Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6457
Peer-review started: March 29, 2021
First decision: April 28, 2021
Revised: May 6, 2021
Accepted: May 26, 2021
Article in press: May 26, 2021
Published online: August 6, 2021
Processing time: 120 Days and 15.6 Hours
Malignant peripheral nerve sheath tumor (MPNST) is a type of spindle cell sarcoma originating from the peripheral nerve, which usually results in the corresponding nerve sign on magnetic resonance imaging (MRI). Patients with MPNST may also have neurofibromatosis type 1.
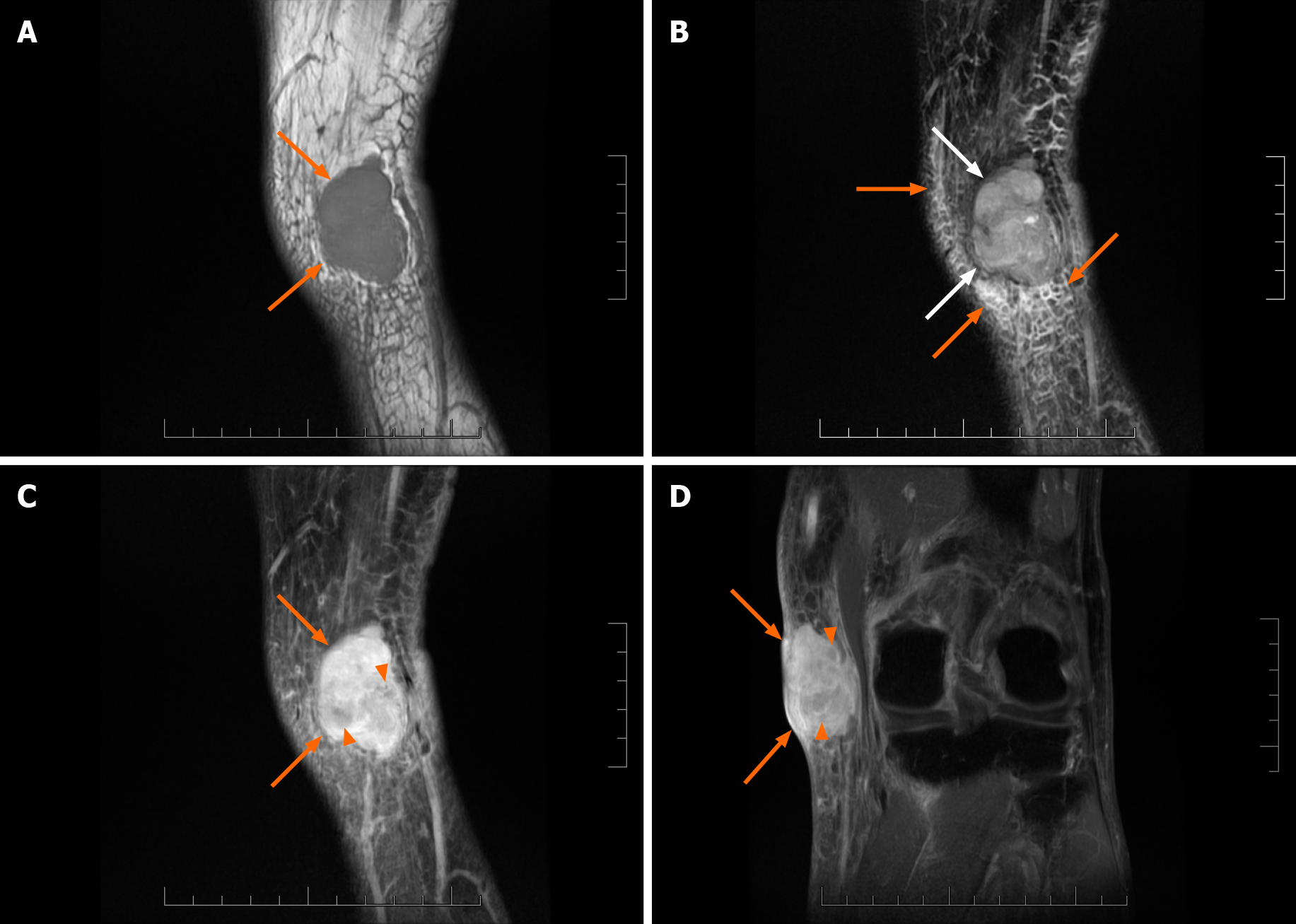
A 78-year-old male was admitted to the hospital due to a tumor in his left knee. He had a previous history of superficial spreading melanoma on the left thigh. Color Doppler ultrasonography showed a hypoechoic mass in the subcutaneous soft tissues of the medial left knee with an abundant rich blood flow. Computed tomography scanning did not show obvious signs of bone destruction, but the skin adjacent to the tumor was slightly thickened. MRI examination revealed that the hypervascular lesion was well-circumscribed, lobulated, invaded the sur
MRI is a useful technique for revealing the biological characteristics of MPNST and provides clinical support for evaluation of the surgical area before operation.
Core Tip: Malignant peripheral nerve sheath tumor is a rare peripheral nerve sarcoma originating from the peripheral nerve. We report the first patient with a malignant peripheral nerve sheath tumor and a clinical history of superficial spreading melanoma. Various imaging examinations showed no signs of nerve origin, but magnetic reso
- Citation: Yang CM, Li JM, Wang R, Lu LG. Malignant peripheral nerve sheath tumor in an elderly patient with superficial spreading melanoma: A case report. World J Clin Cases 2021; 9(22): 6457-6463
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6457.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6457
Malignant peripheral nerve sheath tumor (MPNST) is a type of spindle cell sarcoma originating from the peripheral nerve that is secondary to neurofibromatosis, or heterogeneous differentiation components of the nerve sheath are observed under the microscope. Hence, MPNST was previously called neurogenic sarcoma and neurofi
A 78-year-old male patient was admitted to our hospital due to a soft mass with a rapid growth over 10 d.
In 2020, he accidentally noticed a soft mass the size of a pigeon egg on the inner side of his left knee joint with good mobility and no local redness, swelling or pain. He visited the local clinic, and Color Doppler ultrasound examination suggested that the tumor was a pilomatricoma. No further treatment was performed at that time. However, when the tumor increased over 10 d, the patient felt occasional numbness and discomfort in the left lower extremity.
The patient was previously diagnosed with a superficial spreading melanoma on his left thigh, which was excised in our hospital in 2015. The postoperative positron emission tomography-computed technology examination showed no evidence of regional lymph node metastasis and distant metastasis, so the subsequent radiothe
The patient had no relevant family medical history.
A painless, tough tumor 5 cm × 5 cm × 4 cm in diameter within his left knee joint with a clear boundary, rough surface and hot flushed skin was noted. There were no obvious abnormalities in sensation, blood supply and movement of the left lower limb.
Before operation, the patient’s erythrocyte sedimentation rate increased (30 mm/h, normal range: 0-15 mm/h) and C-reactive protein was slightly high (10.3 mg/L, normal range: 0-10 mg/L). Other laboratory examination results were normal, including hematological, coagulation, kidney and liver functions as well as electro
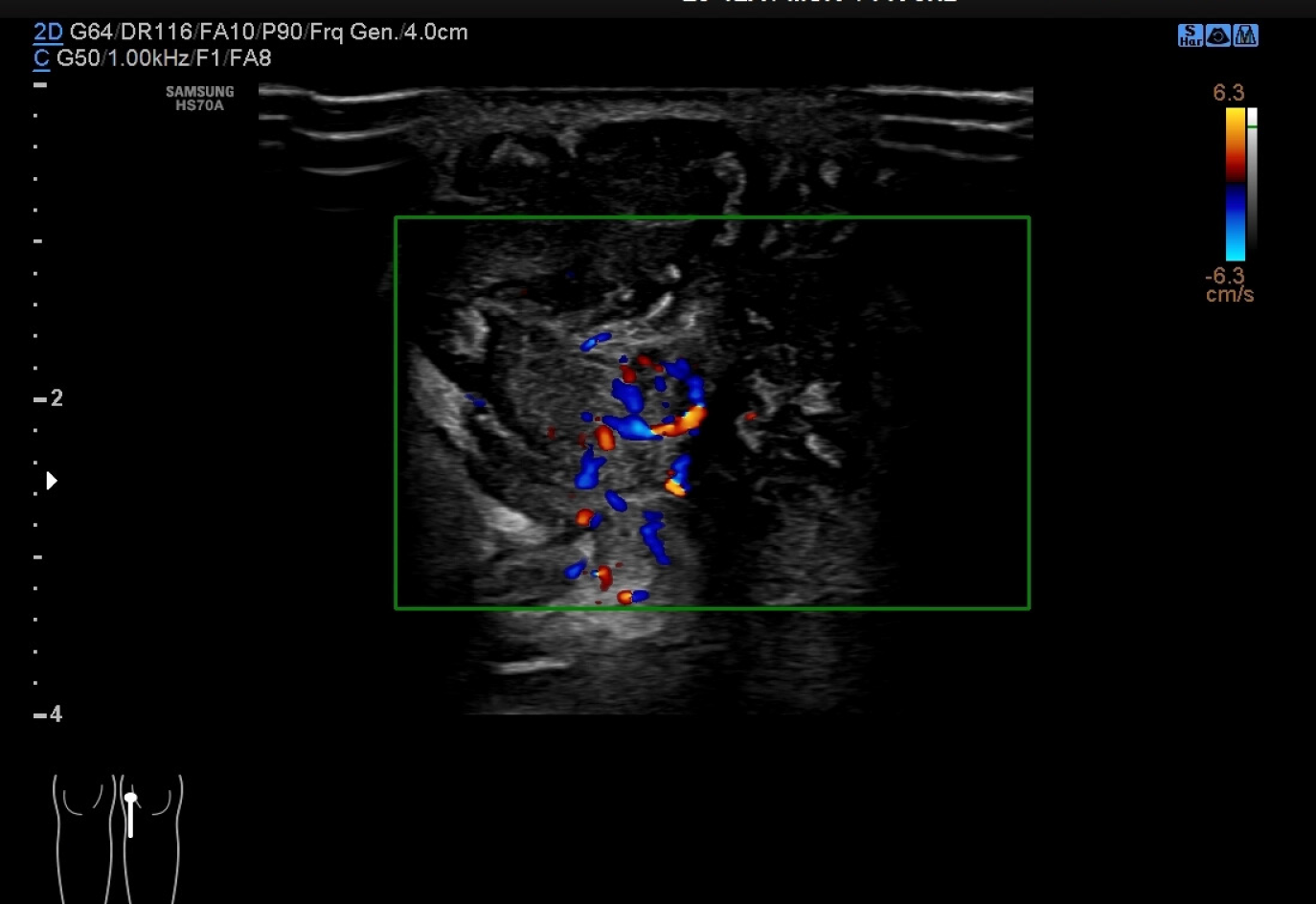
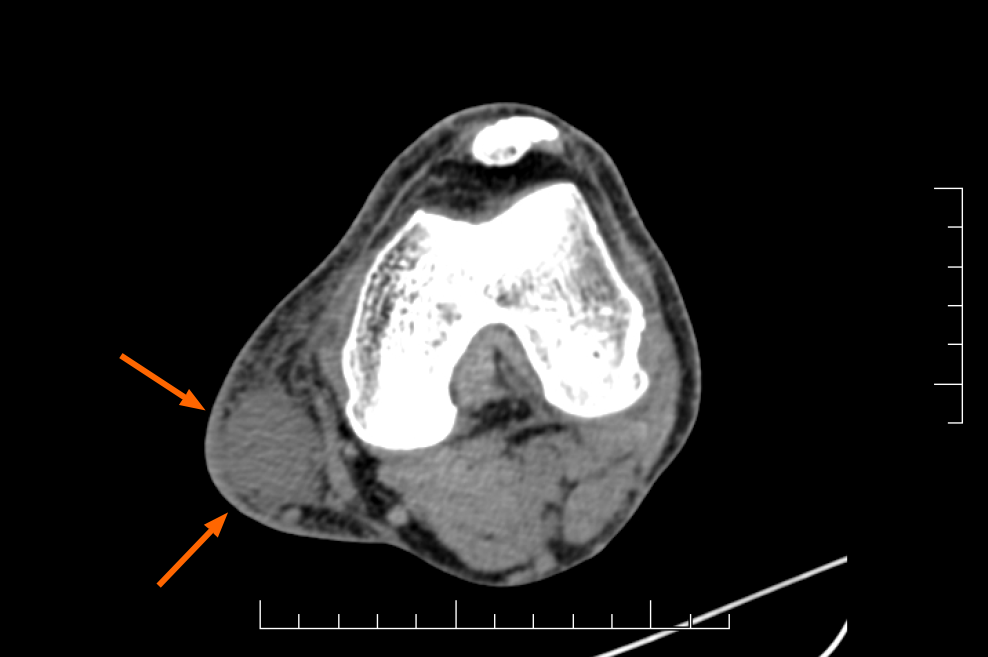
Color Doppler ultrasonography (Figure 1) revealed a hypoechoic mass with an unclear boundary in the subcutaneous soft tissues of the medial left knee with abundant dotted and band-shaped blood flow signals in and around the lesion. Computed tomography scanning (Figure 2) showed a subcutaneous lesion in the left medial femoral area with an average computed tomography value of 34 HU, which was significantly lower than the adjacent soft tissue (62 HU). The skin adjacent to the tumor was slightly thickened, and no obvious signs of bone destruction were ob
Clinical features and all imaging results, especially MRI result, suggested the possi
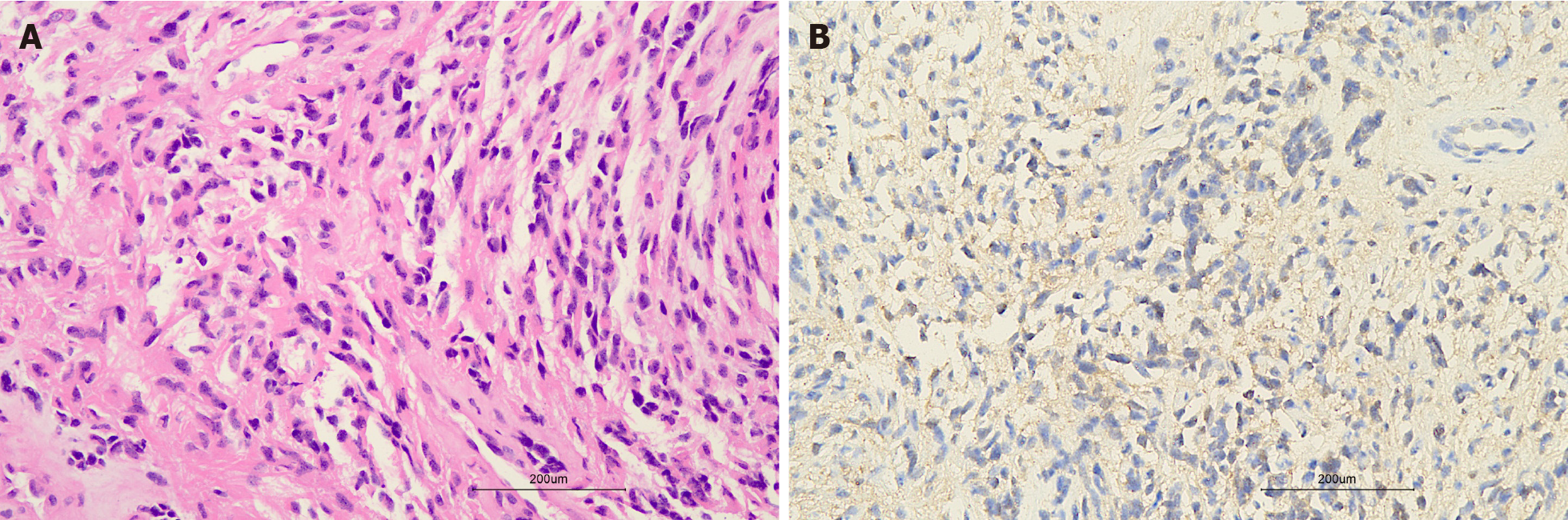
After careful evaluation of the patient’s symptoms, previous medical history and the MRI finding of peripheral tissue edema, the surgeon decided to perform an extended resection of the left knee joint mass. The first frozen section report showed a spindle cell tumor, which was approximately 10.5 cm × 5.0 cm ×2.7 cm in size with positive surgical margins. Hence, the scope of surgical resection was expanded again to the 2 cm of the remaining surrounding tissues, and then the second frozen section report showed negative surgical margins. The postoperative pathological report (Figure 4) suggested MPNST, which was positive for vimentin S100, CD99 (partial positivity) and Ki-67 (approximately 40% positivity in the hot spot area) and negative for HMB 45 and Melan-A.
The patient was in a stable condition without any related complications after ope
MPNST is an uncommon sarcoma and usually demonstrates aggressive biological behavior. In general, appropriate surgery, radiation and chemotherapy are the conventional clinical treatments. However, large tumor size (especially > 5 cm), positive excision margins, high-level tumor grade, association with NF-1, local recurrence and metastases are predictive prognostic markers of inferior consequences of this disease[4]. A small minority of limb MPNSTs are found in the absence of NF-1. In Jordan, a recurrent MPNST occurred at the same site on the forearm of a 51-year-old man who had no preexisting NF-1 but had a positive family history of cancer[5]. Similarly, an elderly man, without a previous diagnosis of NF-1 or positive family history, presented with a giant MPNST in the left axilla and minute lung metastasis before surgery[6]. Both patients died several months after surgery due to the invasive behavior and poor prognosis of MPNST.
Compared with these two cases, tumor size in our patient was smaller, and he received early surgical intervention before the occurrence of symptoms or metastasis. His positive history of melanoma may have been related to the occurrence of MPNST, which is a good reminder for clinicians. It is noteworthy that the MRI result revealed the invasiveness of the tumor and provided clinical evidence for an extended resec
In terms of radiological features, it is generally accepted that all peripheral nerve sheath tumors originate from a single nerve branch[9-11], which leads to corresponding imaging signs. However, in this case, the lesion lacked an entering and exiting nerve sign or a thickened nerve nearby on any of the radiological images. In compliance with our findings, Van Herendael et al[11] suggested that the presence of a nerve sign was less frequent in malignant neurogenic tumors on MR images. There are also other MRI characteristics of MPNSTs, for example large size (> 5 cm), ill-delineated edges, perilesional edema and internal cystic degeneration/necrosis. Combined with the patient’s history, our finding of an abundant blood supply in the lesion was indicative of a malignant tumor. Moreover, the presence of unenhanced focal areas on sagittal contrast-enhanced T1WI in this case was probably caused by intralesional necrosis and hemorrhage[10,11]. Due to the rarity and unspecific imaging manifestations of MPNST, clinical manifestations combined with postoperative pathological results are important for diagnosis of the disease. Specific pathological features, including tightly packed spindle cells with neoplasm necrosis, invasive growth into surrounding tissues and alternating loose and dense cellular areas as well as S100 protein and Ki-67 positive staining[12] were found in our case.
This report describes a patient with a solitary MPNST and a history of melanoma in the lower extremity, without radiological evidence of genetic origin. Clinicians should be aware of the possibility of MPNST in patients with a history of other epithelioid tumors. MRI results could reveal the biological characteristics of the MPNST. Prompt intervention such as extended excision plus postoperative radiotherapy could be beneficial to the outcome of this rare tumor.
We thank our colleagues at the Department of General Surgery and Department of Pathology, Zhuhai Hospital Affiliated with Jinan University, Zhuhai People’s Hospital.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Naswhan AJ S-Editor: Wang JL L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Stucky CC, Johnson KN, Gray RJ, Pockaj BA, Ocal IT, Rose PS, Wasif N. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012;19:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Watson KL, Al Sannaa GA, Kivlin CM, Ingram DR, Landers SM, Roland CL, Cormier JN, Hunt KK, Feig BW, Ashleigh Guadagnolo B, Bishop AJ, Wang WL, Slopis JM, McCutcheon IE, Lazar AJ, Torres KE. Patterns of recurrence and survival in sporadic, neurofibromatosis Type 1-associated, and radiation-associated malignant peripheral nerve sheath tumors. J Neurosurg. 2017;126:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Farid M, Demicco EG, Garcia R, Ahn L, Merola PR, Cioffi A, Maki RG. Malignant peripheral nerve sheath tumors. Oncologist. 2014;19:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 5. | Abdel Al S, Abou Chaar MK, Asha W, Al-Najjar H, Al-Hussaini M. Fungating malignant peripheral nerve sheath tumor arising from a slow-growing mass in the forearm: a case report and review of the literature. J Med Case Rep. 2020;14:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Kusumoto E, Yamaguchi S, Sugiyama M, Ota M, Tsutsumi N, Kimura Y, Sakaguchi Y, Kusumoto T, Ikejiri K, Nakayama Y, Momosaki S. Huge epithelioid malignant peripheral nerve sheath tumor in the left axilla: a case report. Surg Case Rep. 2015;1:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kahn J, Gillespie A, Tsokos M, Ondos J, Dombi E, Camphausen K, Widemann BC, Kaushal A. Radiation therapy in management of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Front Oncol. 2014;4:324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Bernthal NM, Putnam A, Jones KB, Viskochil D, Randall RL. The effect of surgical margins on outcomes for low grade MPNSTs and atypical neurofibroma. J Surg Oncol. 2014;110:813-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Murphey MD, Smith WS, Smith SE, Kransdorf MJ, Temple HT. From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1253-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 359] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Zhai H, Lv Y, Kong X, Liu X, Liu D. Magnetic resonance neurography appearance and diagnostic evaluation of peripheral nerve sheath tumors. Sci Rep. 2019;9:6939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Van Herendael BH, Heyman SR, Vanhoenacker FM, De Temmerman G, Bloem JL, Parizel PM, De Schepper AM. The value of magnetic resonance imaging in the differentiation between malignant peripheral nerve-sheath tumors and non-neurogenic malignant soft-tissue tumors. Skeletal Radiol. 2006;35:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Koeller KK, Shih RY. Intradural Extramedullary Spinal Neoplasms: Radiologic-Pathologic Correlation. Radiographics. 2019;39:468-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |