Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6522
Peer-review started: April 10, 2021
First decision: May 11, 2021
Revised: May 15, 2021
Accepted: May 25, 2021
Article in press: May 25, 2021
Published online: August 6, 2021
Processing time: 108 Days and 18.9 Hours
Postoperative chylothorax is usually regarded as a complication associated with cardiothoracic surgery; however, it is one of the rare complications in orthopedic surgery. This case report describes a female patient who developed chylothorax after a successful L4-S1 transforaminal lumbar interbody fusion surgery. The etiology, diagnosis, and treatment were analyzed and discussed.
A 50-year-old woman was admitted with repeated back and leg pain. She was diagnosed with L4 degenerative spondylolisthesis, L4/L5 and L5/S1 intervertebral disc herniation and L5 instability, and underwent successful posterior L4-S1 instrumentation and fusion surgery. Unfortunately, thoracic effusion was identified 2 d after operation. The thoracic effusion was finally confirmed to be chylous based on twice positive chyle qualitative tests. The patient was discharged after 12-d persisting drainage, 3-d total parenteral nutrition and fasting, and other supportive treatments. No recurring symptoms were observed within 12 mo follow-up.
Differential diagnosis is crucial for unusual thoracic effusion. Comprehensive diagnosis and treatment of chylothorax are necessary. Thorough intraoperative protection to relieve high thoracic pressure caused by the prone position is important.
Core Tip: Differential diagnosis is crucial for idiopathic chylothorax. The diagnosis should be confirmed by laboratory tests and treatment trials. Comprehensive conservative treatments are regarded as the first-line therapy in idiopathic chylothorax patients. To reduce the formation of chyle, fasting and adequate supportive therapy are effective and recommended. Intraoperative protections are necessary to avoid unexpected complications.
- Citation: Huang XM, Luo M, Ran LY, You XH, Wu DW, Huang SS, Gong Q. Chylothorax following posterior low lumbar fusion surgery: A case report. World J Clin Cases 2021; 9(22): 6522-6530
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6522.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6522
Chylothorax, a rare postoperative complication in adult patients, is the result of chyle leakage from the thoracic duct or its collateral branches into the pleural cavity. Chylothorax causes dyspnea, heart failure, hemodynamic disorder, malnutrition, immune suppression, and even death[1]. Chylothorax is usually caused by traumatic and non-traumatic factors including sharp and even blunt trauma to the thorax, iatrogenic factors following surgery, and thoracic tumors[2,3]. In a retrospective analysis of chylothorax in 203 patients, iatrogenic factors including esophagectomy, surgery for congenital heart disease, lung cancer resection and mediastinal mass resection accounted for 38.9% of cases[3]. To date, the clinical diagnosis and treatment of chylothorax are unclear[4-6]. Although postoperative chylothorax is usually regarded as a complication associated with cardiothoracic surgery[4], chylothorax has rarely been confirmed in spinal surgery, where the anterior surgical approach to the cervical, thoracic, or thoracolumbar vertebrae is a risk factor for lymphatic duct injury[7-9]. Interestingly, chylothorax occurred after L4-S1 transforaminal lumbar interbody fusion (TLIF) surgery.
We investigated the diagnosis and treatment of chylothorax. The diagnosis was confirmed by twice positive chyle qualitative tests, and was successfully treated with persistent drainage, total parenteral nutrition and fasting. We speculated that increased intrathoracic pressure caused by the prone position during surgery was involved in the etiology of chylothorax.
A 50-year-old female was admitted due to repeated back and leg pain for more than 2 years.
The patient had persistent back pain without inducement for 2 years with recurrent episodes of lower limb pain without numbness. She had visited another hospital and was diagnosed with L4 spondylolisthesis. Conservative treatments were prescribed without relief. Therefore, she visited our hospital for further treatment.
The patient was previously healthy.
No relevant disorders were identified. The patient had not delivered any children.
At the time of admission, the patient’s vital signs included blood pressure of 113/77 mmHg, heart rate of 85 bpm, and temperature of 36.3˚C (head). Her physical status included height of 1.55 m, weight of 55 kg, and body mass index of 22.89 kg/m2. Spine examination revealed no spinal curve, no decreased muscle strength, and no abnormal reflex. However, L4-L5 interspinous pressing pain was observed accompanied by radiating pain and hypoesthesia in the right medial leg.
Routine blood tests revealed normal blood cell contents. Prothrombin, D-dimer and partial thromboplastin time were normal. Serum C-reactive protein was normal at 1.69 mg/dL (reference range < 5 mg/dL) and interleukin-6 was 1.88 pg/mL (reference range 0-7 pg/mL). Blood biochemistry as well as urinary and fecal analysis were normal.
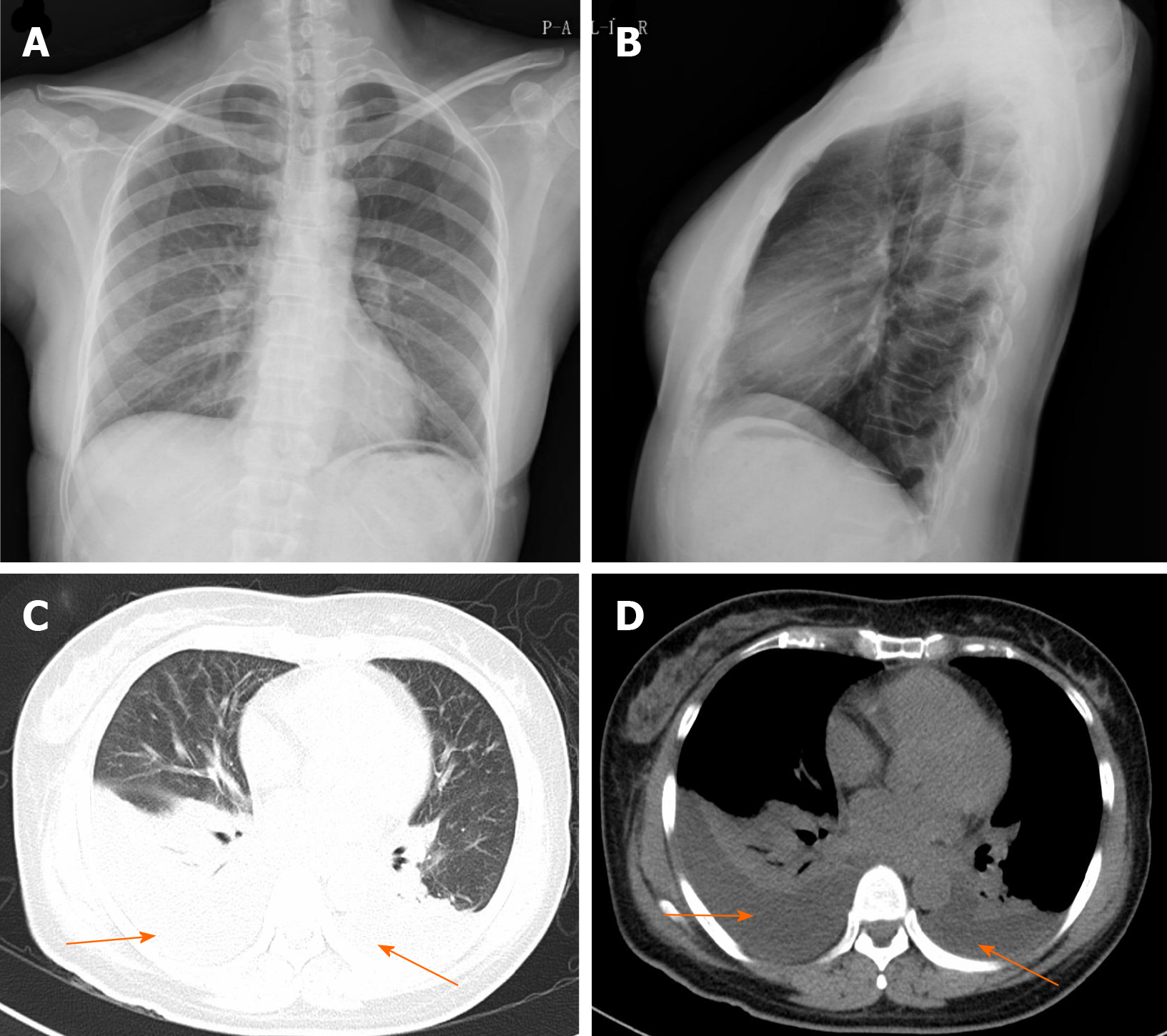
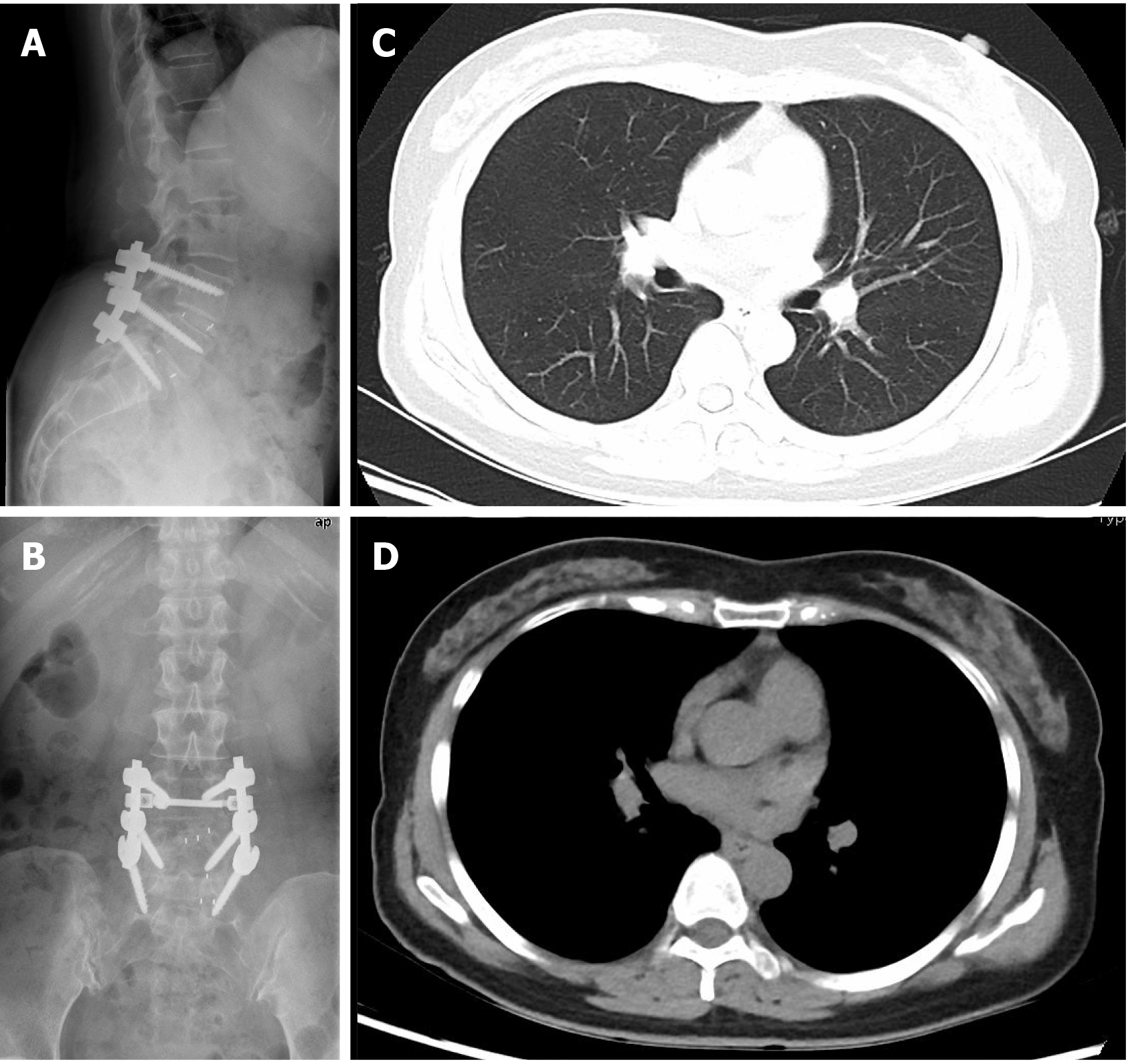
Radiologic examinations including X-ray, computed tomography (CT) and magnetic resonance imaging indicated spondylolisthesis of L4, instability of L5 and disc herniation of L4/L5 and L5/S1. Electrocardiogram, B ultrasound of the abdomen and chest x-ray (Figure 1A and B) were also normal.
The patient was diagnosed with L4 degenerative spondylolisthesis (Meyerding I), L4/L5 and L5/S1 intervertebral disc herniation and L5 instability.
The patient underwent L4-S1 TLIF surgery.
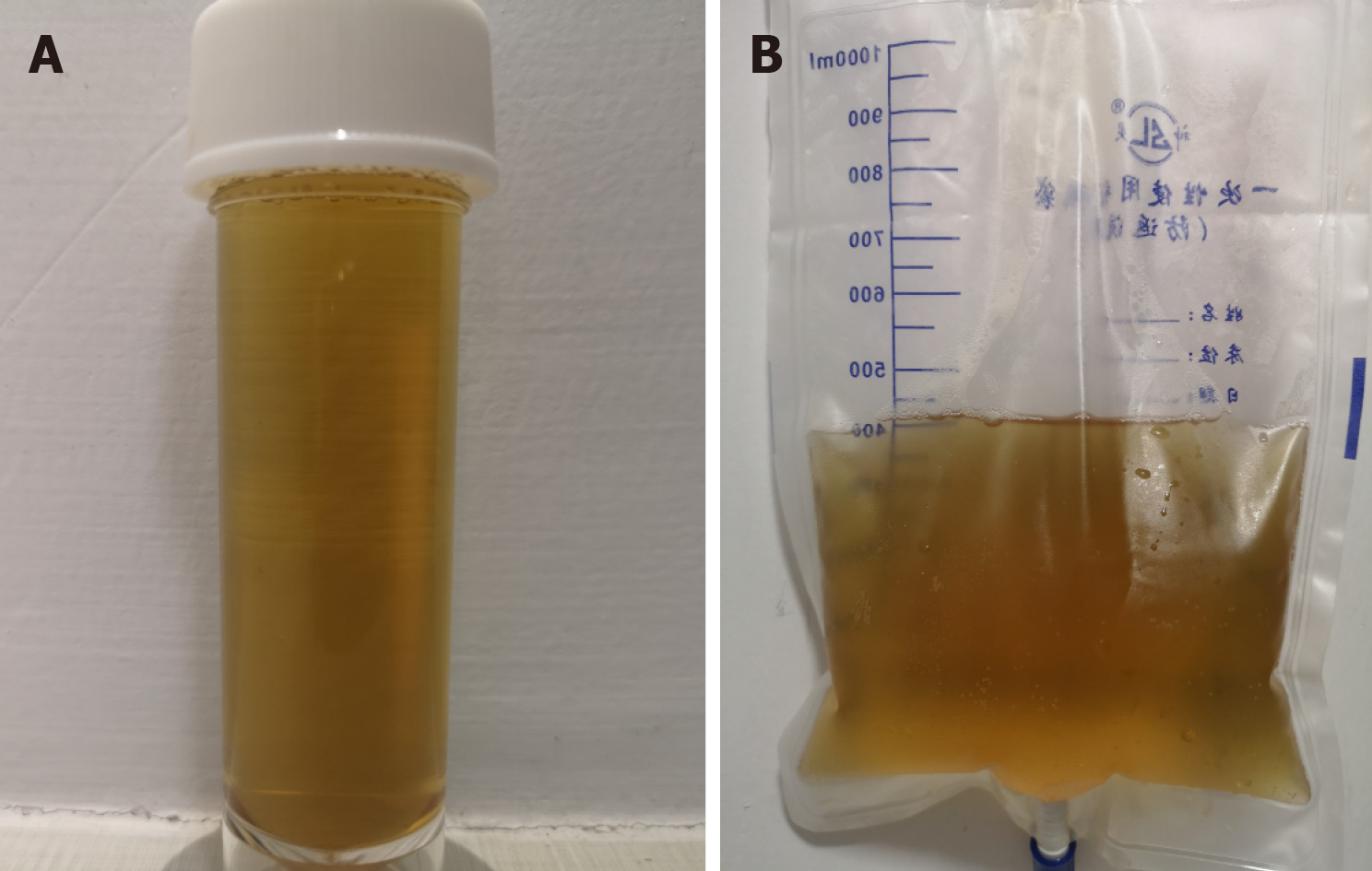
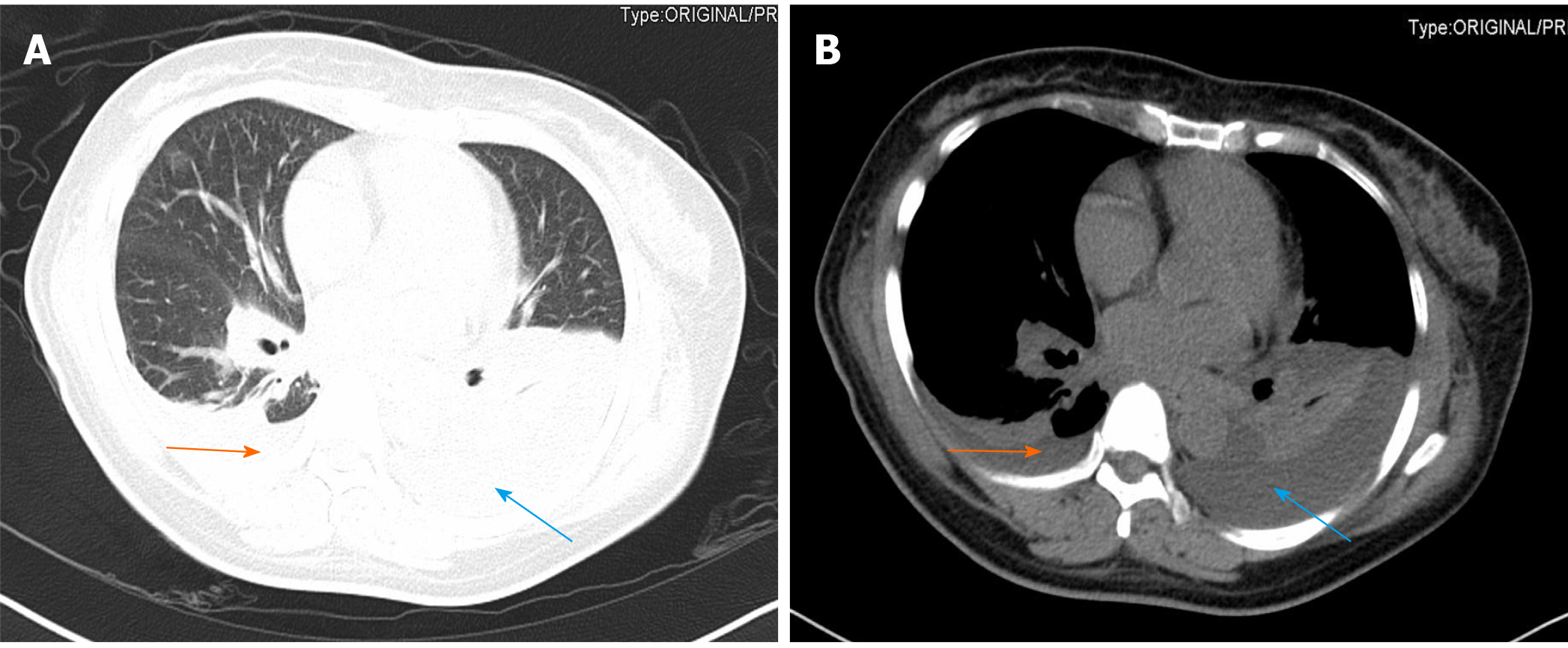
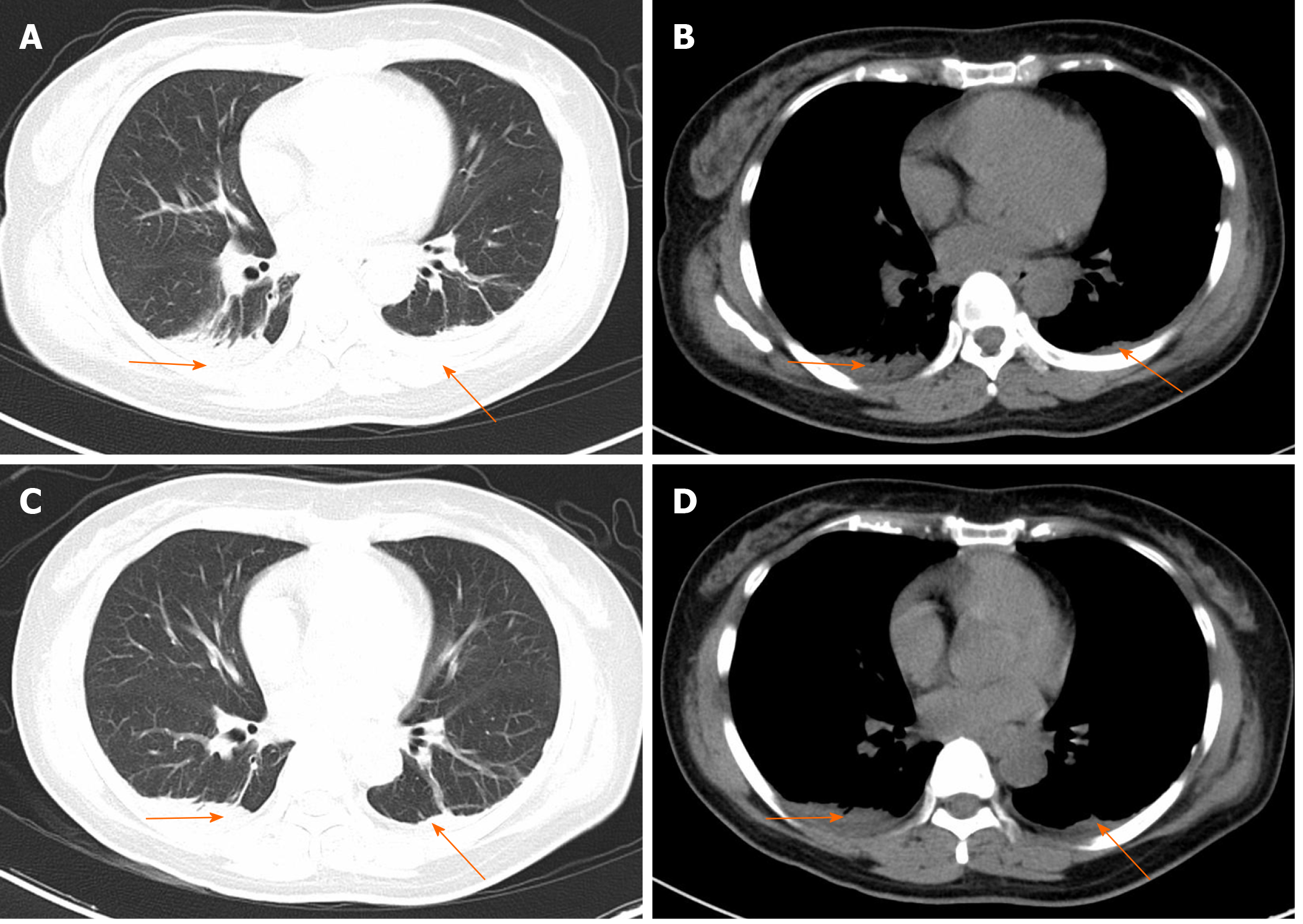
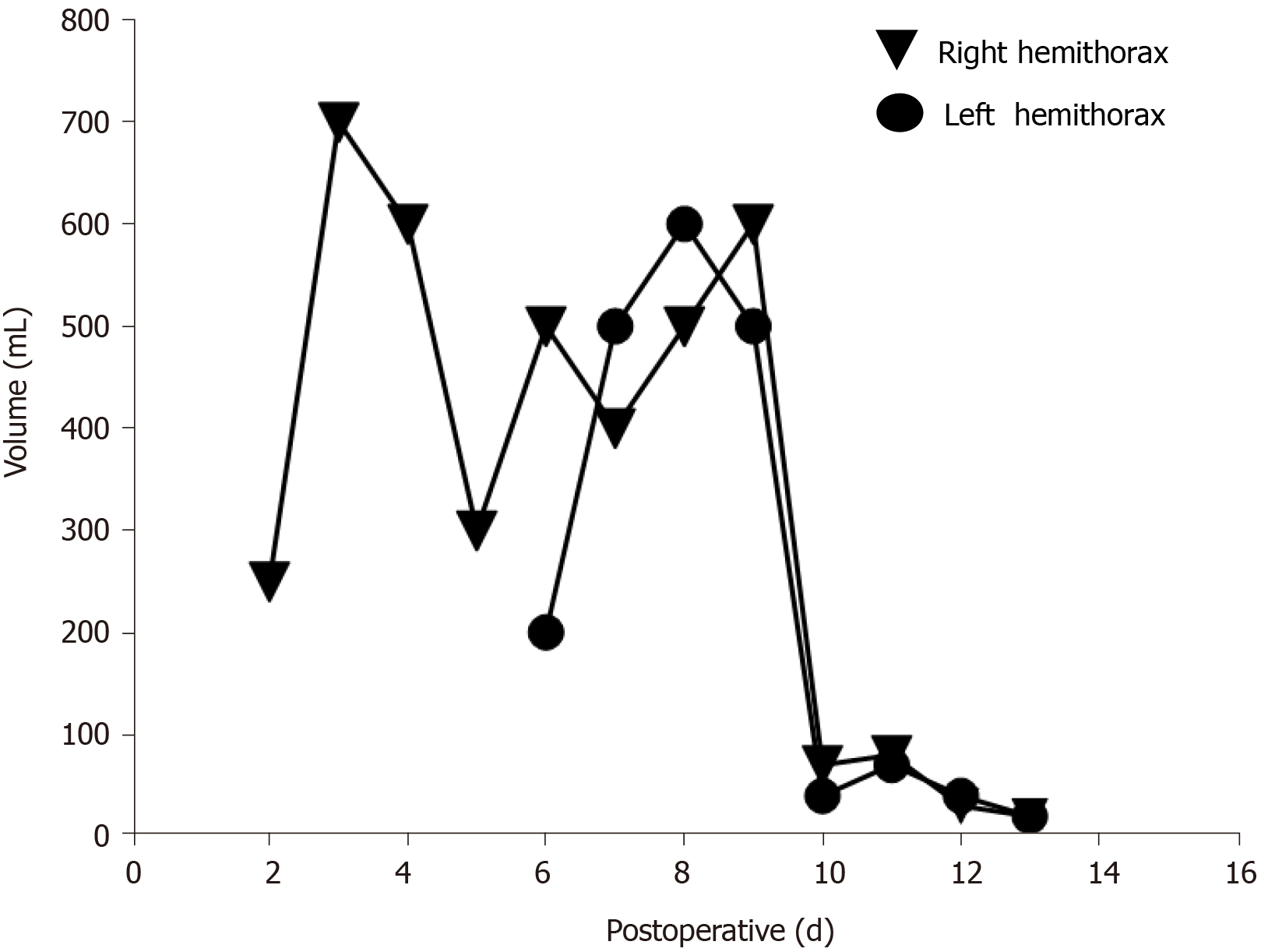
On the first postoperative day, her heart rate and peripheral oxygen saturation (SPO2) were normal. Routine postoperative blood and blood biochemistry tests showed that the concentrations of hemoglobin, total protein, and albumin were 108 g/L (115-150 g/L), 48.8 g/L (65.0-85.0 g/L), and 32.3 g/L (40.0-55.0 g/L), respectively. However, the patient developed hypotension that fluctuated between 80-90/50-60 mmHg, which may have been caused by blood and fluid loss, as well as residual muscle relaxant, due to the 4.5-h operation time. Consequently, the patient was managed with infection prevention, intravenous infusion of crystal and colloidal liquid and albumin, as well as oral feeding. On postoperative day 2, even with oxygen 3 L/min nasally, the patient had severe dyspnea and a dry cough. Physical examination revealed a body temperature of 37 ˚C (head), heart rate of 80 bpm, blood pressure of 90/60 mmHg, and SPO2 of 90%. A high-resolution chest CT scan was immediately performed following detection of reduced respiratory sounds, indicating that there was a small to moderate amount of effusion in both sides of the pleural cavity. However, her preoperative frontal and lateral chest films were normal (Figure 1). Therefore, an intercostal tube was inserted into the right side of the pleural cavity for persistent drainage, and 250 mL of yellowish odorless liquid was drained which looked like interstitial fluid, it was subsequently analyzed and no bacterial infection was found (Figure 2). Her albumin level decreased sharply from 50.6 g/L pre-operation to 32.3 g/L post-operation, resulting in hypo-osmolality, consequently leading to the leakage of tissue fluid from blood vessels to the pleural cavity, which may have been the cause of pleural effusion and hypotension. Unfortunately, the drainage volumes in the first 4 d were 700 mL, 600 mL, 300 mL, and 500 mL, respectively, with no significant decrease. Her vital signs were relatively stable with a temperature of 37 ˚C, heart rate of 80 bpm, blood pressure of 100-111/60-66 mmHg, and SPO2 of about 95%. Repeated chest CT showed a small amount of effusion in the right hemithorax and a moderate amount in the left hemithorax (Figure 3). Another tube was inserted into the left hemithorax for persistent drainage, and 200 mL of pleural effusion was drained, which had the same appearance as that in the right hemithorax (Figure 2B). The concentrations of total protein and albumin were 57.2 g/L and 41.2 g/L, respectively, which demonstrated good nutritional status and excluded the etiology of hypo-osmolality. Laboratory analyses showed that the effusion fluid from the right and left hemithorax was transudative with no bacteria. The diagnosis of chylothorax was confirmed based on twice positive chyle qualitative tests. Conservative treatments were prescribed, including fasting and adequate supportive therapy (such as total parenteral nutrition). The drainage decreased significantly to dozens of milliliters after fasting for 2 d (Figure 4). She was re-fed after 3 d of fasting with no increase in drainage over the next 3 consecutive days (Figure 5). Finally, the tubes were removed and no recurrence was observed after 2 d observation. The patient was successfully discharged on posto
Postoperative chylothorax occurs after cardiothoracic surgery and anterior spinal surgery[3]. However, chylothorax after posterior L4-S1 lumbar instrumentation and fusion surgery has not been reported before.
Anatomically, a typical path of the thoracic duct is from the cisterna chyli at the level of L2, up through the aortic hiatus at T12 level to the thorax. It then ascends along the thoracic spinal column on the right-anterior side between the thoracic aorta and the azygos vein to T5 or T6 level, crossing to the left side of the thoracic vertebrae. It finally joins the left jugular and subclavian veins[10]. Based on the anatomy, rupture or obstruction of the thoracic duct causes chyle leakage resulting in the development of chylous ascites or pleural effusion. The most common traumatic causes of chylothorax are cardiothoracic surgical procedures. Non-traumatic causes include malignancy, tuberculosis, liver cirrhosis, and malformations[3,11,12]. Romero et al[13] reported that chylous ascites could present as chylothorax in patients with liver cirrhosis, due to communications between the thoracic and peritoneal cavity and lower pressure in the pleural cavity. Surgery was not the etiology in our case, as the surgical site was L4 to S1 posteriorly, much lower than the typical cisterna chyli location (L2 level), and TLIF surgery does not expose tissues anterior to the lumbar vertebrae. Additionally, although variations in the lymphatic system are complicated, we excluded this cause due to assurance of no breakthrough of anterior annulus fibrosus.
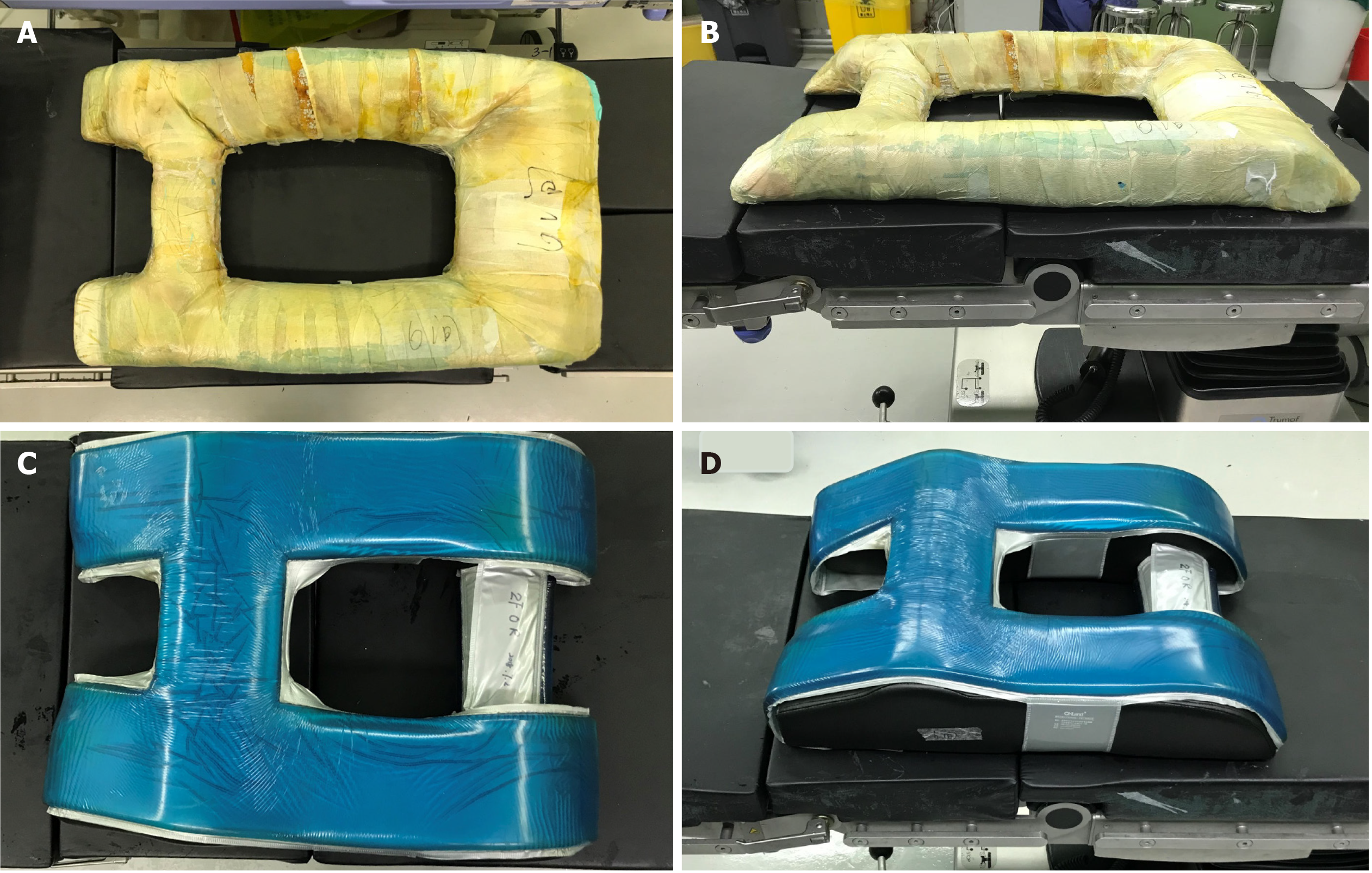
Non-traumatic etiologies (underlying disorders) were also excluded as there was no evidence of these disorders causing chylothorax. With conservative management including drainage, fasting, total parenteral nutrition and other supportive treatments, the patient recovered within 12 d of diagnosis. As the patient refused additional examinations, the specific etiology is unknown. The authors have assumed that the 4.5-h-long prone position during surgery might have induced hyperpressure in the thoracic cavity, causing damage to the thoracic lymphatic system as blunt trauma[14,15]. The procedure for placing a patient in the prone position for posterior lumbar fusion surgery in our department is as follows: The patient is reversed into the prone position, and the self-made, soft, square-frame cushion (Figure 7A and B) is placed under the abdomen to reduce abdominal pressure after the anesthesia takes effect. Thus, the main load-bearing parts are the pubis, bilateral ilia, bilateral abdomen, and lower chest wall. The potential etiology of chylothorax in this patient was thought to be the intraoperative prone position which induced consistent hyperpressure in the thoracic cavity. Therefore, we conclude that intraoperative protection is crucial. Protection of the abdomen alone is not enough in the prone position during surgery, as the chest should be protected to reduce thoracic cavity pressure especially during lengthy operations. Therefore, the self-made cushion was replaced with a newer and better cushion (Figure 7C and D). This patient was considered to have idiopathic chylothorax.
Patients with chylothorax usually have dyspnea (the most common symptom), chest pain, and a nonproductive cough as in most types of pleural effusion[16]. Our patient initially had dyspnea and a dry cough, but no chest pain. A chest CT scan revealed small to moderate effusion in the bilateral pleural cavity. With no previous experience of chylothorax, we initially thought that the chylothorax was caused by hypoproteinemia (albumin concentration of 32.3 g/L). The diagnostic methods used for chylothorax include typical milky appearance of pleural fluid, laboratory analysis, and lymphangiography for location when necessary. White milky pleural effusion is a characteristic but unreliable diagnostic method, as less than half of patients with chylous effusion present with this characteristic appearance, and pseudochylothorax with abundant cholesterol looks milky[17]. There are currently more precise laboratory tests available. Chylomicrons are a sign of chyle postprandially. The gold standard for the diagnosis of chylothorax is the presence of chylomicrons in the effusion. However, the evaluation of chylomicrons is not always available in some laboratories. Alternative and differential diagnostic criteria are as follows: pleural fluid triglyceride > 110 mg/dL; the ratio of pleural fluid to serum triglyceride > 1.0; and the ratio of pleural fluid to serum cholesterol < 1.0[17,18]. In our case, the twice positive chyle qualitative tests indicated a chylothorax, even when the color of the effusion was not typically milky but yellowish. In addition, the patient responded well to drainage, total parenteral nutrition, and fasting. Despite positive qualitative tests, the pleural fluid triglyceride concentration was 14.18 mg/dL; the ratios of both right and left pleural fluid to serum triglyceride and cholesterol were 0.16/0.87 (< 1.0), 0.16/1.06 (< 1.0), 0.76/3.02 (< 1.0) and 0.74/3.54 (< 1.0), respectively. Only the cholesterol ratio met the alternative diagnostic criteria. As chyle is from digested fat and accumulates in gastrointestinal lymphatic vessels, our patient had fasted for 20 h preoperatively and had little food intake postoperatively, which may be the reason why the concentration of triglyceride in the pleural fluid was much lower than the normal reference value. Therefore, triglyceride concentration in pleural effusion is not always greater than 110 mg/dL, especially in postoperative patients who have fasted[19].
The management of chylothorax includes conservative treatment, pleurodesis, surgical ligation, and interventional embolization[5,20]. For non-traumatic cases, conservative management including drainage, total parenteral nutrition, medium-chain fatty acid diet, and fasting are effective[4]. Somatostatin or octreotide is used for congenital chylothorax in child patient most to reduce lipid absorption, which was reported effective[21,22]. However, its dose and course are not well established. We did not prescribe it because strict fasting contributes decreasing chyle most. In the review by Schild and Pieper[23], the success rate of conservative management varied from 16% to more than 75%. If conservative treatment fails after 2 wk or initial drainage output is more than 1000 mL/d, surgical or interventional methods should be considered[23]. In the clinical trial by Haniuda et al[24], which involved seven patients with postoperative chylothorax who had undergone pulmonary resection, six patients received successful surgical treatment after unsuccessful conservative therapy, which proved that aggressive surgical treatment is recommended for post-traumatic or post-surgical chylothorax[24]. Jeong et al[25] compared radiological interventional treatment to conservative treatment, and concluded that the former resulted in a shorter median drainage time, nil per os duration and median length of hospital stay. Unfortunately, interventional treatment is only available in a few large centers. All the treatments above are not complete effective. Refractory chylothorax occurred sometimes. Lai et al[26] introduced a safe and effective modified pleurodesis method for treating refractory chylothorax. Just one patient suffered a recurrence and was cured by second pleurodesis[26]. Combined conservative and surgical or interventional management is preferable to most clinicians[4]. Our patient received 12 d of comprehensive conservative management after the detection of chylothorax, which is consistent with previous reports of chylothorax due to other reasons.
Chylothorax following posterior lumbar fusion surgery is rare. In this case, unusual thoracic effusion mistakenly led us to consider hypo-osmolality. Differential diagnosis is crucial for unusual thoracic effusion. Chylothorax can be diagnosed by a positive chyle qualitative test combined with diagnostic treatment consisting of comprehensive conservative therapies. We believe that thorough intraoperative protection to relieve high thoracic pressure caused by the prone position is important.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Darbari A S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LL
| 1. | Lai FC, Chen L, Tu YR, Lin M, Li X. Prevention of chylothorax complicating extensive esophageal resection by mass ligation of thoracic duct: a random control study. Ann Thorac Surg. 2011;91:1770-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Meignan P, Lakhal W, Binet A, Le Touze A, De Courtivron B, Lardy H, Bonnard C, Odent T. Compressive chylothorax after lumbar spine fracture. Arch Pediatr. 2019;26:168-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Doerr CH, Allen MS, Nichols FC 3rd, Ryu JH. Etiology of chylothorax in 203 patients. Mayo Clin Proc. 2005;80:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg. 2007;32:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Shimizu J, Hayashi Y, Oda M, Morita K, Arano Y, Nagao S, Murakami S, Urayama H, Watanabe Y. Treatment of postoperative chylothorax by pleurodesis with the streptococcal preparation OK-432. Thorac Cardiovasc Surg. 1994;42:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Rosti L, Bini RM, Chessa M, Butera G, Drago M, Carminati M. The effectiveness of octreotide in the treatment of post-operative chylothorax. Eur J Pediatr. 2002;161:149-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Su IC, Chen CM. Spontaneous healing of retroperitoneal chylous leakage following anterior lumbar spinal surgery: a case report and literature review. Eur Spine J. 2007;16 Suppl 3:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Bae JS, Park JH, Jang IT. Bilateral chylothorax following anterior cervical spine surgery. Acta Neurochir (Wien). 2017;159:2019-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Mora de Sambricio A, Garrido Stratenwerth E. Chylothorax following anterior thoraco-lumbar spine exposure. A case report and review of literature. Rev Esp Cir Ortop Traumatol. 2015;59:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Bellier A, Pardo Vargas JS, Cassiba J, Desbrest P, Guigui A, Chaffanjon P. Anatomical variations in distal portion of the thoracic duct-A systematic review. Clin Anat. 2020;33:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Koshima Y, Miyazaki S, Ninomiya J, Kuno Y, Ikeda T. Transudative chylothorax associated with alcoholic cirrhosis. Oxf Med Case Reports. 2019;2019:omz019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Kotaru AC, Rajput AK. Chylothorax From Gorham-Stout Disease. J Bronchology Interv Pulmonol. 2018;25:340-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Romero S, Martín C, Hernandez L, Verdu J, Trigo C, Perez-Mateo M, Alemany L. Chylothorax in cirrhosis of the liver: analysis of its frequency and clinical characteristics. Chest. 1998;114:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Sriprasit P, Akaraborworn O. Chylothorax after Blunt Chest Trauma: A Case Report. Korean J Thorac Cardiovasc Surg. 2017;50:407-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Bottet B, Melki J, Levesque H, Baste JM, Roussel E, Peillon C. [Stretching and chylothorax]. Rev Mal Respir. 2019;36:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Romero S. Nontraumatic chylothorax. Curr Opin Pulm Med. 2000;6:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Staats BA, Ellefson RD, Budahn LL, Dines DE, Prakash UB, Offord K. The lipoprotein profile of chylous and nonchylous pleural effusions. Mayo Clin Proc. 1980;55:700-704. [PubMed] |
| 18. | Hillerdal G. Chylothorax and pseudochylothorax. Eur Respir J. 1997;10:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 122] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Maldonado F, Hawkins FJ, Daniels CE, Doerr CH, Decker PA, Ryu JH. Pleural fluid characteristics of chylothorax. Mayo Clin Proc. 2009;84:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 20. | Itkin M. Lymphatic intervention is a new frontier of IR. J Vasc Interv Radiol. 2014;25:1404-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Long WG, Cai B, Deng JM, Liu Y, Wang WJ, Luo J. Chemical pleurodesis and somatostatin in treating spontaneous chylothorax in pediatric patients: a retrospective analysis and review of the literature. Transl Pediatr. 2020;9:551-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Vass G, Evans Fry R, Roehr CC. Should Newborns with Refractory Chylothorax Be Tried on Higher Dose of Octreotide? Neonatology. 2021;118:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Schild HH, Pieper C. [Chylothorax: Current Therapeutic Options]. Zentralbl Chir. 2019;144:S24-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Haniuda M, Nishimura H, Kobayashi O, Yamanda T, Miyazawa M, Aoki T, Iida F. Management of chylothorax after pulmonary resection. J Am Coll Surg. 1995;180:537-540. [PubMed] |
| 25. | Jeong H, Ahn HY, Kwon H, Kim YD, Cho JS, Eom J. Lymphangiographic Interventions to Manage Postoperative Chylothorax. Korean J Thorac Cardiovasc Surg. 2019;52:409-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Lai Y, Zheng X, Yuan Y, Xie TP, Zhao YF, Zhu ZJ, Hu Y. A modified pleurodesis in treating postoperative chylothorax. Ann Transl Med. 2019;7:549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |