Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6469
Peer-review started: April 7, 2021
First decision: April 23, 2021
Revised: May 6, 2021
Accepted: May 25, 2021
Article in press: May 25, 2021
Published online: August 6, 2021
Processing time: 108 Days and 11.8 Hours
About 20%-30% of newly diagnosed hepatocellular carcinoma (HCC) patients are surgically feasible due to a variety of reasons. Active conversion therapy may provide opportunities of surgery for these patients. Nevertheless, the choice of surgical procedure is controversial after successful conversion therapy. We report a patient with HCC who underwent successful laparoscopic right trisectionectomy after conversion therapy with portal vein embolization and transarterial chemoembolization.
A 67-year-old male patient presented to our hospital with epigastric distention/ discomfort and nausea/vomiting for more than 1 mo. Contrast-enhanced computed tomography scan of the abdomen demonstrated multiple tumors (the largest was ≥ 10 cm in diameter) located in the right liver and left medial lobe, and the left lateral lobe was normal. The future remnant liver (FRL) of the left lateral lobe accounted for only 18% of total liver volume after virtual resection on the three-dimensional liver model. Conversion therapy was adopted after orally administered entecavir for antiviral treatment. First, the right portal vein was embolized. Then tumor embolization was performed via the variant hepatic arteries. After 3 wk, the FRL of the left lateral lobe accounted for nearly 30% of the total liver volume. Totally laparoscopic right trisectionectomy was performed under combined epidural and general anesthesia. The in situ resection was performed via an anterior approach. The operating time was 240 min. No clamping was required during the surgery, and the intraoperative blood loss was 300 mL. There were no postoperative complications such as bile leakage, and the incision healed well. The patient was discharged on the 8th postoperative day. During the 3-mo follow-up, there was no recurrence and obvious hyperplasia of residual liver was observed. Alpha-fetoprotein decreased significantly and tended to be normal.
Due to the different biological characteristics of the liver cancer and the pathophysiological features of the liver from other organs, the conversion treatment should take into account both the feasibility of tumor downstaging and the volume and function of the remnant liver. Our case provides a reference for clinicians in terms of both conversion therapy and laparoscopic right trisectionectomy.
Core Tip: Only 20%-30% of newly-diagnosed hepatocellular carcinoma (HCC) patients are feasible for surgical resection. We report a HCC patient who underwent successful laparoscopic right trisectionectomy after conversion therapy with portal vein embolization and transarterial chemoembolization. There are few reports in the literature. Our case provided reference for clinicians in terms of both conversion therapy and laparoscopic right trisectionectomy.
- Citation: Zhang JJ, Wang ZX, Niu JX, Zhang M, An N, Li PF, Zheng WH. Successful totally laparoscopic right trihepatectomy following conversion therapy for hepatocellular carcinoma: A case report. World J Clin Cases 2021; 9(22): 6469-6477
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6469.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6469
Primary liver cancer is the fourth most frequently diagnosed malignancy and the second most frequent cause of cancer-related deaths in China[1,2]. Primary liver cancer includes three major pathological types: hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and HCC-ICC mixed type, among which HCC accounts for 85%-90%. Surgical resection remains the treatment of choice for HCC patients and the most important strategy for achieving long-term survival. However, as most HCC patients have underlying cirrhosis or are already in the advanced stages when diagnosed, only 20%-30% of newly diagnosed HCC patients are eligible for surgical resection[3]. Compared with other therapies, surgical resection can lead to better quality of life and higher survival rates in liver cancer patients; therefore active conversion therapy to enable subsequent surgical intervention should be a therapeutic goal, and a variety of conversion methods are available.
Surgical resection includes liver transplantation, traditional open partial hepatectomy, and laparoscopic (or robotic) hepatectomy. Liver transplantation is limited by its indications and the availability of donor organs, while partial hepatectomy is the mainstream surgery at present. The past three decades have witnessed advances in laparoscopic liver resection from partial hepatectomy to regular lobectomy and then segmentectomy (resection of segments I, VII, and VIII). Now most liver surgeries can be performed laparoscopically. Compared with conventional open hepatectomy, laparoscopic surgeries can achieve superior short-term outcomes, with equivalent long-term prognosis[4,5]. However, there are few reports on the application of totally laparoscopic anatomical right hepatic trisectionectomy in the treatment of liver cancer, especially for giant HCC.
Here, we report an HCC patient who underwent successful laparoscopic right trisectionectomy after conversion therapy.
A 67-year-old male patient was presented to our hospital with epigastric distention/ discomfort and nausea/vomiting.
The patient’s symptoms started 1 mo prior with epigastric distention/discomfort and nausea/vomiting.
He denied any history of hypertension, diabetes, coronary heart disease, and infectious diseases such as hepatitis or tuberculosis.
He denied any history of personal and family history.
The patient’s temperature was 36.3 ℃, heart rate was 74 beats per min, respiratory rate was 20 breaths per min, blood pressure was 120/75 mmHg. On physical examination, a fixed, painless and relatively hard abdominal mass with a diameter of 8 cm was palpated in the right upper quadrant.
The results of laboratory examinations at admission are shown in Table 1.
| Parameters | Values | Normal limits |
| PT | 10.8 | 9-13 |
| ALB | 40.6 g/L | 35-52 g/L |
| AFP | ≥ 2000 ng/mL | 0-9 ng/mL |
| TBIL | 22.1 µmol/L | 3–20 µmol/L |
| DBIL | 8.6 µmol/L | 0-6.8 µmol/L |
| AST | 32.6 U/L | 15-40 U/L |
| ALT | 27.4 U/L | 9-50 U/L |
| HBsAg | Positive | Negative |
| HBeAb | Positive | Negative |
| HBcAb | Positive | Negative |
| Hepatitis B DNA | 2.27E+03 | < 500 |
| HA | 268.7 ng/mL | 0-120 ng/mL |
| CIV | 187.5 ng/mL | 0-95 ng/mL |
| Child-Pugh score | 5 | 5 |
| ICGR 15 | 4.8% | ≤ 10% |
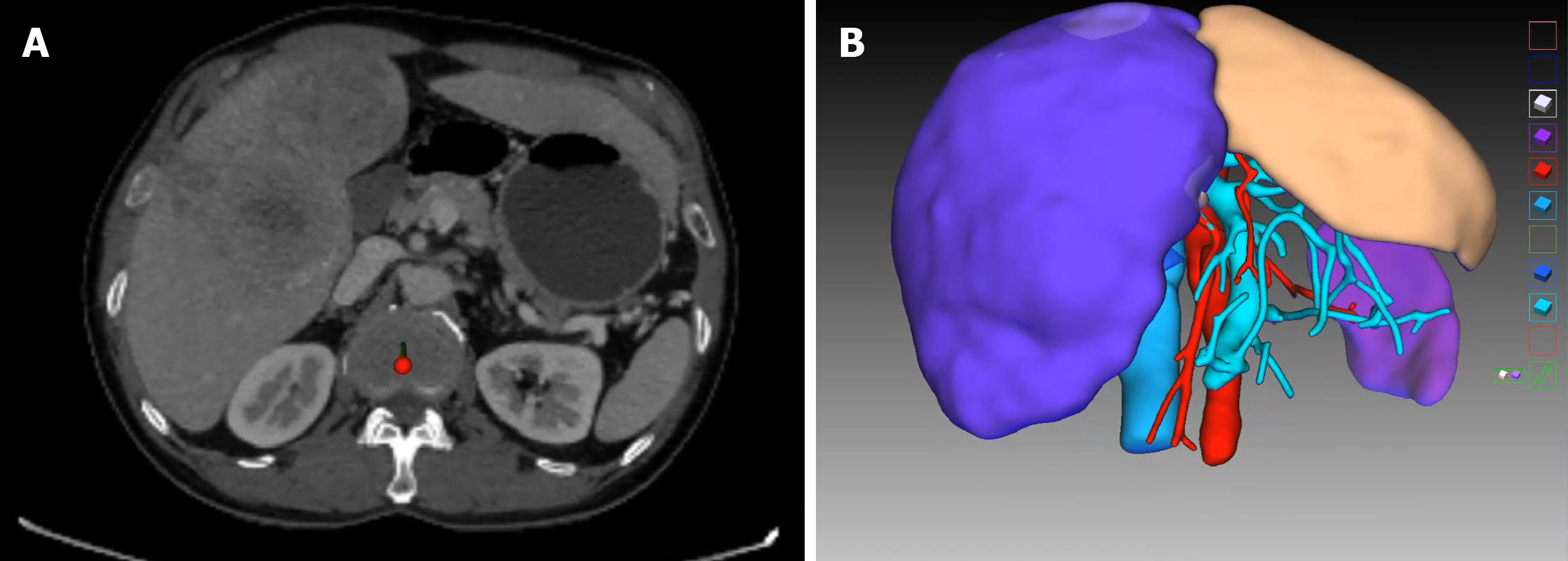
Contrast-enhanced computed tomography scan of the abdomen demonstrated variations of the hepatic artery; the left branch of the hepatic artery arose from the abdominal trunk and the right branch of the hepatic artery originated from the superior mesenteric artery, multiple tumors (the largest was ≥ 10 cm in diameter) were located in the right liver and the left medial lobe, and the left lateral lobe was normal. The future remnant liver (FRL) of the left lateral lobe accounted for only 18% of total liver volume after virtual resection on the three-dimensional (3D) liver model (Figure 1).
Radiotherapy is not recommended as the first choice of treatment for patients with diffuse tumor distribution but can be used as a complementary or adjuvant treatment.
Surgery is the preferred treatment for HCC. If there is no indication for surgery, targeted therapy or systemic chemotherapy can be considered.
Portal vein embolization (PVE) and trans-arterial chemoembolization (TACE) can be considered for follow-up surgery.
Based on experts’ advice, we should take the lead in conversion therapy. If successful, we should carry out laparoscopic right trisectionectomy.
The final diagnosis of the presented case was HCC.
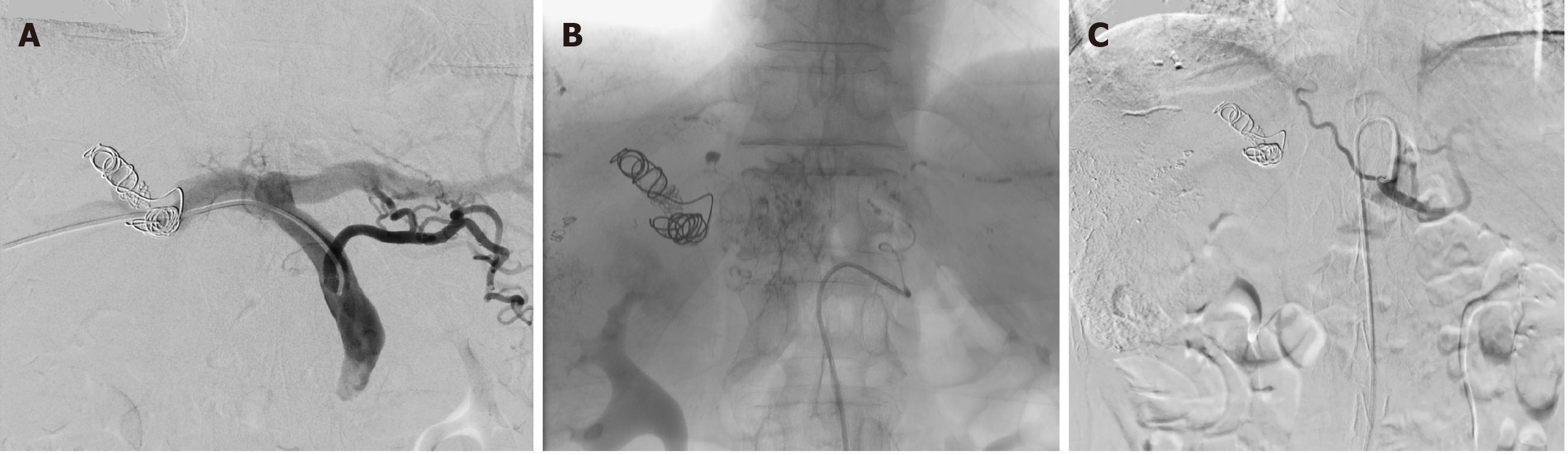
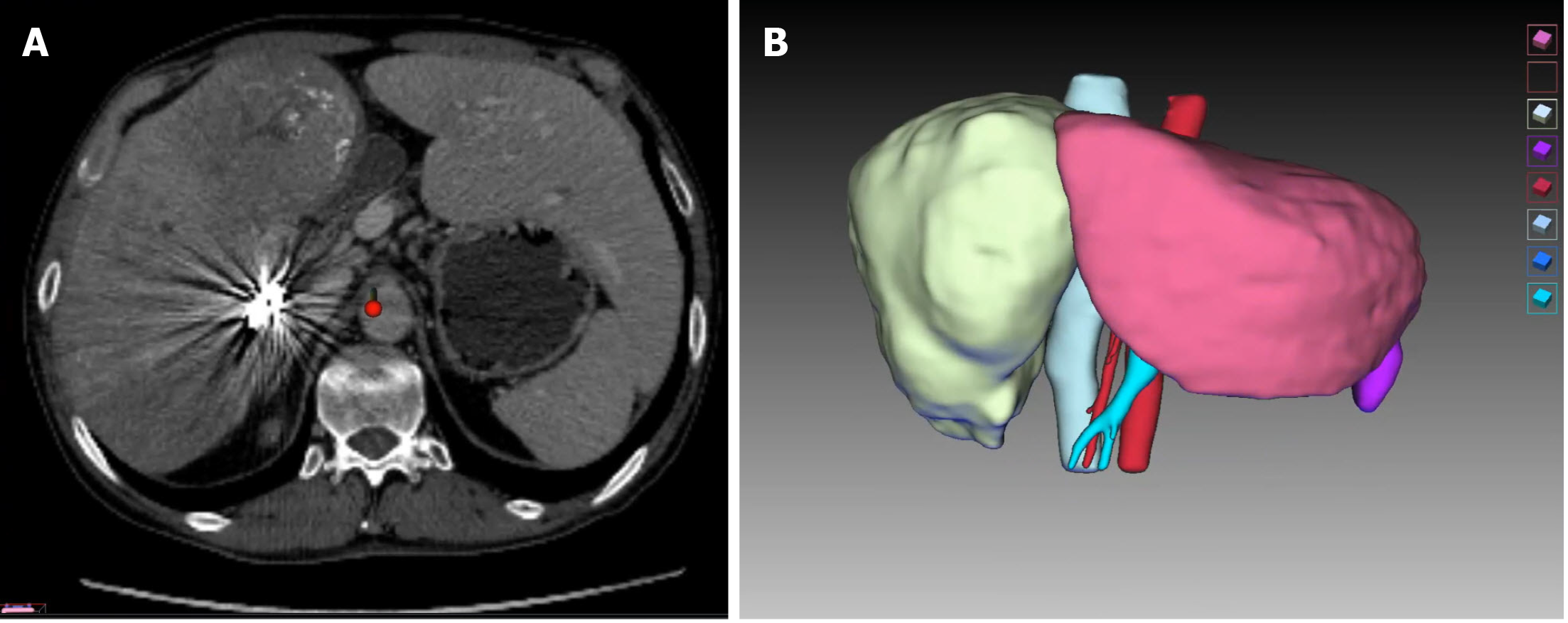
After multidisciplinary evaluation, conversion therapy was adopted after orally administered entecavir for antiviral treatment. The right portal vein was embolized first; after the liver function returned normal 1 wk later, tumor embolization was performed via the variant hepatic arteries (Figure 2). After 3 wk, a second abdominal CECT revealed obvious hyperplasia of the left lateral lobe, and the FRL of the left lateral lobe accounted for nearly 30% of the total liver volume (Figure 3). The results of laboratory examinations before surgery are shown in Table 2. The Child-Pugh score was still A.
| Parameters | Values | Normal limits |
| PT | 10.7 | 9-13 |
| ALB | 41.7 g/L | 35-52 g/L |
| AFP | 3000 ng/mL | 0-9 ng/mL |
| TBIL | 16.8 µmol/L | 3-20 µmol/L |
| DBIL | 8.8 µmol/L | 0-6.8 µmol/L |
| AST | 42.6 U/L | 15-40 U/L |
| ALT | 54.3 U/L | 9-50 U/L |
| Hepatitis B DNA | < 500 | < 500 |
| HA | 149.20 ng/mL | 0-120 ng/mL |
| CIV | 161.49 ng/mL | 0-95 ng/mL |
| Child-Pugh score | 5 | 5 |
| ICGR 15 | 10.1% | 10% |
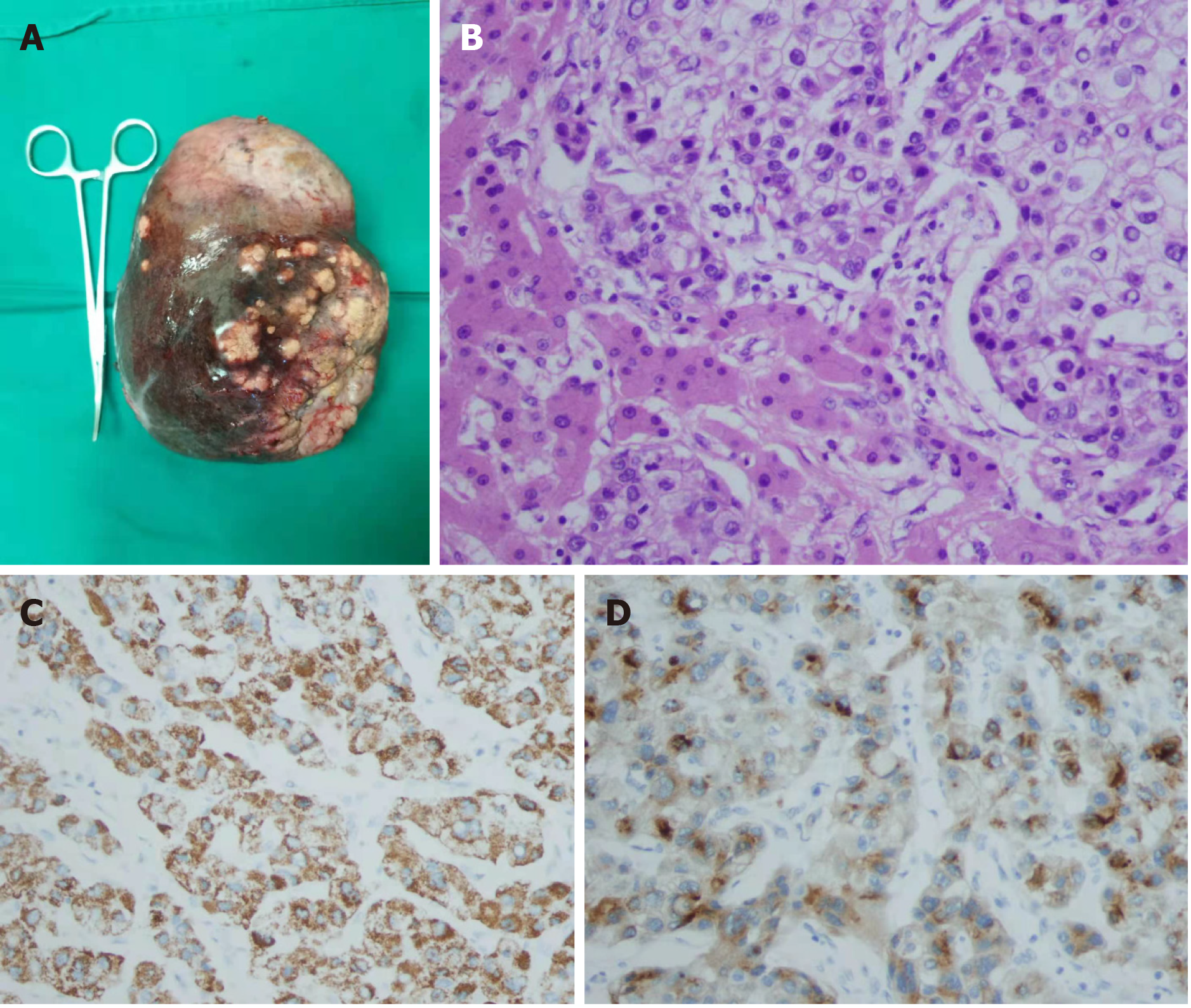
Totally laparoscopic right trisectionectomy was performed under combined epidural and general anesthesia, and the technique was approved by the ethics committee of our hospital. The in situ resection was performed via an anterior approach. The first porta hepatis was first exposed. The clamping band was reserved. The liver parenchyma was dissected along the right side of the sagittal branch of the portal vein, and the middle hepatic vein and the root of the right hepatic vein were exposed in turn. After the right hepatic artery in the first hepatic hilum and the right branch of the portal vein were dissected, the middle hepatic vein and the right hepatic vein were dissected with a cutting stapler under laparoscope. Finally, the third hepatic hilum and the perihepatic ligament were proceeded (Figure 4). The operating time was 240 min. No clamping was required during surgery, and the intraoperative blood loss was 300 mL. The resected specimens were observed with the naked eye and under microscope (Figure 5).
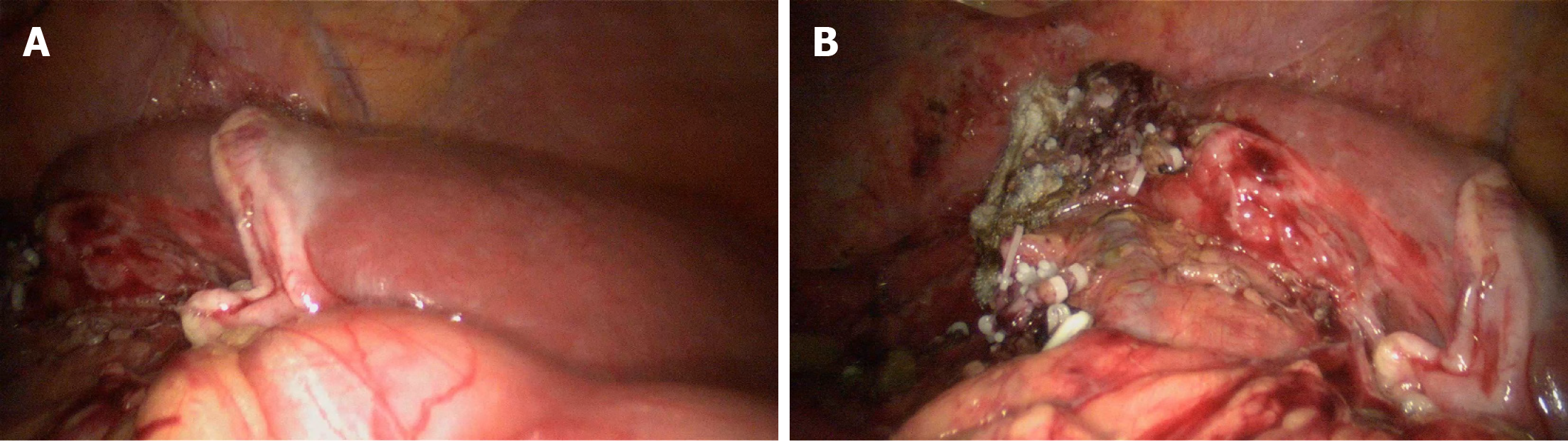
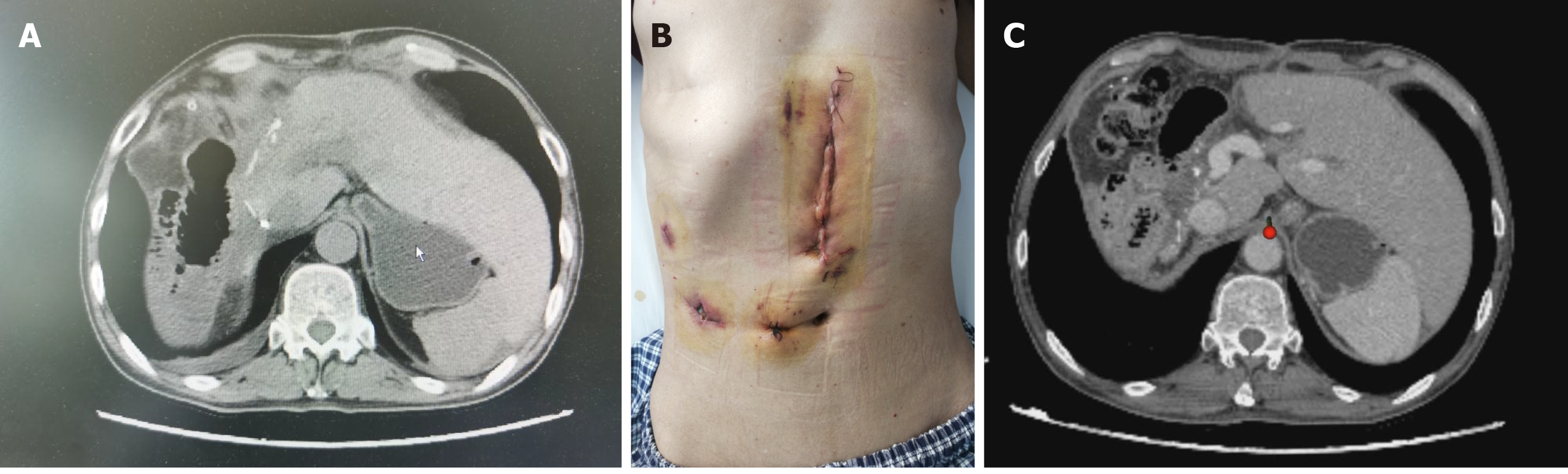
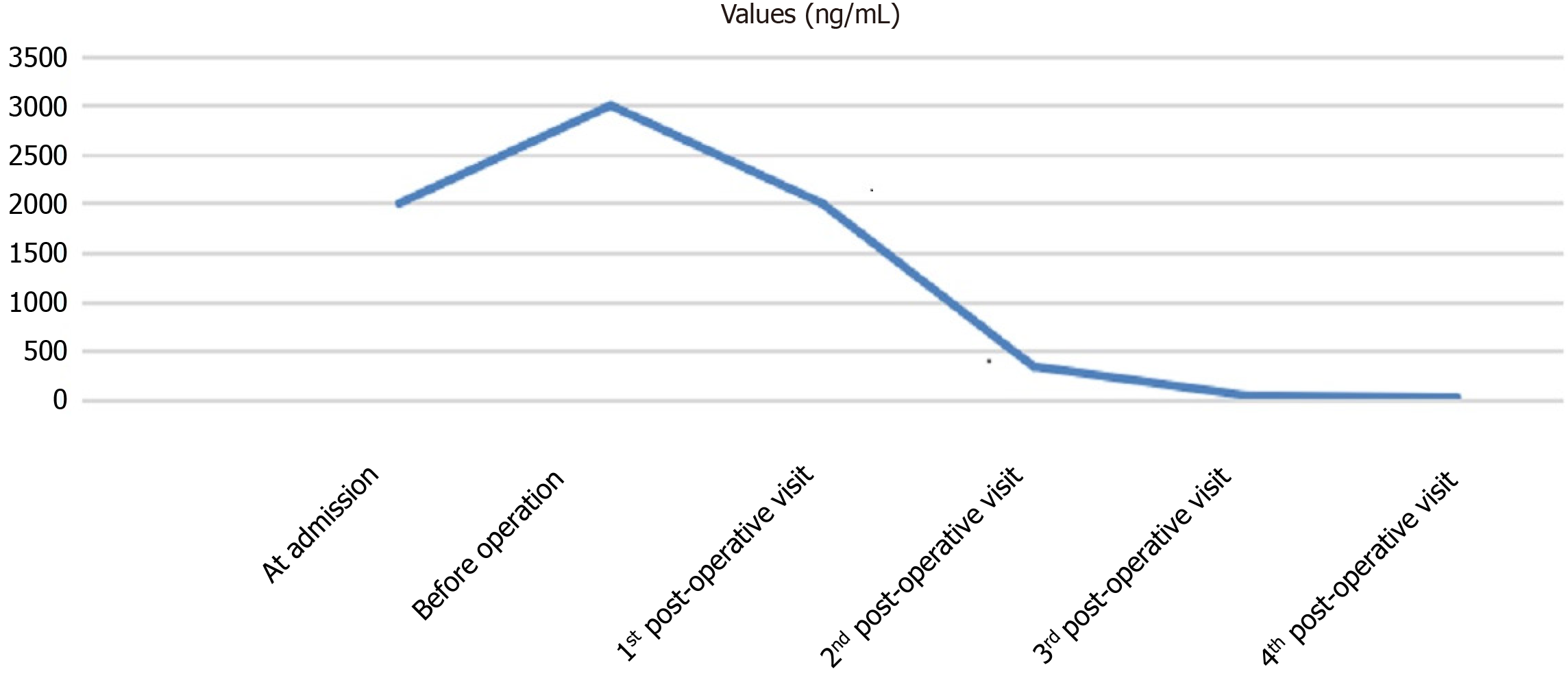
There were no postoperative complications such as bile leakage, and the incision healed well. The patient was discharged on the 8th postoperative day (Figure 6A and B). TACE was applied prophylactically once after surgery. During the 3-mo follow-up, there was no recurrence and obvious hyperplasia of residual liver was observed (Figure 6C). Alpha-fetoprotein (AFP) decreased significantly and tended to be normal (Figure 7).
For patients with Barcelona Clinic Liver Cancer stage B or China Liver Cancer Staging stage Ⅱb liver cancer[3], surgical resection is not superior to non-surgical treatments (e.g., TACE). However, for tumors confined to the same segment or the ipsilateral half of the liver, surgical resection may achieve better outcomes than other treatment modalities[6,7]. In the present case, as the tumor was not only located in the right lobe but also in the left medial lobe (Figure 5A), an anatomical right trisectionectomy was required. Since there was an inadequate volume of FRL, successful conversion therapy was the only way to enable the subsequent surgery. Due to the different biological characteristics of the liver cancer and the pathophysiological features of the liver from other organs, the conversion treatment should take into account both the feasibility of tumor down staging and the volume and function of the remnant liver.
Tumor downstaging can be achieved using a variety of techniques, which can be applied in a combined or sequential manner. Among them, TACE is the most commonly used treatment and is considered to have the highest success rate[8]. In addition, TACE also allows the early detection of undetectable lesions by other imaging modalities. In the present case, if TACE revealed the presence of a lesion in the remaining left lateral lobe, surgery would have a limited value. Solutions for the inadequate volume of FRL include the associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), a two-stage hepatectomy, and PVE. ALPPS can induce rapid hypertrophy of FRL and provide a high resection rate; however, it is associated with high complication and mortality rates and should be performed with caution. After multidisciplinary consultations, PVE combined with TACE was applied in our case, with the aim of achieving radical resection. Compared with PVE alone, PVE plus TACE accelerates the regeneration of FRL tissue while avoiding or delaying the growth of tumors in the left medial lobe. The interval from PVE to surgery was nearly 4 wk, during which the ratio of FRL to standard liver volume increased from 18% to 30%. Despite the successful conversion, tumors progressed during the waiting period; AFP increased from ≥ 2000 ng/mL at admission to ≥ 3000 ng/mL before surgery, which might be related to the varying tumor diameters and the extensive distribution of the tumors in liver tissue (Figure 5A). TACE combined with systematic drug administration may help to address this problem.
For patients with giant HCC (> 10 cm in diameter) requiring anatomical right trisectionectomy, surgical resection by laparoscopic approach alone is rarely reported in the literature. Safety is the first concern during a laparoscopic procedure, followed by the “tumor-free” principle[4,5]. In 2014, Ban et al[9] developed a difficulty scoring system (DSS-BAN) based on 90 patients undergoing laparoscopic surgery for HCC at three medical centers according to the extent of liver resection, tumor location, tumor size, preoperative liver function, and tumor proximity to major vessels. In DSS-BAN, performing laparoscopic liver resection with a difficulty score of 10 requires experience with more than 50 cases of laparoscopic liver resection. Our team had experience with more than 100 cases, which ensured the successful operation in our current case[10]. Another technical challenge during the operation is to ensure sufficient tumor margin to prevent tumor rupture. The preoperative 3D reconstruction, precise evaluation and intraoperative ultrasound if necessary can help determine the resection margin. If the resection margin is close to important vessels/ducts, removal of the tumor exactly along the resection margin is not required, but a pathologically negative margin should be ensured. Laparoscopic in situ liver resection was performed via an anterior approach to avoid compression of tumors. During the operation, the liver parenchyma between the left lateral lobe and left medial lobe was divided firstly, followed by the division of right hepatic pedicle, middle hepatic vein, right hepatic vein, and vessels at the third porta of liver. Finally, the perihepatic ligament was transected to remove the specimen. These steps were expected to avoid the iatrogenic hematogenous spread of cancer cells. Postoperative morphological examination showed that the resected specimen was intact and unbroken, and the pathological examination showed that the resection margin was negative for tumor cells. The surgical procedure adhered to the tumor-free principle, and the results were equivalent to traditional open surgery.
Our case provided reference for clinicians in terms of both conversion therapy and laparoscopic right trisectionectomy. No recurrence has been noted, but the follow-up period is still short. The patient has been treated with prophylactic TACE once after surgery. There is no evidence on whether additional TACE sessions or other techniques are required to prevent recurrence. In addition, there are also controversies on the treatment options for potential recurrence. In conclusion, there are still many concerns about this case, and follow-up will be continued.
In this case, as the tumor was not only located in the right lobe but also in the left medial lobe (Figure 5A), an anatomical right trisectionectomy was required. Since there was an inadequate volume of FRL, successful conversion therapy was the only way to enable the subsequent surgery. Due to the different biological characteristics of the liver cancer and the pathophysiological features of the liver from other organs, the conversion treatment should take into account both the feasibility of tumor downstaging and the volume and function of the remnant liver. Our case provided reference for clinicians in terms of both conversion therapy and laparoscopic right trisectionectomy.
First of all, I would like to thank the patient in this case for his support and approval of this article. Second, I would like to express my gratitude to the authors of this article and thank them for their help and dedication from the end. Without them, I would not be able to complete this work. I also want to thank the government for providing us with a strong backing. Finally, I would like to thank all the staff in the editorial department, and thank you for all your valuable comments.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Martínez-Pérez A S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LL
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65: 87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21362] [Article Influence: 2136.2] [Reference Citation Analysis (3)] |
| 2. | Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, Mullany EC, Redford SB, Liu H, Naghavi M, Hay SI, Murray CJL, Liang X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2384] [Article Influence: 397.3] [Reference Citation Analysis (1)] |
| 3. | Department of Medical Administration, National Health and Health Commission of the People's Republic of China. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 4. | Cheung TT, Han HS, She WH, Chen KH, Chow PKH, Yoong BK, Lee KF, Kubo S, Tang CN, Wakabayashi G. The Asia Pacific Consensus Statement on Laparoscopic Liver Resection for Hepatocellular Carcinoma: A Report from the 7th Asia-Pacific Primary Liver Cancer Expert Meeting Held in Hong Kong. Liver Cancer. 2018;7:28-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, Aroori S, Belli G, Besselink M, Briceno J, Gayet B, D'Hondt M, Lesurtel M, Menon K, Lodge P, Rotellar F, Santoyo J, Scatton O, Soubrane O, Sutcliffe R, Van Dam R, White S, Halls MC, Cipriani F, Van der Poel M, Ciria R, Barkhatov L, Gomez-Luque Y, Ocana-Garcia S, Cook A, Buell J, Clavien PA, Dervenis C, Fusai G, Geller D, Lang H, Primrose J, Taylor M, Van Gulik T, Wakabayashi G, Asbun H, Cherqui D. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 494] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 6. | Metussin A, Patanwala I, Cross TJ. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. J Hepatol. 2015;62:747-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Fuster J. A Snapshot of the Effective Indications and Results of Surgery for Hepatocellular Carcinoma in Tertiary Referral Centers: Is It Adherent to the EASL/AASLD Recommendations? Ann Surg. 2015;262:e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 518] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 9. | Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, Kaneko H, Wakabayashi G. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 10. | Liu YB, Meng XK, Ren JJ, Li J, Qiao JL, Zhang JJ. Comparison of safety and short-term efficacy between laparoscopic hepatectomy and open hepatectomy. Zhonghua Ganzang Waike Shoushuxue Dianzi Zazhi. 2020;9:547-551. [DOI] [Full Text] |