Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6478
Peer-review started: April 2, 2021
First decision: April 28, 2021
Revised: May 16, 2021
Accepted: June 1, 2021
Article in press: June 1, 2021
Published online: August 6, 2021
Processing time: 116 Days and 14.9 Hours
Primary small cell esophageal carcinoma (PSCEC) is aggressive and rare, with a worse prognosis than other subtypes esophageal carcinoma. No definitive and optimum standard guidelines are established for treating it. Herein, we report a case of PSCEC, including a current literature review of PSCEC.
A 79-year-old male was diagnosed PSCEC with multiple lymph node metastasis thorough computed tomography, positron emission tomography-computed tomography, endoscopy and pathology. Surgery was not suitable for this patient. He was treated with etoposide 100 mg/m2 and cisplatin 25 mg/m2 on days 1-3, every 3 wk for 4 cycles. The tumor and lymph nodes became smaller and dysphagia and vomiting symptoms improved. The patient could not tolerate subsequent chemotherapy (CT) because of hematological toxicity; therefore, we performed immunotherapy (durvalumab, 1500 mg) every 4 wk. At present the patient has received 12 cycles immunotherapy over about 1 year. He is still receiving treatment and follow-up.
PSCEC with multiple lymph nodes metastasis does not always indicate surgery. CT may extend survival time and improve the quality of life in the absence of surgery. Immunotherapy or immunotherapy plus CT may also work as a treatment for PSCEC.
Core Tip: A 79-year-old male was diagnosed primary small cell esophageal carcinoma and multiple lymph nodes metastasis thorough computed tomography, positron emission tomography-computed tomography, endoscopy and pathology. Surgery was not suitable for this patient. Instead, he was treated with etoposide and cisplatin chemotherapy regiment, every 3 wk for 4 cycles, which caused the tumor and lymph nodes to shrink. The patient could not tolerate subsequent chemotherapy due to hematological toxicity; therefore, we performed immunotherapy (durvalumab, 1500 mg) every 4 wk. At present, the patient has received 12 cycles immunotherapy over about 1 year and continues treatment and follow-up.
- Citation: Wu YH, Zhang K, Chen HG, Wu WB, Li XJ, Zhang J. Primary small cell esophageal carcinoma, chemotherapy sequential immunotherapy: A case report. World J Clin Cases 2021; 9(22): 6478-6484
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6478.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6478
Primary small cell esophageal carcinoma (PSCEC) is a rare disease, accounting for only 0.8% to 3.1% of all esophageal malignancies[1,2]. The first case was reported in 1952 by McKeown, at present, only about 300 cases of PSCEC have been reported in the world medical literature[3].
PSCEC has an aggressive progression, with early metastasis and high malignancy, and the prognosis is poorer than other subtypes esophageal carcinoma[1]. However, at present, there are no definitive and optimum standard treatment guidelines for PSCEC, though most experts recommend radical esophagectomy, chemotherapy (CT) and radiotherapy (RT) for PSCEC either alone or in combination[4,5]. With the wide application of immunotherapy, perhaps it will become a new treatment[6,7]. Herein we report the case of a 79-year-old man with a PSCEC treated with CT followed by immunotherapy, the tumor has stopped enlarging and has not metastasized, and the patient is no longer experiencing discomfort.
A 79-year-old male was hospitalized for progressive dysphagia, frequent vomiting, and weight lost (approximately 5 kg in 1 mo).
The patient began to experience dysphagia 1 mo prior to examination. Symptoms of obstruction and dysphagia were aggravated, with intermittent chest pain during this month. Food intake decreased, and patient lost 5 kg of weight during this month. Acid reflux and heartburn symptoms were not present, and he did not cough when he swallowed or drank.
The patient is healthy without a history of hypertension or diabetes.
He was a nonpassive smoker. He did not have a family history of malignancy.
Physical examination does not identify any enlarged lymph nodes in the neck or supraclavicular regions. Cardiopulmonary examination is almost normal without any positive signs.
Blood neuron-specific enolase (NSE) level was 20.80 ng/mL higher than the normal value (16.3 ng/mL), other tumor markers (alpha fetoprotein, CEA, CA-125, CA-199) were all within the normal range.
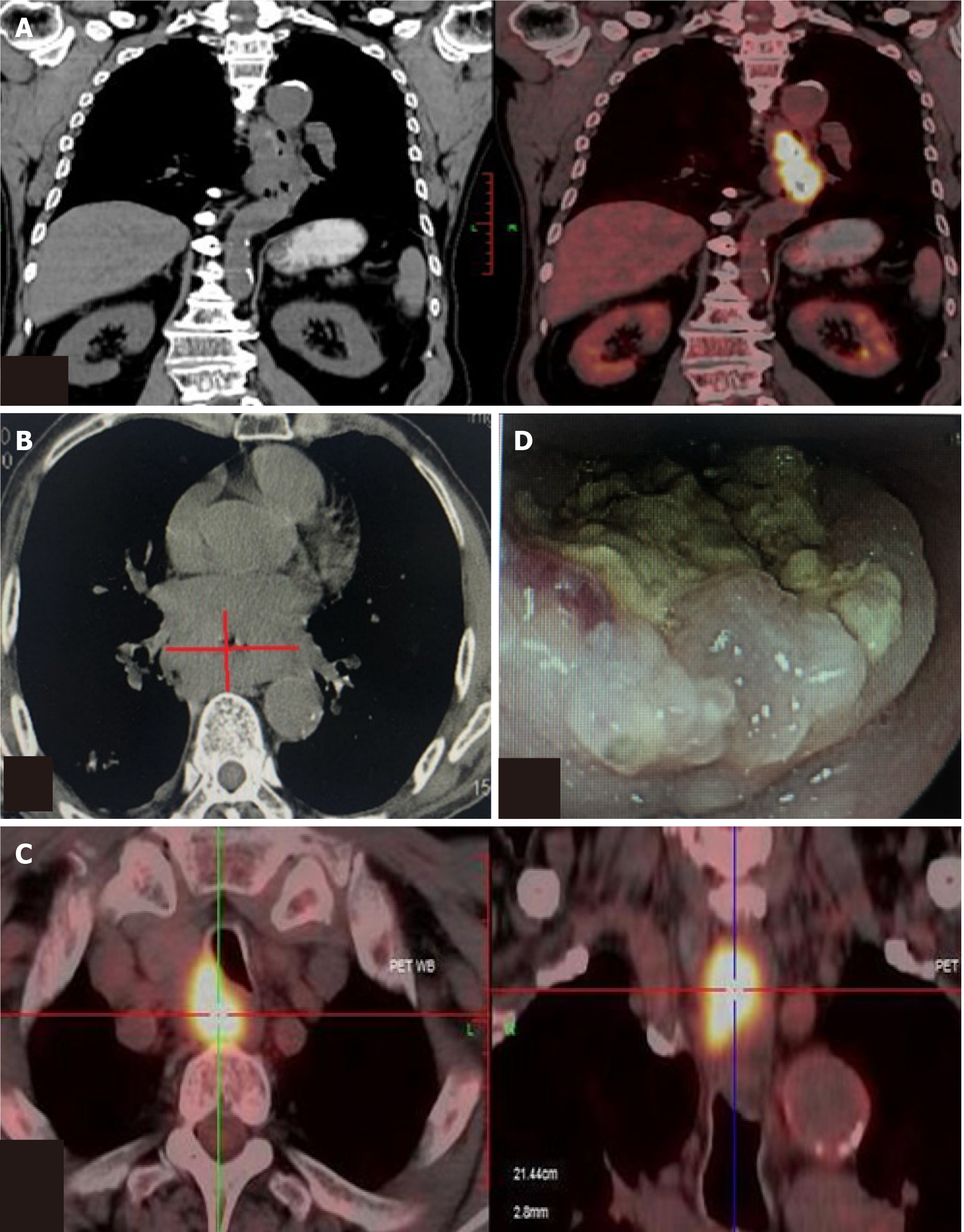
Positron emission tomography-computed tomography and computed tomography demonstrated a tumor in the middle and lower esophagus. Multiple mediastinal lymph nodes were enlarged and were regarded as metastasis (Figure 1A-C). Endoscopy revealed a carcinoma in the esophagus beginning at 28 cm from his teeth and extending to 38 cm, the carcinoma almost blocked 3/4 of esophageal cavity (Figure 1D).
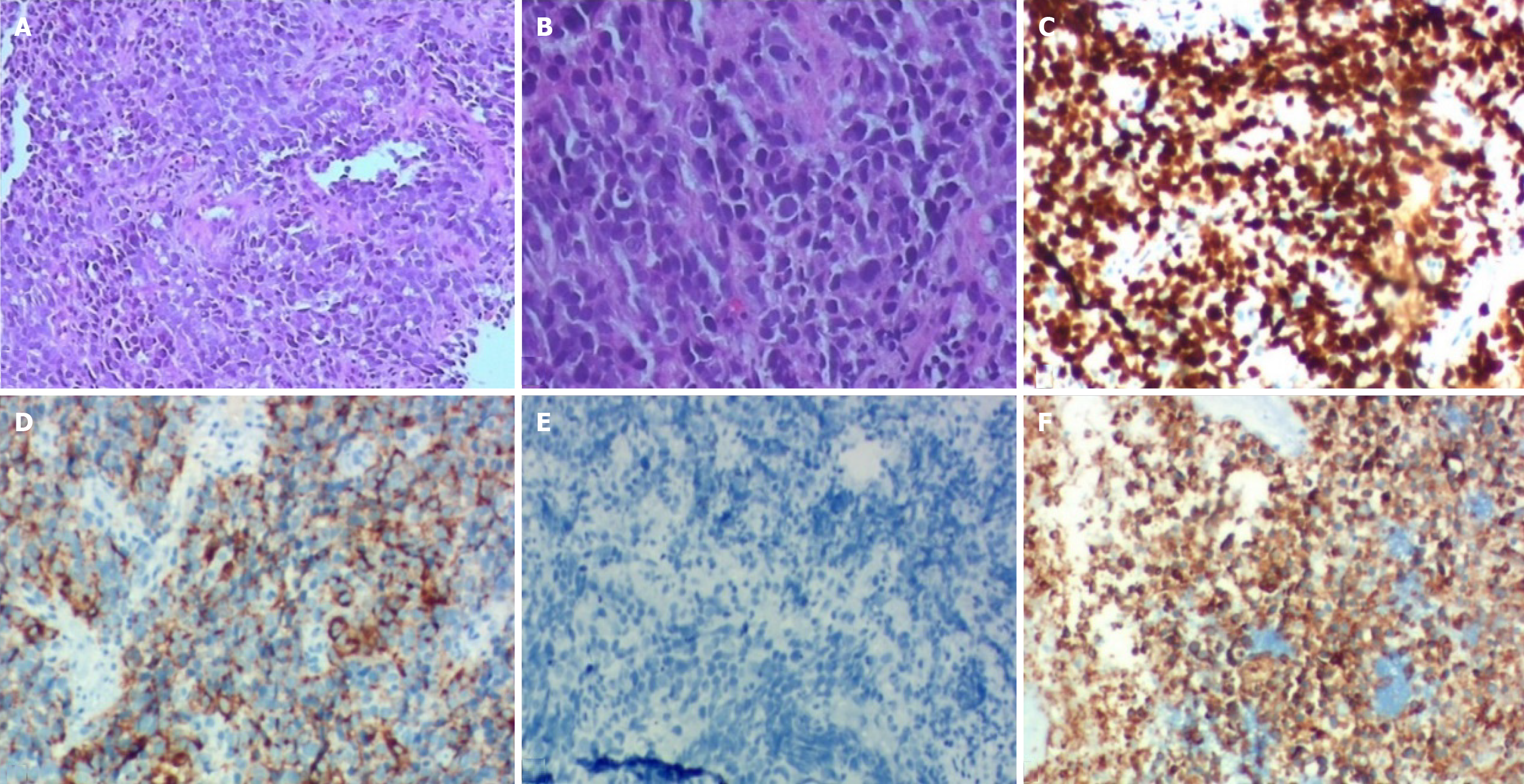
Microscopic examination of hematoxylin-eosin staining tumor slices showed the tumor cells had small oval and spindle cell shape nuclei, ill-defined cell borders, and inconspicuous nucleoli (Figure 2A and B). Immunohistochemistry (IHC) studies showed the tumor Ki-67 index > 80%, Cg-A-positive, p40-positive, Syn-positive (Figure 2C-F).
PSCEC, TNM: cT3N2M0, stage III.
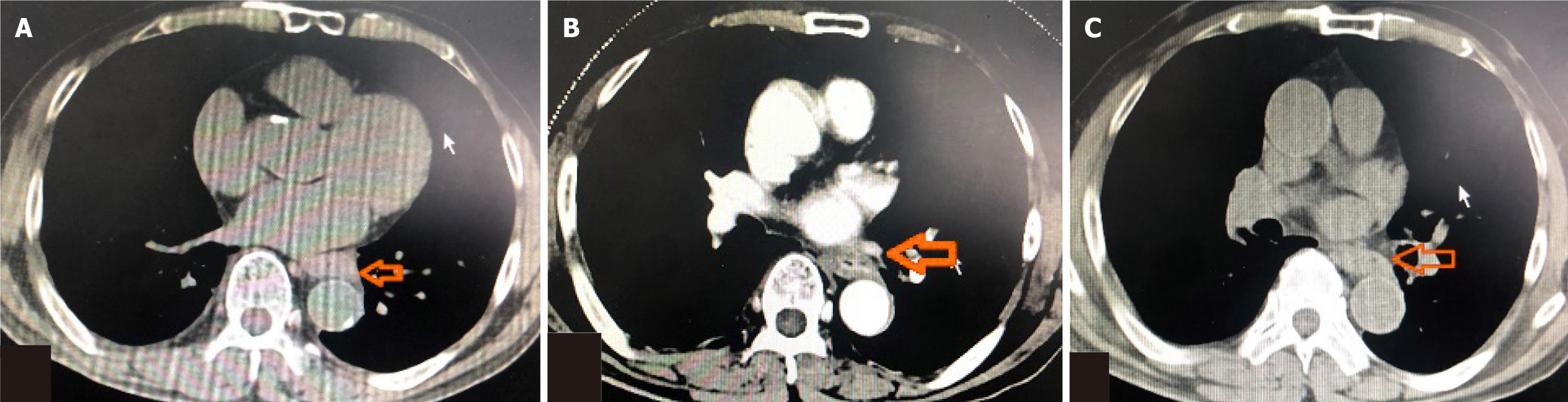
Stage III PSCEC was not suitable for surgery, and the patient did not tolerate concurrent CT plus RT; therefore, he was treated with etoposide 100 mg/m2 and cisplatin 25 mg/m2 on days 1-3, every 3 wk. After one cycle, dysphagia decreased and frequent vomiting resolved. It was partial response according to the Recist 1.1 guidelines, and after four cycles of CT, dysphagia completely resolved (Figure 3A). NSE levels in blood also decreased by 6.4 ng/mL. Due to CT toxicities, subsequent therapy was switched to immunotherapy (durvalumab 1500 mg) every 4 wk. According to imaging, the tumor remained stable after 4 cycles of CT and 8 cycles immunotherapy (Figure 3B and C), and NSE blood levels remained normal. Patient did not experience any immune-related adverse events (irAEs) or cancer progressions symptoms for about 1 year. The patient was followed every 3 mo with chest imaging, abdominal ultrasound, brain magnetic resonance and monitored monthly with blood tests.
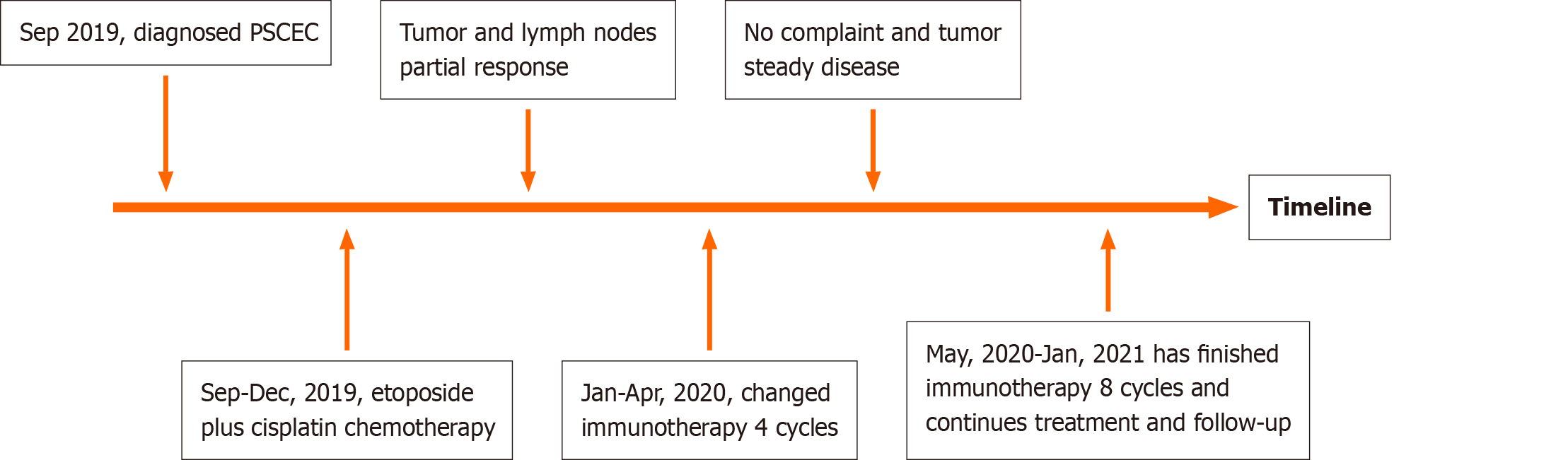
The patient has received 12 cycles of durvalumab (1500 mg). The tumor and lymph nodes remain stable, and no new metastasis have been identified. the patient has not reported any more dysphagia or frequent vomiting, and continues follow-up and treatment. A timeline showed the whole medical procedure of this case (Figure 4).
PSCEC is a rare esophageal malignancy. Because of aggressive biologic behavior and early widespread metastasis, it is generally regarded to have a poor prognosis[8]. The management, treatment, and follow-up strategies are still not sufficiently standardized. Other studies described a median survival of only 8 mo for patients with local disease (LD) and 3 mo for patients with extensive disease (ED)[9].
PSCEC lacks characteristic manifestations in the early stage, which is similar to other types of esophageal carcinoma. Most patients are at an advanced stage at the time of diagnosis, and symptoms include dysphagia, obstruction and/or frequent vomiting, and weight loss.
World Health Organization histological criteria (2004) for small cell lung cancer (SCLC) or SCEC include small, round, oval or spindle-shaped cells with scant cytoplasm, ill-defined cell borders, finely granular nuclear chromatin, and absent or inconspicuous nucleoli. IHC shows positive expression of the tumor markers CK8, AE1/AE3, EMA, Syn, NSE, CD56, Cg-A, and TTF-1[3,10,11]. Wang et al[10] showed that high Ki-67 expression was an independent favorable prognostic factor for PSCEC patients[12]. It is generally believed that the Ki-67 index is > 50% in small carcinomas and this tumor Ki-67 index is > 80%. Syn and NSE are expressed in all gastrointestinal small cell carcinomas. This case was Cg-A positive and Syn-positive, and NSE levels were elevated in blood.
PSCEC is like SCLC, with local and distant lymphatic and hematogenous metastasis at first diagnosis, suggesting routine surgery for PSCEC may not be necessary. However, some studies show that esophagectomy can produce the best overall survival for patients with localized or local-advanced cancer, compared to chemoradiotherapy (CRT) or CT alone[13].
Some analysis suggest that surgery could achieve clinical benefits only for patients with LD, combined RT or/and CT might be to extend the survival time patients with regional and extensive[14]. Sun et al[15] reported some patients who received surgery and postoperative adjuvant CT achieved a survival of 10 years. PSCEC is a chemical sensitive disease, and CT is one of the most important methods for treating it; therefore, it is believed to be the cornerstone of the multimodality therapy, improving the survival time both in LD and ED patients.
Tumor TNM staging has been seen as a significant prognostic factor for survival in patients. Stage I/IIA, the median survival time (MST) for patients who undergo surgery is 29 mo, as compared to 17.4 mo for those who do not receive surgery. Patients with stage II do not prolong the overall survival with radical surgery alone. Patients with stage IIB/III disease who receive CT have a better MST than patients without CT (13.0 mo vs 6.1 mo), while those who receive CT combined with RT have a longer MST than patients who receive surgery combined with CT (25.7 mo vs 12.3 mo)[16]. CT can greatly improve the survival time of patients with stage III and IV disease[3]. The MST of patients with stage IV disease after CT is three times that of patients who don’t receive CT.
Commonly, CT regimens are platinum-based combined with etoposide or irinotecan, while some use docetaxel in combination with platinum[14,17]. In general, PSCEC CT regimens are the same as those used for SCLC. At present, etoposide combined with platinum is first-line CT for PSCEC, and a few studies have showed that docetaxel combined with platinum as a first-line treatment is also effective[18]. Doxorubicin, cyclophosphamide and vincristine should be considered as second-line treatments.
Programmed death-ligand 1 (PD-L1) inhibition (durvalumab 1500 mg) plus EP (etoposide and platinum) has become the new standard for first-line therapy of SCLC; the 12-mo progress-free survival (PFS) was improved with PD-L1 plus EP vs EP alone (18% vs 5%)[19,20]. Immunotherapy plus CT can improve outcomes in patients with esophageal squamous cell cancer [21]. But no related studies reported immunotherapy or immunotherapy plus CT treated PSCEC. According to histological pathological criteria between SCLC and SCEC, we administered durvalumab (1500 mg every 4 wk) to the patient. Tumor and lymph nodes were unchanged after about 1 year. The patient also did not experience any irAEs, and the PFS reached 12 mo.
PSCEC in the neck or upper segment is treated with RT rather than surgery. Patients are treated with RT to relieve these symptoms quickly. CT and RT alone or combi
Several deficiencies in the whole course of treatment remain for our patient. First, CRT was not performed, which might decrease tumor size, and may have a better outcome than CT alone. Second, we did not confirm PD-L1 levels in the tumor or note any tumor pathological changes after immunotherapy. Third, the follow-up time is relatively short and a long-term follow-up is necessary to evaluate the therapeutic efficacy.
PSCEC is characterized by early metastasis, advanced stage at diagnosis, and poor prognosis. The prognosis depends on the tumor TNM stage, and the choice of local and/or systemic treatment. PSCEC with stage III or higher are not surgical candidates; however, CT play a role in extending survival time and improving the quality of life. Immunotherapy or immunotherapy plus CT could become another treatment for PSCEC.
We thank the patient for permitting us to use his data to complete this article.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oda M S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Tustumi F, Takeda FR, Uema RH, Pereira GL, Sallum RA, Cecconello I. Primary neuroendocrine neoplasm of the esophagus - Report of 14 cases from a single institute and review of the literature. Arq Gastroenterol. 2017;54:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Xu L, Li Y, Liu X, Sun H, Zhang R, Zhang J, Zheng Y, Wang Z, Liu S, Chen X. Treatment Strategies and Prognostic Factors of Limited-Stage Primary Small Cell Carcinoma of the Esophagus. J Thorac Oncol. 2017;12:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Schuerle T, Aoun E, Farah K. Small cell carcinoma of the oesophagus: a rare cause of dysphagia. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Fan H, Lu P, Xu L, Qin Y, Li J. Synchronous occurrence of hereditary gastric adenocarcinoma, gastrointestinal stromal tumor, and esophageal small cell and squamous carcinoma in situ: an extremely rare case report. BMC Cancer. 2017;17:720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Sahai P, Baghmar S, Nath D, Arora S, Bhasker S, Gogia A, Sikka K, Kumar R, Chander S. Extrapulmonary Small Cell Carcinoma - a Case Series of Oropharyngeal and Esophageal Primary Sites Treated with Chemo-Radiotherapy. Asian Pac J Cancer Prev. 2015;16:7025-7029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Zhao Q, Yu J, Meng X. A good start of immunotherapy in esophageal cancer. Cancer Med. 2019;8:4519-4526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Baba Y, Nomoto D, Okadome K, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Baba H. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. 2020;111:3132-3141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 8. | Markogiannakis H, Theodorou D, Toutouzas KG, Larentzakis A, Pattas M, Bousiotou A, Papacostas P, Filis K, Katsaragakis S. Small cell carcinoma arising in Barrett's esophagus: a case report and review of the literature. J Med Case Rep. 2008;2:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Ku GY, Minsky BD, Rusch VW, Bains M, Kelsen DP, Ilson DH. Small-cell carcinoma of the esophagus and gastroesophageal junction: review of the Memorial Sloan-Kettering experience. Ann Oncol. 2008;19:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Wang N, Li X, Luo H, Sun Y, Zheng X, Fan C, Wang H, Ye K, Ge H. Prognostic value of pretreatment inflammatory biomarkers in primary small cell carcinoma of the esophagus. Thorac Cancer. 2019;10:1913-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Fujihara S, Kobayashi M, Nishi M, Yachida T, Yoshitake A, Deguchi A, Muraoka A, Kobara H, Masaki T. Composite neuroendocrine carcinoma and squamous cell carcinoma with regional lymph node metastasis: a case report. J Med Case Rep. 2018;12:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Liu D, Xu X, Wen J, Xie L, Zhang J, Shen Y, Jiang G, Chen J, Fan M. Integrated Genome-Wide Analysis of Gene Expression and DNA Copy Number Variations Highlights Stem Cell-Related Pathways in Small Cell Esophageal Carcinoma. Stem Cells Int. 2018;2018:3481783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Chen B, Yang H, Ma H, Li Q, Qiu B, Hu Y, Zhu Y. Radiotherapy for small cell carcinoma of the esophagus: outcomes and prognostic factors from a retrospective study. Radiat Oncol. 2019;14:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Xiao Q, Xiao H, Ouyang S, Tang J, Zhang B, Wang H. Primary small cell carcinoma of the esophagus: Comparison between a Chinese cohort and Surveillance, Epidemiology, and End Results (SEER) data. Cancer Med. 2019;8:1074-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Sun KL, He J, Cheng GY, Chai LX. Management of primary small cell carcinoma of the esophagus. Chin Med J (Engl). 2007;120:355-358. [PubMed] |
| 16. | Zou B, Li T, Zhou Q, Ma D, Chen Y, Huang M, Peng F, Xu Y, Zhu J, Ding Z, Zhou L, Wang J, Ren L, Yu M, Gong Y, Li Y, Chen L, Lu Y. Adjuvant Therapeutic Modalities in Primary Small Cell Carcinoma of Esophagus Patients: A Retrospective Cohort Study of Multicenter Clinical Outcomes. Medicine (Baltimore). 2016;95:e3507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Jeene PM, Geijsen ED, Muijs CT, Rozema T, Aleman BMP, Muller K, Baas JM, Nuyttens JJ, Wouterse S, Braam PM, Oppedijk V, Ceha HM, Cnossen J, Spruit P, Bongers EM, Berbée M, Mook S, Hulshof MCCM. Small Cell Carcinoma of the Esophagus: A Nationwide Analysis of Treatment and Outcome at Patient Level in Locoregional Disease. Am J Clin Oncol. 2019;42:534-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Akiyama Y, Iwaya T, Shioi Y, Endo F, Chiba T, Otsuka K, Nitta H, Koeda K, Mizuno M, Uesugi N, Kimura Y, Sasaki A. Effectiveness of neoadjuvant chemotherapy with cisplatin and irinotecan followed by surgery on small-cell carcinoma of the esophagus: A case report. Int J Surg Case Rep. 2015;17:121-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Pacheco JM. Immunotherapy for extensive stage small cell lung cancer. J Thorac Dis. 2020;12:6212-6224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2750] [Cited by in RCA: 3244] [Article Influence: 405.5] [Reference Citation Analysis (0)] |
| 21. | Kakeji Y, Oshikiri T, Takiguchi G, Kanaji S, Matsuda T, Nakamura T, Suzuki S. Multimodality approaches to control esophageal cancer: development of chemoradiotherapy, chemotherapy, and immunotherapy. Esophagus. 2021;18:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |