by severe preeclampsia, diamniotic-dichorionic twins (one survived and one died), abnormal liver function, fatty liver, bilateral hydronephrosis with dilatation of the upper ureter, and thrombocytopenia. The infant’s Apgar scores at 1, 5, and 10 min were 6, 9, and 9, respectively.
Upon physical examination, prematurity, poor responsiveness, a temperature of 36.1 °C, a pulse of 158/min, a respiratory rate of 65/min, irregular spontaneous breathing, and retraction were noted, while no abnormalities in the heart and abdomen were observed, and bowel sounds were normal.
On May 20, i.e., 2 d after removal of the UAC/UVC, the patient’s abdomen was slightly distended and bloated but soft to palpation, bowel sounds were normal, no redness or ecchymosis on the abdominal wall was identified, and no abnormal mass was palpable.
On May 20, i.e., 2 d after removal of the UAC/UVC, peripheral blood examination showed a hemoglobin level of 94 g/L (normal range for reference: 170-210 g/L), hematocrit of 25.8% (normal range for reference: 58%-59%), C-reactive protein (CRP) level of 15.6 mg/L (normal range for reference: 0-8 mg/L), procalcitonin level of 0.74 ng/mL (normal range for reference: 0-0.5 ng/mL), coagulation function prothrombin time of 14.6 s (normal range for reference: 7.6-13.6 s), and activated partial thromboplastin time of 55.9 s (normal range for reference: 15.2-35.2 s).
On May 20, i.e., 2 d after removal of the UAC/UVC, chest X-ray showed that the tip position of the PICC was in the 7th-8th thoracic vertebrae. Abdominal X-ray revealed inflation of the small intestine, but no obvious dilatation of the intestinal lumen or effusion was observed (Figure 2). Color Doppler ultrasound showed partial thrombosis of the abdominal aorta (2 cm × 0.3 cm) and abdominal effusion, but no thrombus was noted in the portal vein, splenic vein, or superior mesenteric vein. On May 21, abdominal X-ray showed that the range of intestinal inflation increased over previous measurements (Figure 3).
ELBW, very preterm infant, neonatal respiratory distress syndrome, neonatal intrauterine infection, neonatal asphyxia, anemia, neonatal gastrointestinal bleeding, abdominal aortic thrombosis, and neonatal NEC.
The patient was treated with fasting and gastrointestinal decompression (11 d), and meropenem, piperacillin, tazobactam, vancomycin, and fluconazole were administered to prevent infection (before obtaining the results of resistant blood cultures, we chose appropriate antibiotics based on clinical experience, after which we used antibiotics according to the results of a chemosensitivity test). Leukocyte-depleted suspended red blood cells (four times), fresh frozen plasma (twice), and irradiated apheresis platelets (once) were infused. Symptomatic and supportive treatments were administered, including vitamin K1 for hemostasis, recombinant human erythropoietin and oral folic acid to prevent and treat anemia, and low-molecular-weight heparin sodium for anticoagulant therapy (from May 20 to June 1, the dosage of low-molecular-weight heparin sodium was adjusted according to the anti-Xa activity during treatment).
Examination on the 66th day after admission (July 14) showed that the patient had good responsiveness, a peripheral oxygen saturation range of 95%-96% without oxygen inhalation, and good digestion of the planned volume of milk, and no abdominal distension, fever, hypopnea, vomiting, or hematochezia was noted. Abdominal ultrasound showed that the partial thrombosis of the abdominal aorta (0.4 cm × 0.2 cm, 0.2 cm × 0.1 cm × 0.15 cm) significantly decreased in size.
After 67 d of hospitalization (July 15), the patient fully recovered and was discharged. After discharge, an outpatient follow-up in the Department of Vascular Surgery outpatient clinic was recommended to the patient’s parents to closely observe the blood supply to the lower limbs and dynamically monitor the abdominal aorta with color Doppler ultrasound. At the follow-up, the patient was found to have recovered well, and the partial thrombosis of the abdominal aorta had disappeared.
The patient’s main treatments and management process during hospitalization are shown in Table 1.
| Date | Events | Auxiliary examination | Handling | Treatment | Feeding |
| May 9 | Umbilical artery catheter (UAC) and umbilical venous catheter (UVC) placement | (1) Chest X-ray showed that the tip positions of the UAC/UVC were in the 6th-7th thoracic vertebrae (Figure 1); and (2) Routine blood examination: Hemoglobin (HGB), 203 g/L; hematocrit (HCT), 59.4%; C-reactive protein (CRP), 2.1 mg/L | (1) Routine daily tubing maintenance; (2) Tube flushed and sealed with 0.5 IU/mL heparin solution q6h; and (3) The UAC was used for continuous invasive arterial blood pressure monitoring and continuous infusion of 0.5 IU/mL heparin solution; the UVC was used for intravenous medication and parenteral nutrition administration | Nasal continuous positive airway pressure, cefoperazone sodium/sulbactam sodium, ampicillin, caffeine citrate, vitamin K1 | Completely hydrolyzed protein formula, 0.5 mL q12h; tube feeding |
| May 15 | Invasive arterial blood pressure monitoring was stopped according to the doctor’s plan, and the UAC was maintained for blood sampling | (1) Routine daily tubing maintenance; and (2) Tube flushed and sealed with 0.5 IU/mL heparin solution q6h | Nasal continuous positive airway pressure, cefoperazone sodium/sulbactam sodium, ampicillin, caffeine citrate, vitamin K1 | Completely hydrolyzed protein formula, 0.5 mL q8h; tube feeding | |
| May 18 | The UAC and UVC were removed, and a peripherally inserted central catheter (PICC) was inserted | (1) Chest X-ray showed that the tip position of the PICC was at the upper edge of the 6th thoracic vertebra (cannulation through an upper limb); and (2) Routine blood examination: HGB, 150 g/L; HCT, 43.9%; CRP, 2.9 mg/L | (1) Routine daily tubing maintenance; and (2) Tube flushed and sealed with 1 IU/mL heparin solution q6h | Nasal continuous positive airway pressure, cefoperazone sodium/sulbactam sodium, ampicillin, caffeine citrate, vitamin K1 | Completely hydrolyzed protein formula, 0.5 mL q6h; tube feeding |
| May 19 | Significant abdominal distension, visible bowel pattern, weakened bowel sounds | Feeding was paused once | Nasal continuous positive airway pressure, cefoperazone sodium/sulbactam sodium, ampicillin, caffeine citrate, vitamin K1 | Feeding was paused once | |
| May 20 | Poor response, an elevated heart rate range of 175-180/min (normal temperature), abdominal distension but soft to palpation, normal bowel sounds; a total of 24 g of currant jelly stool | (1) Chest X-ray: the tip position of the PICC was at the 7th-8th thoracic vertebrae; (2) Abdominal X-ray: the small intestine showed inflation, but no obvious dilatation of the intestinal lumen or effusion was noted (Figure 2); (3) Color Doppler-mode ultrasound showed a partial thrombosis of the abdominal aorta (2 cm × 0.3 cm) and abdominal effusion; (4) Routine blood examination: HGB, 94 g/L; HCT, 25.8%; CRP, 15.6 mg/L; PCT, 0.74 ng/mL; and (5) Coagulation function: prothrombin time (PT) 14.6 s; activated partial thromboplastin time (APTT) 55.9 s | (1) A crossmatch test was performed immediately; active blood transfusion and plasma transfusion; (2) The position of the PICC was adjusted, and the heart rate decreased to 160/min; a recheck showed that the tip position of the PICC was at the 6th thoracic vertebra; (3) Surgery consultation; and (4) Fasting, gastrointestinal decompression | (1) Cefoperazone sodium/sulbactam sodium was stopped; (2) Meropenem and fluconazole were added; (3) Low-molecular-weight heparin sodium q12h anticoagulant therapy was added; and (4) Other treatments were the same as before | Fasting; gastrointestinal decompression |
| May 21 | A total of 15 g of dark-red currant jelly stool | (1) Abdominal X-ray showed that the range of intestinal inflation increased over previous measurements (Figure 3); and (2) Routine blood examination: HGB, 113 g/L; HCT, 31.7% | (1) Reexamination of routine blood parameters and abdominal X-ray; and (2) Blood transfusion | Additional diagnosis: abdominal aortic thrombosis, neonatal necrotizing enterocolitis (stage IIA) | Fasting; gastrointestinal decompression |
| May 22 | Anti-Xa activity: 0.4 IU/mL (on the lower side) | The dose of low- molecular-weight heparin sodium was increased | Additional diagnosis: abdominal aortic thrombosis, neonatal necrotizing enterocolitis (stage IIA) | Fasting; gastrointestinal decompression | |
| May 25 | Slight abdominal distention, audible bowel sounds, and a small amount of brown stool was produced after an enema | Blood routine examination: HGB, 106 g/L; HCT, 27.5%; CRP, 0.7 mg/L | A crossmatch test was performed immediately; transfusion | Meropenem was replaced with piperacillin | Fasting; gastrointestinal decompression |
| May 26 | Dark green stool | B-ultrasound: Partial thrombosis of the abdominal aorta (0.6 cm × 0.2 cm, 0.3 cm × 0.2 cm) | Meropenem was replaced with piperacillin | Fasting; gastrointestinal decompression | |
| May 31 | Dark green stool | Gastrointestinal decompression was stopped; feeding started | Completely hydrolyzed protein formula, 0.5 mL q6h; tube feeding | ||
| June 1 | Soft abdomen, normal bowel sounds | Blood routine examination: HGB, 83 g/l; B-ultrasound: partial thrombosis of the abdominal aorta (0.5 cm × 0.2 cm, 0.2 cm × 0.1 cm) | Transfusion | Low-molecular-weight heparin sodium was stopped; Piperacillin was stopped; The PICC was removed | The amount of milk was increased appropriately |
| July 14 | B-ultrasound: partial thrombosis of the abdominal aorta (0.4 cm × 0.2 cm, 0.2 cm × 0.1 cm × 0.15 cm) | The amount of milk was increased appropriately | |||
| July 15 | Body weight: 1790 g; milk intake: 37 mL q3h | Discharge | |||
Because of the rapid velocity and high pressure of the blood flow in the aortic lumen, aortic thrombosis is rare in clinical practice. The mechanism of thrombosis is not clear; few relevant reports are available, and the condition is rare in neonates[3]. In adults, thrombosis is mainly secondary to severe atherosclerosis, arteritis, trauma, aneurysm, dissection, malignant tumors, or pregnancy and a hypercoagulable state, resulting in poor arterial supply to the abdomen and lower extremities; the most common site is the lower abdominal aorta, and a mural thrombus is often formed[4,5]. This patient was a 28+6-wk-old (gestational age) premature infant without a notable medical history, such as atherosclerosis. She had a poor response and an elevated heart rate range of 175-185/min. The patient had a history of hematochezia on the 11th day of hospitalization. Chest X-ray showed that the tip position of the PICC was at the 7th-8th thoracic vertebrae. Furthermore, B-mode ultrasound showed partial thrombosis of the abdominal aorta (2 cm × 0.3 cm).
Based on the patient’s clinical manifestations, physical examination, auxiliary examinations, and treatment, the response was speculated to have been related to the catheters: (1) The increased heart rate was related to the excessive depth of the PICC, and the heart rate was gradually reduced to 160/min by adjusting the position of the PICC; and (2) The partial thrombosis of the abdominal aorta was related to the UAC. On the first day of admission (May 9), UAC/UVC insertion was performed, and after successful catheterization, the catheter tips were located in the abdominal aorta and the inferior vena cava. Abdominal X-ray showed that the location was at the 6th-7th thoracic vertebrae. The UAC was used for continuous invasive arterial blood pressure monitoring, which was stopped 6 d later; however, the UAC was retained for arterial blood sampling (at that time, continuous infusion of the heparin solution was stopped, and the heparin solution was routinely used only for flushing and sealing the tube q6h). Three days later (May 18), the UAC and UVC were removed. Throughout the UAC indwelling period (May 9-18), blood samples (including for blood gas analysis, routine blood examination, CRP measurement, and other examinations) were collected from the UAC several times, and 0.5 IU/mL heparin solution was used for positive pressure tube sealing after each blood sample collection. Therefore, the reason for abdominal aortic thrombosis in this patient may have been due to the collection of multiple blood samples via the UAC, which may have led to the formation of tiny blood clots on the inner wall of the heparin cap junction that were pushed into the abdominal aorta during pulse flushing for positive pressure tube sealing.
In view of the abdominal aortic thrombosis caused by the UAC, low-molecular-weight heparin sodium q12h was immediately administered for anticoagulation. After 12 d of treatment, B-mode ultrasound suggested that the thrombus was significantly reduced compared with previous observations.
The branches of the abdominal aorta include the superior mesenteric artery (which supplies blood to the right half of the intestine) and inferior mesenteric artery (which supplies blood to the left half of the intestine)[1]. When a thrombus forms in the abdominal aorta, the blood supply to the superior and inferior mesenteric arteries is blocked, which in turn impairs blood circulation in the intestinal wall. Ischemia leads to increased vascular permeability in the intestinal submucosa and to ischemia, necrosis, and shedding of the intestinal mucosa, and a large volume of bloody exudates flows into the intestinal lumen to form bloody/currant jelly stools[6].
At present, no standardized therapeutic regimen or clinical guidelines are available for the treatment of abdominal aortic thrombosis, and treatment mainly focuses on microcirculation improvement, thrombolysis, anticoagulation, and keeping the patient quiet. In severe cases, interventional therapy or surgical treatment may be required[7]. Because of the risk of exacerbated ischemia caused by distal embolism due to embolus detachment or intestinal perforation and necrosis caused by a new embolism, this disease requires immediate treatment once diagnosed, but the choice of treatment options depends on the location of the thrombus and the condition of the patient. This patient was a 28+6-wk-old (gestational age) premature infant with a history of umbilical arterial and venous catheterization. Currant jelly/bloody stools occurred within 48 h after extubation. Physical examination revealed slight abdominal distension without signs of peritoneal irritation. Bowel sounds were active. Ultrasound showed thrombosis near the superior mesenteric artery branch of the abdominal aorta, but the superior mesenteric artery was unobstructed. No signs of intestinal necrosis or ischemia and no indications for surgery were identified. Therefore, low-molecular-weight heparin sodium anticoagulation therapy was administered. In this case, thrombosis occurred in the lower segment of the opening of the abdominal aorta to the superior mesenteric artery. Once thrombus detachment occurs, the thrombus may embolize the bilateral renal arteries, inferior mesenteric artery, bilateral common iliac arteries, and lower limb arteries. Therefore, during intravenous medication treatment, the abdominal aorta must be dynamically monitored by color Doppler ultrasound, and the blood supply to the lower limbs and the functions of various organs must be closely observed. The integrity of the skin is protected to prevent pressure ulcers, but the infant should be moved as little as possible to prevent thrombus detachment; at the same time, changes in the skin color and skin temperature of the abdominal wall and lower limbs, the presence or absence of symptoms of gastrointestinal bleeding, changes in the urine volume and color, and lower limb arterial pulses should be monitored. In this case, the patient was administered anticoagulation treatment with low-molecular-weight heparin sodium. Twelve days later, abdominal aortic thrombosis was significantly reduced compared to previous observations, and no complications, such as distal limb and organ embolism caused by embolus detachment, occurred; therefore, the therapeutic effect was considered good. From low-molecular-weight heparin sodium discontinuation (June 1) until discharge, digestion of the planned volume of milk was good, bowel sounds were normal, and no abdominal distension or hematochezia was noted. The patient was discharged on July 15 as planned. Since her discharge from the hospital, the patient has been followed at the Department of Vascular Surgery outpatient clinic; she has had no signs of abdominal distension or hematochezia and has recovered well, and the thrombus has essentially disappeared.
Arterial thromboses are rare and severe in newborns and are mostly caused by arterial catheterization. NEC caused by UAC-associated abdominal aortic embolism is also rare[6]. The treatment and management of affected neonates were reviewed herein. Although multiple blood samples were collected via the UAC, routine UAC flushing was missed only once. Considering that this patient was an ELBW infant, the concentration of heparin solution used for flushing was 0.5 IU/mL, which was different from the conventional concentration of 1 IU/mL. Did missing one flush contribute to the thrombosis? Do different heparin concentrations have any effect on thromboprophylaxis? Does the UAC need to be retained for blood sampling after invasive arterial blood pressure monitoring is stopped (along with continuous infusion of a heparin solution during monitoring)? All of these questions need to be answered in further studies. Since 72 h after removal of the PICC and umbilical arterial and venous catheters is the risk period for thrombosis, medical staff must be competent in early identification, early intervention, and active treatment of the primary disease to reduce the occurrence of related complications.
Manuscript source: Unsolicited manuscript
Specialty type: Nursing
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Syahputra DA, Wang Y, Yang WY S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Li JH
| 1. | Shao X, Ye H, Qiu X. Practice of neonatology. 5th ed. Beijing: People's Medical Publishing House, 2019. |
| 2. | Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol. 2013;40:27-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Kahlberg A, Montorfano M, Cambiaghi T, Bertoglio L, Melissano G, Chiesa R. Endovascular Stent-Grafting of the Ascending Aorta for Symptomatic Parietal Thrombus. J Endovasc Ther. 2016;23:969-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Yang S, Yu J, Zeng W, Yang L, Teng L, Cui Y, Shi H. Aortic floating thrombus detected by computed tomography angiography incidentally: Five cases and a literature review. J Thorac Cardiovasc Surg. 2017;153:791-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Yuan YS, Wang ZQ, Jia CF, Yang ZQ, Sun XX, Zou YJ. Accidental detection of floating thrombus in the ascending aorta by pulmonary artery computed tomography angiography: a case report. Zhongguo Xunhuan Zazhi. 2018;33:403. [DOI] [Full Text] |
| 6. | Jeican II, Ichim G, Gheban D. Intestinal ischemia in neonates and children. Clujul Med. 2016;89:347-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Huang TH, Bao Y. Pulmonary embolism combined with thoracic aortic thrombosis: a case report. Linchuang Feike Zazhi. 2016;21:193-194. [DOI] [Full Text] |