Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6485
Peer-review started: March 28, 2021
First decision: April 28, 2021
Revised: May 9, 2021
Accepted: May 27, 2021
Article in press: May 27, 2021
Published online: August 6, 2021
Processing time: 121 Days and 9.2 Hours
Hydrocephalus following dural tear after spinal surgery is rare. Although a few cases of obstructive hydrocephalus caused by subdural fluid collection and communicating hydrocephalus associated with meningitis have been reported, the mechanism remains uncertain. Herein we describe a patient complicated with hydrocephalus after cervical laminoplasty in whom subdural fluid collection in the cervical spine and posterior cranial fossa rather than chronic meningitis was the main mechanism.
A 45-year-old man underwent cervical laminoplasty for cervical spondylotic myelopathy at a local hospital. Ten days postoperatively, a high fever occurred and magnetic resonance imaging (MRI) showed cerebrospinal fluid (CSF) leakage. Pseudomeningocele liquid test showed high levels of protein and white blood cell (WBC) count with negative bacterial culture. The patient was treated with short-term intravenous antibiotic and discharged with normal body temperature. The patient was uneventful during the first 8 mo follow-up although repeated MRI showed persistent pseudomeningocele. At the 9th mo postoperatively, the patient gradually presented with dizziness and headache accompanied by recurrent weakness of his left arm. Imaging examinations demonstrated hydrocephalus and a cystic lesion around the cervical spinal cord. CSF test from lumbar puncture indicated chronic meningitis. MRI on 1 d after pseudomeningocele drainage showed a significant decrease in the cystic volume, suggesting that the cystic lesion would be subdural fluid collection rather than adhesive arachnoiditis. After dural defect repair, the patient’s symptoms completely resolved and hydro
Subdural fluid collection rather than meningitis contributes to the hydrocephalus formation after cervical laminoplasty.
Core Tip: Hydrocephalus following dural tear after spinal surgery is rare, and the mechanism remains uncertain. Although this case is not the first case of subdural fluid collection or chronic meningitis accompanied with hydrocephalus after spinal surgery, it is the first case of hydrocephalus accompanied with both subdural fluid collection and chronic meningitis. It confirmed that subdural fluid collection rather than meningitis mainly contributes to hydrocephalus after cervical laminoplasty for the first time. Combined with this case and literature review, it provided a reliable explanation for the mechanism of hydrocephalus after spinal surgery.
- Citation: Huang HH, Cheng ZH, Ding BZ, Zhao J, Zhao CQ. Subdural fluid collection rather than meningitis contributes to hydrocephalus after cervical laminoplasty: A case report. World J Clin Cases 2021; 9(22): 6485-6492
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6485.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6485
Previous studies have shown the incidences of dural tear after spinal surgery ranging from 1.7% to as high as 17.4%[1,2]. Moreover, destruction of the cerebrospinal fluid (CSF) barrier due to dural tears may result in serious complications, such as pseudomeningocele, meningitis, arachnoiditis, hemorrhage, and extremely rare hydro
The patient is a 45-year-old man who presented with dizziness and headache accompanied by recurrent weakness of his left arm at the 9th mo after cervical laminoplasty.
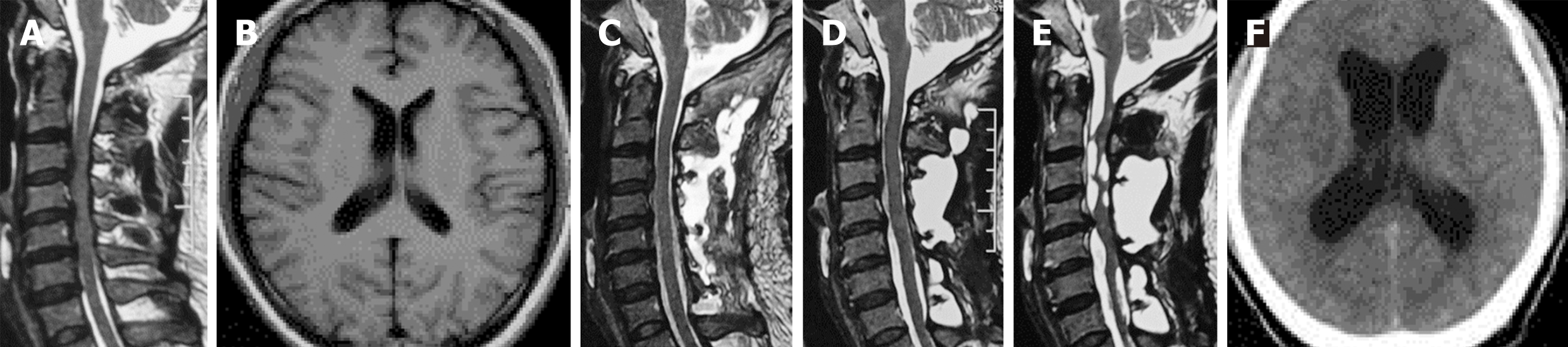
The patient presented with symptoms of aggravated sensory and motor disturbances in the limbs and unstable walking. He underwent cervical laminoplasty for cervical spondylotic myelopathy at a local hospital (Figure 1A). Although the patient complained of dizziness, the brain magnetic resonance imaging (MRI) before surgery did not reveal any abnormalities (Figure 1B). After the surgery, the patient felt a significant improvement in his condition. However, on the 10th day after operation, a high fever occurred, accompanied by abnormal blood test [white blood cells (WBCs): 13.22 × 109/L; neutrophils: 10.42 × 109/L; C-reactive protein: 18.55 mg/L; ESR: 37 mm/h]. The skin around the incision was slightly red, but there was no pressure pain or exudation. Cervical MRI was performed due to local doctors' concerns about possible surgical site infection, and unexpectedly revealed occult CSF leakage. Pseudomeningocele fluid test showed high levels of protein and WBC count (Table 1). Although the patient had no obvious symptoms of neurologic deficits and meningeal irritation, and bacterial culture of pseudomeningocele fluid was negative, mild acute central nervous system infection could not be excluded. Therefore, he was treated with short-term intravenous antibiotic and discharged with normal body temperature.
| Date | Sample | WBCs | RBCs | Glucose | Protein | Chloride | CSF culture | Implication |
| 1 | Cyst puncture | WBCs: 450 × 106/L | 0 | 5.92 mmol/L | 1.478 g/L | Normal | Negative | Acute meningitis |
| 2 | Lumbar puncture | WBCs: 303 × 106/L; monocytes, 14% | 0 | Normal | 4.24 g/L | 117 mmol/L | Negative | Chronic meningitis |
| 3 | Lumbar puncture | WBCs: 10 × 106/L | 0 | 5.09 mmol/L | 2.02 g/L | 130 mmol/L | Negative | Chronic meningitis |
The patient was uneventful during the first 8 mo follow-up although repeated MRI showed persistent pseudomeningocele (Figure 1C and D). However, at the 9th mo, the patient gradually presented with dizziness and headache accompanied by recurrent weakness of his left arm. MRI at this time revealed pseudomeningocele, as well as cystic lesion around the cervical spinal cord and medulla oblongata (Figure 1E). And cranial computed tomography (CT) scans showed marked enlargement of the ventricular system (Figure 1F). Then, he was admitted to our hospital for further treatment.
The patient had no previous history of any illnesses.
The patient had no relevant personal or family history.
Physical examination showed no obvious abnormality except decreased muscle strength of the left upper limb.
CSF analysis at our hospital indicated chronic meningitis (Table 1). Repeated bacterial culture of CSF was negative. The hematology test was normal.
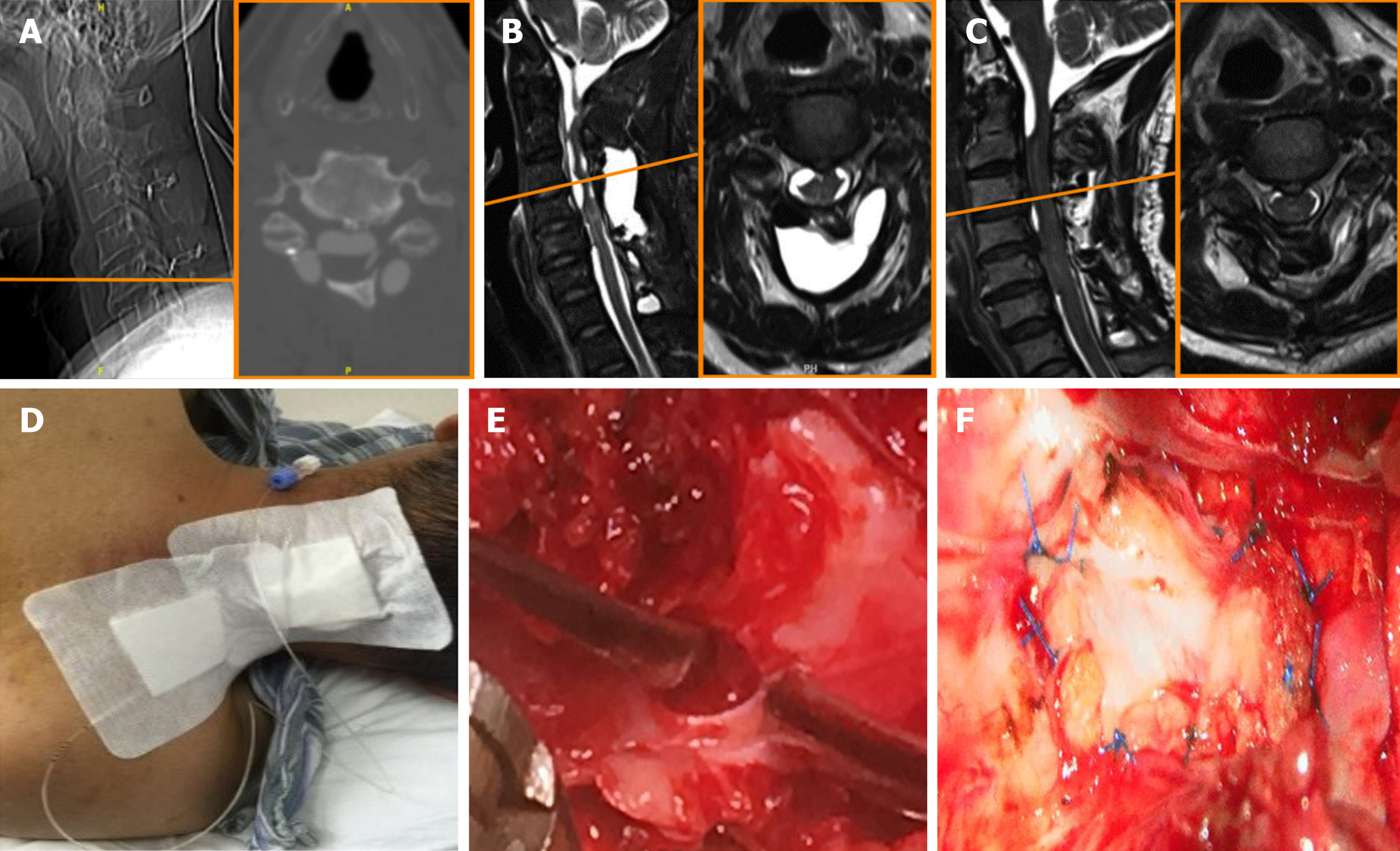
On the second day after admission, computed tomography myelography showed that the dural-arachnoid defect was located at the level of C5, near the lower edge of the fixed plate (Figure 2).
The patient was diagnosed with hydrocephalus, chronic meningitis, and CSF leakage. We concluded that subdural fluid collection was the main cause of patient’s discomfort.
Dural repair was used to eliminate the source of subdural fluid collection (Figure 2E and F). No antibiotic treatment was given because the patient had no obvious fever or meningeal irritation.
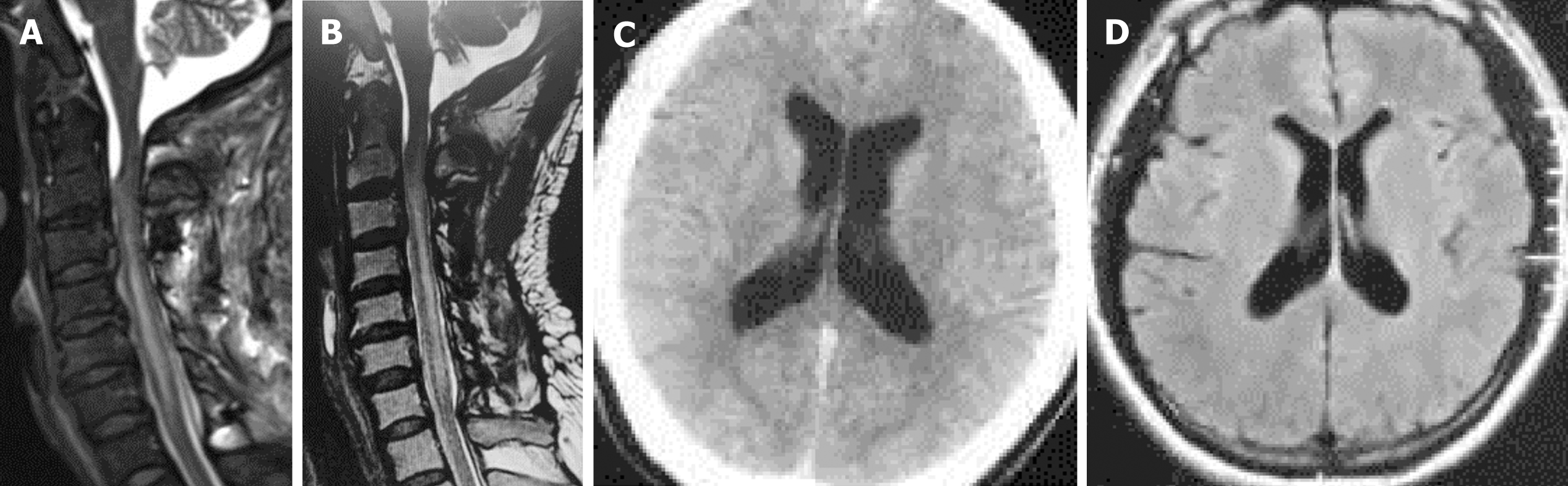
The patient showed an immediate improvement in his disease after the surgery. Postoperative cervical MRI showed significantly decreased pseudomeningocele and subdural fluid collection (Figure 3A). During the follow-up period, repeated imaging examination revealed that subdural fluid collection disappeared completely (Figure 3B), and ventricular size gradually returned to normal (Figure 3C and D). CSF analysis at the 21-mo follow-up showed that the levels of protein and WBC count decreased significantly without long term antibiotic treatment (Table 1).
Previous cases have found that hydrocephalus can develop following cervical laminoplasty and fusion[3-6], thoracic and lumbar decompression[7-12], intraspinal tumors resection[13-17], and even cervical myelogram[18] (Table 2). There are two types of hydrocephalus. One is obstructive hydrocephalus. Different etiologies, including oppressive effect of subdural fluid collection or cerebellar enlargement due to cerebellar hemorrhage[7,13], obstruction of clot formation after brisk bleed enters the subarachnoid space and ventricle system[18], had been reported.
| Ref. | Age (yr), sex | Spine procedure | Dural tear | Fever | CSF test | Risk factors of hydrocephalus | Intervention | Outcome |
| Bland and McDonald[14], 1992 | 58, M | Cervical tumor resection | Yes | None | Elevated CSF protein and red blood cell count | Elevated CSF protein, subarachnoid hemorrhage | VP shunt | Full recovery |
| Maezawa et al[4], 1996 | 69, F | Cervical laminoplasty | Yes | None | Elevated protein (64 mg/L) | Systemic hypertension, elevated CSF protein level, subdural hydroma, suboccipital arachnoiditis | VP shunt | Full recovery |
| Aghi et al[18], 2004 | 52, F | Cervical myelogram | None | None | Elevated leukocytes and erythrocytes | Hemorrhage in cervical subdural space | EVD, suboccipital craniectomy, and C1–C2 laminectomies | Full recovery |
| Koerts et al[10], 2008 | 45, M | Lumbar surgery | Yes | None | Moderate increase of WBCs, elevated protein level (69 mg/L) and lactate | Multiple lumbar surgery, CSF infection, and spinal adhesive arachnoiditis | EVD | Full recovery |
| Morofuji et al[11], 2009 | 51, M | Thoracic decompression | Yes | None | None | Remote cerebellar hemorrhage | Suboccipital decompression | Full recovery |
| Lindley et al[3], 2011 | 14, M | Oc–C2 fusion + rhBMP | None | None | None | Intense inflammatory response to rhBMP, wound seroma formation, Epidural fluid extending from the surgical site into the epidural space | EVD, wound exploration, and drain | Full recovery |
| 8,M | Oc–C1–C2 fusion + rhBMP | None | None | None | Full recovery | |||
| Stovell et al[6], 2013 | 63, F | C1-C2 fixation | Yes | None | None | Potential subarachnoid blood, injury of vessel | VP shunt | Full recovery |
| Cavanilles et al[7], 2013 | 65, F | Luambar fusion and decompression | Yes | None | None | Caudal sagging of cerebellum, mass effect with compression in the posterior fossa | EVD | Mild motor deficits |
| Kaloostian et al[9], 2013 | 77, M | T11–S1 posterior decompression and instrumented fusion | Yes | None | None | Subarachnoid blood in the cerebellar folia | VP shunt | Cognitive defects |
| 81, M | L4–5 decompression | Yes | None | None | Cerebellar hemorrhage | Ventriculostomy | Died | |
| 64, F | L1~S1 posterior decompression and instrumented fusion | Yes | None | None | Large cerebellar hemorrhage, brainstem compression, and hydrocephalus | - | Died soon | |
| Matsushima et al[5],2016 | 65, M | Cervical laminoplasty | Yes | None | Elevated protein (75 mg/L) | Increased CSF protein levels, spinal cord subarachnoidal hemorrhage | Dural repair and VP shunt | Full recovery |
| Endriga et al[8], 2016 | 62, F | Lumbar decompression | Yes | None | None | Subarachnoid hemorrhage, extensive subdural fluid collection, pseudomeningoceole | VP shunt | Full recovery |
| Benedetto et al[13], 2016 | 31, M | Cervical tumor resection | Yes | None | None | Subdurall fluid collections | Dural repair | Full recovery |
| Esfahani et al[15], 2017 | 7, M | Cervical neurenteric cyst resection | Yes | High fever | None | Contamination of high cytokeratin content or other debris in the CSF, chemical meningitis | VP shunt | Full recovery |
| Kobayashi et al[16], 2018 | 39, M | Cervical tumor resection | Yes | None | None | Aseptic meningitis, microhemorrhage, and fibrinogenic components | VP shunt and dural repair | Full recovery |
| Prior et al[17], 2018 | 6, F | Lumbar tumor resection | Yes | None | None | Possible dissemination of fat droplets in the subarachnoid spaces, aseptic | VP shunt and dural repair | Full recovery |
| Tan et al[12], 2018 | 76, F | L3-S1 laminectomies and fusion | Yes | None | None | Intraventricular hemorrhage | EVD | Full recovery |
The other is communicating hydrocephalus. There are possible relationships between postoperative communicating hydrocephalus and subarachnoid hemorrhage, infection, contamination of the CSF with blood, multiple surgeries, increased CSF protein levels, high blood pressure, and meningitis[4,5,8,10,19]. Researchers speculated that these factors may lead to the obstruction of arachnoid granulation and arachnoid villi, the reduction of CSF compartment compliance, and the rise of CSF circulation resistance, which in turn causes the disorder of CSF absorption and circulation[16,20,21]. However, most of them are speculative conclusions without direct evidence.
This is the first case of hydrocephalus accompanied with both subdural fluid collection and chronic meningitis after spinal surgery. In this case, it is significant to differentiate subdural fluid collection from spinal adhesive arachnoiditis. Spinal adhesive arachnoiditis is a disease characterized by inflammation and scarring of the arachnoid membrane of the spinal cord, and only surgical intervention may provide temporary relief[22]. Reduced volume of cystic lesion and improved condition after pseudomeningocele drainage successfully conformed its subdural fluid collection nature. Dural repair eliminated the patient’s discomfort, and hydrocephalus gradually disappeared, suggesting that subdural fluid collection rather than meningitis contributes to hydrocephalus. At the 21-mo follow-up, the patient still had asymptomatic meningitis with decreased CSF protein and WBC compared with before, which further confirmed the dominant role of subdural fluid collection in the formation of hydrocephalus. Subdural fluid collection in this case bought about the compression and backward displacement of the medulla oblongata, and caused the stenosis of the fourth ventricle outlet and following hydrocephalus. In addition, it also hindered the flow of CSF below the cervical spine and reduced the compliance of the CSF circulation system, which further promoted the formation of hydrocephalus[23].
Notably, the occurrence of chronic meningitis is a complex process, and one third of patients are still unable to determine the specific pathogenic factors[24]. In this case, further etiological analysis could not be accessed because of the lack of pathogen detection methods. Considering the long-term asymptomatic state of the patient and the gradual decrease of CSF protein level and WBC, it is reasonable to keep observation and follow-up of the patient, which can avoid excessive examination and overtreatment.
The development of hydrocephalus after cervical laminoplasty in this case was mainly caused by subdural fluid collections rather than meningitis, which provides original insight into the pathogenesis of hydrocephalus after spinal surgery. Priority could be given to the relief of obstruction in similar cases permitted under patients’ condition.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chrastina J S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Tafazal SI, Sell PJ. Incidental durotomy in lumbar spine surgery: incidence and management. Eur Spine J. 2005;14:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Wolff S, Kheirredine W, Riouallon G. Surgical dural tears: prevalence and updated management protocol based on 1359 lumbar vertebra interventions. Orthop Traumatol Surg Res. 2012;98:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Lindley TE, Dahdaleh NS, Menezes AH, Abode-Iyamah KO. Complications associated with recombinant human bone morphogenetic protein use in pediatric craniocervical arthrodesis. J Neurosurg Pediatr. 2011;7:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Maezawa Y, Baba H, Annen S, Uchida K, Imura S, Handa Y. Development of hydrocephalus after cervical laminoplasty for ossification of the posterior longitudinal ligament: case report. Spinal Cord. 1996;34:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Matsushima K, Hashimoto R, Gondo M, Fukuhara H, Kohno M, Jimbo H. Perifascial Areolar Tissue Graft for Spinal Dural Repair with Cerebrospinal Fluid Leakage: Case Report of Novel Graft Material, Radiological Assessment Technique, and Rare Postoperative Hydrocephalus. World Neurosurg. 2016;95:619.e5-619.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Stovell MG, Pillay R. Subarachnoid hemorrhage and acute hydrocephalus as a complication of C1 lateral mass screws. Spine. 38:E1162-E1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Cavanilles-Walker JM, Tomasi SO, Sgier F, Kröber M. Remote cerebellar haemorrhage after lumbar spine surgery: case report. Arch Orthop Trauma Surg. 2013;133:1645-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Endriga DT, Dimar JR 2nd, Carreon LY. Communicating hydrocephalus, a long-term complication of dural tear during lumbar spine surgery. Eur Spine J. 2016;25 Suppl 1:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Kaloostian PE, Kim JE, Bydon A, Sciubba DM, Wolinsky JP, Gokaslan ZL, Witham TF. Intracranial hemorrhage after spine surgery. J Neurosurg Spine. 2013;19:370-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Koerts G, Rooijakkers H, Abu-Serieh B, Cosnard G, Raftopoulos C. Postoperative spinal adhesive arachnoiditis presenting with hydrocephalus and cauda equina syndrome. Clin Neurol Neurosurg. 2008;110:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Morofuji Y, Tsunoda K, Takeshita T, Hayashi K, Kitagawa N, Suyama K, Nagata I. Remote cerebellar hemorrhage following thoracic spinal surgery. Neurol Med Chir (Tokyo). 2009;49:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Tan LA, Kasliwal MK, An HS, Byrne RW. Obstructive Hydrocephalus Due to Intraventricular Hemorrhage After Incidental Durotomy During Lumbar Spine Surgery. Spine (Phila Pa 1976). 2018;43:E316-E319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Benedetto N, Cagnazzo F, Gambacciani C, Perrini P. Subdural fluid collection and hydrocephalus following cervical schwannoma resection: hydrocephalus resolution after spinal pseudomeningocele repair: case report. J Neurosurg Spine. 2016;25:762-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Bland LI, McDonald JV. Hydrocephalus following spinal cord schwannoma resection. Arch Neurol. 1992;49:882-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Esfahani DR, Burokas L, Brown HG, Hahn YS, Nikas D. Management of an unusual, recurrent neurenteric cyst in an infant: case report and review of the literature. Childs Nerv Syst. 2017;33:1603-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Kobayashi K, Ando K, Ito K, Tsushima M, Morozumi M, Tanaka S, Machino M, Ota K, Ishiguro N, Imagama S. A case of delayed hydrocephalus from cerebrospinal fluid leak after resection of a cervical spinal schwannoma. Nagoya J Med Sci. 2018;80:605-609. [PubMed] |
| 17. | Prior A, Severino M, Rossi A, Pavanello M, Piatelli G, Consales A. Acute Communicating Hydrocephalus as Spinal Cord Surgery Complication in Patient with Lumbar Lipomyelocele. World Neurosurg. 2018;115:468-472.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Aghi M, Coumans JV, Brisman JL. Subarachnoid hematoma, hydrocephalus, and aseptic meningitis resulting from a high cervical myelogram. J Spinal Disord Tech. 2004;17:348-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Mactier H, Galea P, McWilliam R. Acute obstructive hydrocephalus complicating bacterial meningitis in childhood. BMJ. 1998;316:1887-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Massicotte EM, Del Bigio MR. Human arachnoid villi response to subarachnoid hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg. 1999;91:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Ellington E, Margolis G. Block of arachnoid villus by subarachnoid hemorrhage. J Neurosurg. 1969;30:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 102] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Wright MH, Denney LC. A comprehensive review of spinal arachnoiditis. Orthop Nurs. 2003;22:215-9; quiz 220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Morandi X, Amlashi SF, Riffaud L. A dynamic theory for hydrocephalus revealing benign intraspinal tumours: tumoural obstruction of the spinal subarachnoid space reduces total CSF compartment compliance. Med Hypotheses. 2006;67:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Helbok R, Broessner G, Pfausler B, Schmutzhard E. Chronic meningitis. J Neurol. 2009;256:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |