Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6254
Peer-review started: April 2, 2021
First decision: April 28, 2021
Revised: May 7, 2021
Accepted: June 3, 2021
Article in press: June 3, 2021
Published online: August 6, 2021
Processing time: 116 Days and 11.5 Hours
Endoscopic retrograde pancreatic drainage (ERPD) and stent implantation has become the major treatment method for pancreatic pseudocysts. However, it is associated with a high recurrence rate and infection.
To manage pancreatic pseudocysts by sequential therapy with endoscopic naso-pancreatic drainage (ENPD) combined with ERPD and evaluate the treatment outcome.
One hundred and sixty-two cases of pancreatic pseudocyst confirmed by endoscopic examination at our hospital between January 2014 and January 2020 were retrospectively analyzed. There were 152 cases of intubation via the duodenal papilla, of which 92 involved pancreatic duct stent implantation and 60 involved sequential therapy with combined ENPD and ERPD (two-step procedure). The success rate of the procedure, incidence of complications (infection, bleeding, etc.), recurrence, and length and cost of hospitalization were compared between the two groups.
The incidence of infection was significantly higher in the ERPD group (12 cases) than in the two-step procedure group (2 cases). Twelve patients developed infection in the ERPD group, and anti-infection therapy was effective in five cases but not in the remaining seven cases. Infection presented as fever and chills in the two-step procedure group. The reoperation rate was significantly higher in the ERPD group with seven cases compared with zero cases in the two-step procedure group (P < 0.05). Similarly, the recurrence rate was significantly higher in the ERPD group (19 cases) than in the two-step procedure group (0 cases).
Sequential therapy with combined ENPD and ERPD is safe and effective in patients with pancreatic pseudocysts.
Core Tip: In recent years, with the development of endoscopic technology, endoscopic pancreatic pseudocyst drainage has become the major treatment method for pancreatic pseudocyst. We reported 60 cases of pancreatic pseudocyst treated by sequential therapy with endoscopic naso-pancreatic drainage combined with endoscopic retrograde pancreatic drainage, and evaluated the treatment effect. The two-step method can significantly reduce the incidence and recurrence rate of postoperative infection.
- Citation: He YG, Li J, Peng XH, Wu J, Xie MX, Tang YC, Zheng L, Huang XB. Sequential therapy with combined trans-papillary endoscopic naso-pancreatic and endoscopic retrograde pancreatic drainage for pancreatic pseudocysts. World J Clin Cases 2021; 9(22): 6254-6267
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6254.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6254
Pancreatic pseudocyst is often secondary to acute or chronic pancreatitis, pancreatic injury, and pancreatic duct obstruction[1-5]. It is reported that 10%-20% of acute pancreatitis cases are complicated with pancreatic pseudocyst and 20%-40% of chronic pancreatitis cases are complicated with pancreatic pseudocyst[2,4,6-9]. Failure of conservative treatment of pancreatic pseudocyst leads to symptoms such as abdominal pain, abdominal distension, fever and chills, and digestive tract obstruction, and additional treatments, including puncture drainage, surgery, and endoscopic treatment (trans-gastric and trans-duodenal drainage), are required[1-3,9,10]. However, no single treatment is completely effective. Open surgery or laparoscopic pancreatic pseudocyst-stomach anastomosis and pancreatic cysto-jejunostomy are associated with extensive tissue injury and a long recovery period. Moreover, the postoperative recurrence and complication rates are 2.5%-5% and 30%, respectively[2]. No significant differences in the success rate and complication rate between open surgery and laparoscopic pancreatic pseudocyst-stomach anastomosis have been observed[11,12]. Percutaneous pancreatic pseudocyst drainage is typically performed under the guidance of ultrasound and computed tomography. Percutaneous drainage is performed when patients are not eligible for surgery and when the pancreatic pseudocyst is not connected to the main pancreatic duct and not close to the gastrointestinal tract. However, these situations are rare[3,12]. Recovery of the fistula is hampered when the pancreatic pseudocyst and the pancreatic duct form a pancreas–skin fistula after percutaneous drainage. The incidence of pancreas–skin fistula, the recurrence rate of pancreatic pseudocyst, and the long duration of a guide pipe result in a high infection rate[13,14]. With endoscopic ultrasound-guided drainage, there is a possibility of persistent non-healing, bleeding, perforation, stent displacement, or stent blockage. Moreover, there is an increased risk of retrograde infection of the gastrointestinal tract due to foreign matter and recurrence after stent removal[15-17]. After endoscopic trans-papillary pancreatic duct stent drainage, pancreatic juice enters the duodenum, which meets the physiologic requirements. The digestive tract symptoms are mild after the procedure. However, there is a risk of infection following blockage of the pancreatic duct stent[18].
In recent years, with the development of endoscopic technology, endoscopic pancreatic pseudocyst drainage has become the major method for treating pancreatic pseudocyst[2,3,9,19]. In most previously reported studies, the pancreatic duct stent was placed under an endoscope, and endoscopic ultrasound trans-gastric drainage was chosen for the management of pancreatic pseudocyst. A naso-pancreatic duct is not routinely implanted. It is reported that the tube can be dredged if the naso-pancreatic duct is blocked during naso-pancreatic drainage, which may reduce complications, such as infections, when the tube is obstructed[18,20,21]. However, prolonged naso-pancreatic drainage is inconvenient in patients as they may remove the naso-pancreatic duct, resulting in failure of drainage[18,21]. Therefore, there is a need to overcome the limitations of the currently used methods for managing pancreatic pseudocyst.
Herein, we hypothesized that a two-step trans-papillary procedure involving endoscopic naso-pancreatic drainage (ENPD) and endoscopic retrograde pancreatic drainage (ERPD) sequential therapy for pancreatic pseudocyst may reduce the infection-related complications seen with single stent implantation, address concerns related to tube blockage, and reduce patient discomfort due to long-term single naso-pancreatic duct implantation. The aim of this study was to evaluate the effectiveness and safety of the two-step procedure for pancreatic pseudocysts.
Data were retrospectively obtained from patients with pancreatic pseudocyst who were admitted to the Department of Hepatobiliary Surgery of the Second Affiliated Hospital of Army Medical University between January 2014 and January 2020. This experimental study strictly followed the Declaration of Helsinki and the International Theoretical Guidelines for Biomedical Research Involved with Humans and was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Army Medical University. All patients provided informed consent. After successful surgery, the patients received anti-infection, nutrition, supportive, and enzyme suppression treatments.
The inclusion criteria were: Age > 16 years; pancreatic pseudocyst confirmed by imaging examinations [B-mode ultrasound, computed tomography (CT), or magnetic resonance cholangiopancreatography (MRCP)]; history of pancreatitis and the formation of a pancreatic pseudocyst (time of cyst formation > 6 wk), with a cyst diameter > 6 cm[1,15]; and significant symptoms: Repeated abdominal pain, abdominal distension, fever, and possible pancreatic pseudocyst compression against surrounding tissues and organs causing jaundice, and digestive symptoms.
The exclusion criteria were: Pancreatic tumor (confirmed by imaging examinations); acute stage of pancreatitis, acute suppurative cholangitis, and other acute inflammation or combination with cyst infection.
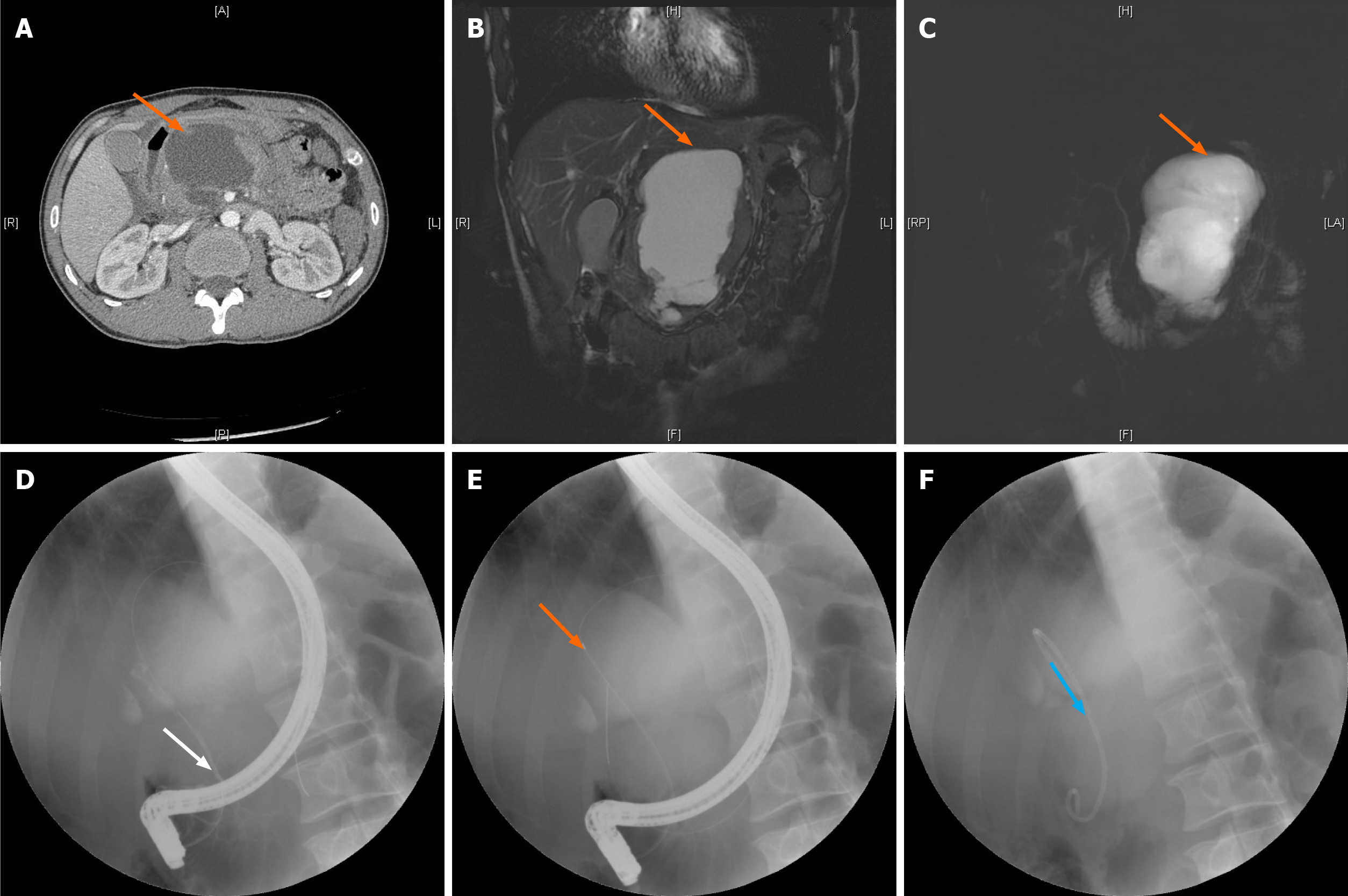
The patient was placed in the left prone position to monitor vital signs. After duodenal papilla intubation, with the help of a guide wire, radiography was performed (a small amount of contrast agent was injected to identify the condition of the bile and pancreatic ducts and the relationship between the pancreatic pseudocyst and pancreatic duct). Endoscopic sphincterotomy (EST) and endoscopic pancreatic sphincterotomy (EPS) were adopted to implant the pancreatic duct stent through the guide pipe into the pancreatic duct under the guidance of the guide wire (Figure 1). The following precautions were taken: The system stent was used if it is long enough; a self-made stent was used if the system stent was not used; the length of the pancreatic duct stent implanted was determined by the measurement between the intersection of the cyst wall and the distal pancreatic duct (pancreatic duct rupture point) and papillary orifice, which is the effective length of the self-made stent (not including the pig tail). The stent was placed more than 2 cm inside the cyst to avoid the stent falling out and entering the pancreatic duct later and thus reducing the drainage effect. When the stent was released, the pig tail was located in the intestine on the side of the duodenal papilla to prevent the stent from moving to the pancreatic duct.
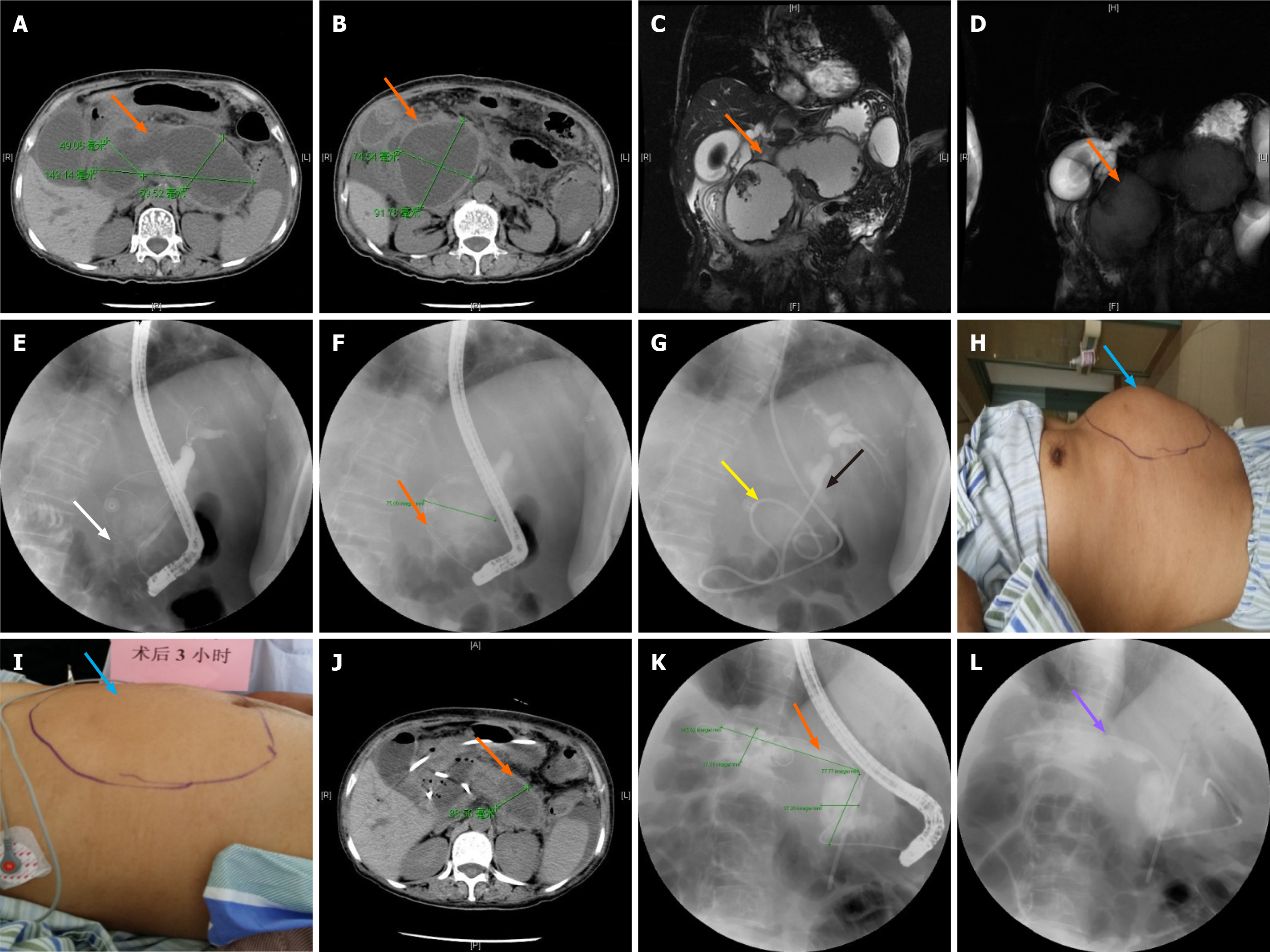
The patient was placed in the left prone position to inspect the duodenum and the duodenum papilla. After guide wire insertion, radiography was performed (a small amount of contrast agent was injected to identify the condition of the bile and pancreatic ducts and the relationship between the pancreatic pseudocyst and pancreatic duct. Bile duct disease was treated if it was detected during the operation). EST and EPS were adopted to implant the naso-pancreatic duct (naso-biliary drainage was used as the naso-pancreatic duct). Under the guidance of the guide wire, the duct was passed through the duodenal papilla orifice and into the pancreatic duct. The cyst fluid was seen draining from the naso-biliary duct, which was then pumped back to confirm that the cyst fluid could be extracted. After insertion of the naso-pancreatic duct, changes in abdominal signs and vital signs of the patients were observed, the color and volume of the drainage fluid in the naso-pancreatic duct were observed, and the drainage fluid in the naso-pancreatic duct was sampled for bacteria culture. Abdominal CT was performed after 7 d to observe changes in the pancreatic pseudocyst. Placement of the pancreatic duct stent was performed using the same precautionary measures as followed in the ERPD group. The patients were discharged within 1-3 d if no discomfort was recorded (Figure 2).
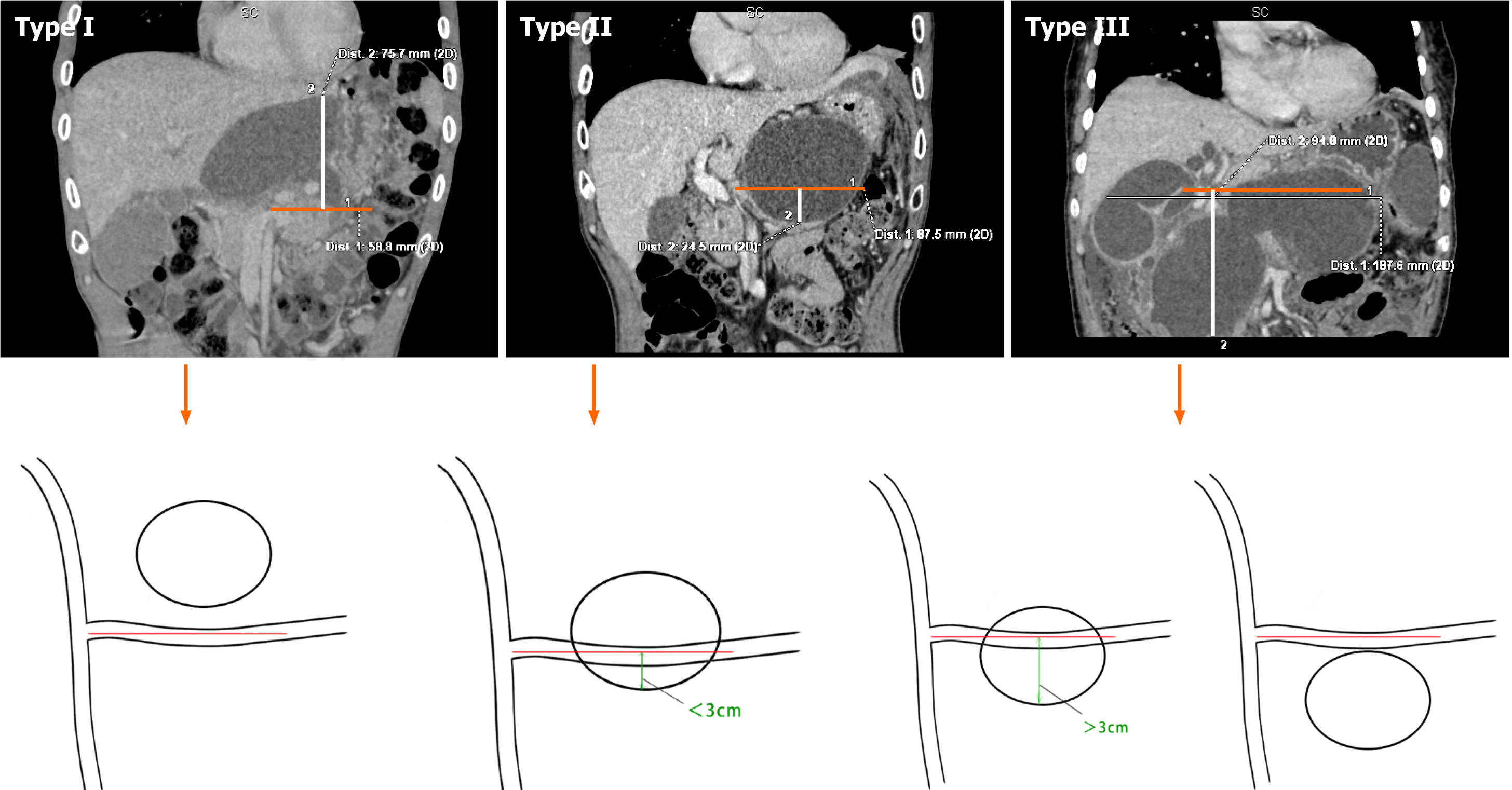
We used our Xin-Qiao classification based on the relationship between the pancreatic pseudocyst and the splenic vein. As pancreatitis with pancreatic pseudocyst is not identified in most pancreatic ducts and the splenic vein is fixed in most cases, we used the splenic vein as the reference plane (Figure 3).
SPSS26.0 (SPSS Inc., IBM, Armonk, NY, United States) statistical software package was used for data analyses in this study. Measurement results are presented as the mean ± SD, and the independent sample t-test was used for comparisons between the two groups. Enumeration data are expressed as incidence, and the χ2 test was used for comparisons between the two groups. ANOVA was used to compare the means of multiple samples, and P < 0.05 was considered statistically significant.
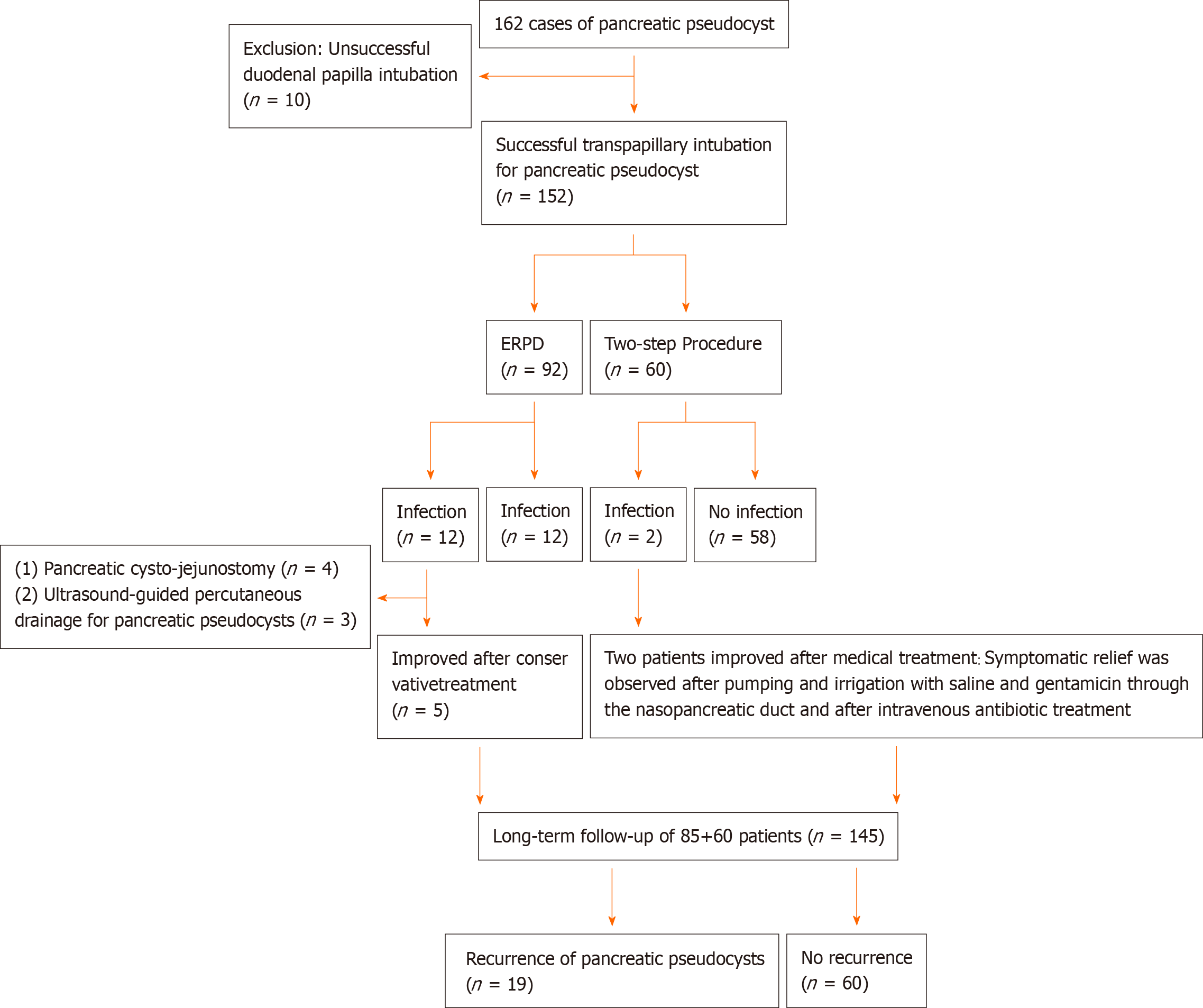
A total of 162 patients with pancreatic pseudocysts were included in this study, ten of whom were excluded due to duodenal papilla intubation failure (Figure 4). The remaining 152 patients who underwent successful duodenal papilla intubation were divided into an ERPD group (92 cases) and a two-step procedure group (60 cases). Baseline characteristics of the enrolled patients are listed in Table 1. There were no differences in age, sex, or etiology and location of the pancreatic pseudocyst between the two groups (P > 0.05). All patients were followed by telephone calls or at the outpatient clinic after discharge. The follow-up included checking for recurrence of symptoms and repeat imaging examinations (B-mode ultrasound, CT, and MRCP). The follow-up period ranged from 3 mo to 2 years (every 3 mo) after discharge. The pancreatic duct stent was electively changed or removed based on the examination results.
| Variable | Grouping by surgery | χ2 / t | P value | ||
| ERPD | Two-step procedure | ||||
| Gender | Male | 58 | 35 | 0.339 | 0.560 |
| Female | 34 | 25 | |||
| Age (yr) mean ± SD | - | 47.7 ± 16.21 | 47.42 ± 15.34 | 0.066 | 0.947 |
| Source/Location | Biliary | 39 | 35 | 9.249 | 0.100 |
| High fat | 28 | 16 | |||
| Alcoholic | 14 | 9 | |||
| Post-operation | 3 | 0 | |||
| Trauma | 6 | 0 | |||
| Pancreatic fistula | 2 | 0 | |||
| Part | Body of the pancreas | 30 | 15 | 1.705 | 0.426 |
| Head of the pancreas | 20 | 18 | |||
| Tail of the pancreas | 42 | 27 | |||
Infection occurred in 12 (13.04%) cases in the ERPD group and two (3.33%) cases in the 2-step procedure group, and the difference in the incidence of infection between the two groups was statistically significant (P < 0.05). No postoperative complications, such as intestinal perforation, bleeding, or pancreatic fistula, were observed in either group (Table 2).
| Variable | Classification | Grouping by surgery | χ2 / t | P value | |
| ERPD | Two-step procedure | ||||
| Complications | Infection | 12 | 2 | 4.095 | 0.043 |
| None | 80 | 58 | |||
| Rate of reoperation | Reoperation method (puncture/open surgery) | 7 (4/3) | 0 | 4.786 | 0.029 |
| None | 85 | 60 | |||
| Recurrence rate | Recurrence | 19 | 0 | 14.161 | 0.000 |
| None | 73 | 60 | |||
| Size of pancreatic pseudocyst (cm), mean ± SD | - | 8.73 ± 3.71 | 13.25 ± 4.11 | -4.483 | 0.000 |
| Length of stay (d) | - | 8.61 ± 3.07 | 12.15 ± 1.99 | -19.157 | 0.000 |
| Treatment cost (USD), mean ± SD | - | 7875.05 ± 5221.5 | 7715.21 ± 3030.57 | 0.126 | 0.900 |
In the ERPD group, anti-infection treatment was effective in five of 12 cases with infection but not in the remaining seven cases, and urgent ultrasound-guided percutaneous drainage was performed in three of these seven cases and open cyst-jejunum anastomosis in four cases. In the two-step procedure group, infection presented as fever and chills in two cases. Symptomatic relief was observed after pumping and irrigation with saline and gentamicin through the naso-pancreatic duct and after intravenous antibiotic treatment. The reoperation rate in the ERPD group was 7.6% (7 cases), and was 0% in the two-step procedure group, and the difference was statistically significant (P < 0.05) (Table 2).
All patients were followed by telephone calls or at the outpatient clinic after discharge for recurrence of symptoms and repeat imaging examinations. The follow-up period ranged from 3 mo to 2 years (every 3 mo) after discharge. The pancreatic duct stent was electively changed or removed based on the examination results. In the ERPD group, recurrence was noted in 19 (20.65%) of 92 cases, but no recurrence was noted in the two-step procedure group (P < 0.05).
The hospital stay in the ERPD group and two-step procedure group was 8.61 ± 3.07 d and 12.15 ± 1.99 d, respectively (P < 0.05). There was no significant difference in treatment costs between the two groups (7875.05 ± 5221.5 USD and 7715.21 ± 3030.57 USD, respectively, P > 0.05).
Classification of pseudocyst type was carried out according to the location of the pancreatic pseudocyst in relation to the splenic vein as pancreatitis with pancreatic pseudocysts cannot be identified in most pancreatic ducts and the splenic vein is relatively fixed in most cases. Therefore, we used the splenic vein as the reference plane. Of 152 patients, 7 had type I, 64 had type II, and 81 had type III. Infection occurred in 14 of 152 patients, including two cases with type II (14.28%) and 12 cases with type III (85.72%). The incidence of infection in type III was significantly higher than that in type I and type II (P < 0.05) (Table 3). The CT cycle threshold between the ERPD group and the two-step procedure group was not significantly different (P > 0.05) (Table 4). The mean CT cycle threshold of 14 patients with infection was 15.60 Hounsfield units (HU), and the mean CT cycle threshold of 138 patients without infection was 11.73 HU. The CT cycle threshold of patients with infection was significantly higher than that of patients without infection (P < 0.05). In addition, the larger the CT cycle threshold, the higher the risk of infection (Table 5).
| Group | Complications | Total | χ2 | P value | |||
| Infection | None | ||||||
| ERPD group | Classification | I | 0 | 7 | 7 | 6.271 | 0.043 |
| II | 2 | 37 | 39 | ||||
| III | 10 | 36 | 46 | ||||
| Total | 12 | 80 | 92 | ||||
| Two-step procedure | Classification | I | 0 | 0 | 0 | 1.379 | 0.240 |
| II | 0 | 24 | 24 | ||||
| III | 2 | 34 | 36 | ||||
| Total | 2 | 58 | 60 | ||||
| Total | Classification | I | 0 | 7 | 7 | 6.339 | 0.042 |
| II | 2 | 61 | 64 | ||||
| III | 12 | 70 | 81 | ||||
| Total | 14 | 138 | 152 | ||||
| Group | Number of cases | Average value (HU) | Standard deviation | Mean square | F | P value |
| ERPD group | 92 | 12.796 | 6.4624 | 36.857 | 0.755 | 0.388 |
| Two-step procedure group | 60 | 14.636 | 8.6450 | 48.789 | ||
| Total | 152 | 13.205 | 6.9711 |
| Group | Number of cases | Average value (HU) | Standard deviation | Mean square | F | P value |
| Infection | 14 | 15.600 | 8.3953 | 222.425 | 4.862 | 0.031 |
| No infection | 138 | 11.731 | 5.5476 | 45.747 | ||
| Total | 152 | 13.205 | 6.9711 |
Surgery was the standard procedure for the treatment of pancreatic pseudocysts[3,5,10]. However, this has gradually been replaced by minimally invasive, non-surgical methods due to the significant trauma, longer hospital stay, high costs, etc. associated with conventional surgery[22]. With the rapid development of endoscopic techniques, endoscopic drainage of pancreatic pseudocysts has become the main treatment for pancreatic pseudocysts[2,3,19]. Endoscopic drainage of pancreatic pseudocysts[1-3] includes endoscopic trans-gastric ultrasonography-guided puncture drainage for the treatment of pancreatic pseudocysts, ERPD, and ENPD.
Endoscopic trans-gastric ultrasonography-guided puncture drainage for the treatment of pancreatic pseudocysts may carry the risk of bleeding (27.8%)[23], perforation, and peritonitis (11%)[15], etc., and may increase the risk of intra-cystic or intra-abdominal infection due to inadequate stent drainage or infection after prolonged stent placement[16,17] and the rate of recurrence due to stent displacement or removal (12%)[15]. In addition, treatment costs are high and there is a lack of qualified endoscopic ultrasonography technicians and good-quality devices, especially in underdeveloped areas. Beckingham et al[24] reported that the pancreatic duct stent prevented the occurrence of infection, sepsis, and other complications[25]. Our study also confirmed that there was a high incidence of infection and a higher rate of reoperation after ERPD. Bhasin et al[18] reported 11 cases of pancreatic pseudocysts treated by ENPD, of which naso-pancreatic ducts were successfully placed in ten cases (90.9%), with good outcome and a low incidence of infection complications. When the naso-pancreatic duct is blocked, the drainage fluid can be sampled through the naso-pancreatic duct and submitted for bacterial culture, and antibiotics can be selected according to culture test results[25]. In addition, the naso-pancreatic duct is used for suctioning and flushing to solve the problem of infection due to the inadequate drainage of pancreatic fluid. However, prolonged placement of the naso-pancreatic duct is inconvenient in patients and reduces their quality of life; moreover, there is a chance that the naso-pancreatic duct may fall out, resulting in drainage failure and the need for reoperation[18,21].
Inadequate trans-gastric-pancreatic drainage, especially pancreatic drainage from the tail of the pancreas, is the trigger factor for pancreatic pseudocysts, and the establishment of normal physiological channels in the pancreatic duct is the main factor affecting the treatment outcome and prognosis of patients with pancreatic pseudocysts[5,26-28]. Moreover, trans-gastric puncture drainage in the treatment of pancreatic pseudocysts increases the risk of bleeding, intestinal fistula, gastric fistula, extravasation of pancreatic fluid, and infection due to foreign body reflux in the digestive tract[15-17,23]. Lin et al[29] reported that the trans-papillary treatment of pancreatic pseudocysts can offer good results. However, simple pancreatic duct stenting is not suitable for pancreatic pseudocysts with thick cystic fluid, massive necrotic tissues, and infection. As it is challenging to carry out complete drainage, inadequate drainage of pancreatic fluid can result in pancreatic pseudocyst infection and pancreatic abscess due to pancreatic duct obstruction. Therefore, ultrasound-guided percutaneous drainage of pancreatic pseudocysts or internal drainage of pancreatic pseudocysts by open surgery is required. Although a naso-pancreatic duct can be placed in the pancreatic duct through the duodenal papilla alone, the blocked drainage channel can be unblocked by flushing. However, a relatively long period of naso-pancreatic drainage will cause extreme inconvenience to the patient, and the patient may accidentally remove the naso-pancreatic duct and thus cause drainage failure[18,21]. Therefore, our strategy of treating pancreatic pseudocysts using a sequential procedure can lead to cyst collapse in the early phase due to rapid naso-pancreatic drainage, thus avoiding repeated stent replacement due to infection caused by blocked pancreatic duct stent drainage in the treatment of pancreatic pseudocysts. After about a week of drainage, the cyst is basically occluded. At this time, replacement of the pancreatic duct stent can further promote the healing and adhesion of the cyst, which can reduce the discomfort caused to the patient by prolonged placement of the naso-pancreatic duct and establish a normal and adequate pancreatic drainage channel. This preliminary clinical study showed that the patients recovered well after surgery without extra complications, and this technique is expected to be a novel and effective treatment strategy.
In this study, the incidence of infection in the ERPD group was 13.04%, the postoperative recurrence rate was 20.65%, and the rate of reoperation in infected patients after anti-infective treatment was 7.6%. The incidence of infection in the two-step procedure group was 3.3%. In this group, infection could be avoided by naso-pancreatic duct aspiration and dilution of the fluid in the cyst by rinsing with sterile normal saline. The pancreatic pseudocysts were observed to be significantly reduced following a repeat CT examination (reduced to within 20% of the original volume), and pancreatic duct stenting could be performed on the designated date. No reoperations were performed in this group, and follow-up revealed no recurrence in any of the patients after treatment. It is critical that rinsing with normal saline be carried out slowly and gently through the naso-pancreatic duct to avoid excessive force and pressure, which may lead to nosocomial infection and cyst rupture. In patients who developed infection after ENPD, gentamicin or metronidazole was used for rinsing. In addition, a negative pressure aspirator can be used to maintain vacuum aspiration when naso-pancreatic drainage is adequate. In patients with suspected duct obstruction whose naso-pancreatic ducts are rinsed with normal saline, a guide wire can be used to unblock the duct under fluoroscopy or the naso-pancreatic duct can be replaced. In this study, drainage (color and amount of drainage fluid) can be observed by means of ENPD to facilitate rinsing and unblocking after drainage blockage and to avoid infection caused by drainage blockage due to inadequate drainage. After ENPD, a repeat abdominal CT examination allows us to understand the degree of pancreatic pseudocyst shrinkage before ERPD is re-performed. In this way, infection and other complications will be reduced, and pancreatic pseudocysts will be treated completely with a low recurrence rate.
Pancreatic pseudocysts located in the head of the pancreas have a lower success rate of intubation than those located in the tail of the pancreas[15]. Our study also confirmed that intubation in the tail of the pancreas had a high success rate, as pseudocysts located in the head of the pancreas compress the duodenum and narrow the enteric cavity, thus making intubation difficult.
Appropriate cyst classification is also important for the selection of treatment. In this study, we found that postoperative infection due to the treatment of pancreatic pseudocysts using ENPD or ERPD was closely related to the location, size, and CT cycle threshold of the pancreatic pseudocysts, and the Xin-Qiao classification was then proposed. In the 24 cases of infection, we analyzed the following possible reasons for infection due to pancreatic pseudocysts with pancreatic duct stents inserted. First, type I cystic fluid can easily drain into the duodenum via ENPD or ERPD under the influence of gravity. Type II cystic fluid flows out via ENPD or ERPD under the influence of gravity, but there is still some cystic fluid below the stent plane, and the cystic fluid accumulating in the cyst cannot be easily drained. After invasive surgery, stenting can easily carry bacteria into the cyst and cause infection or retrograde intestinal bacterial growth can cause infection in the cyst, which was observed in two (14.28%) of 14 cases of infection. In terms of type III, because most of the cystic fluid is lower than the stent drainage plane, it is not easy to drain via ENPD or ERPD, so bacteria in the cystic fluid grow, and the cystic fluid accumulates in the cyst for a long time and causes infection. This vicious circle continues with inadequate stent drainage, which was found in 12 (85.72%) of 14 cases of infection.
Second, among the 14 patients with infection, the mean CT cycle threshold was 15.6 HU, while the mean CT cycle threshold of patients without infection was 11.73 HU. A larger CT cycle threshold of pancreatic pseudocysts indicate that the fluid in the cyst has more necrotic liquefied tissues, and the fluid is more turbid and viscous. Therefore, the stent or the drainage duct can be more easily blocked, and the risk of infection after stent placement is higher. However, as for the comparison between the ERPD group and the two-step procedure group, the CT cycle threshold in the combination group was higher than that in the ERPD group, but the proportion of infection was smaller, which may be related to the fact that we first used ENPD for active suction of the cystic fluid or rinsed and diluted the fluid to reduce its density in the cyst, thus avoiding blockage of the naso-pancreatic duct. The following suggestions are made based on this retrospective analysis: For type I cysts, pancreatic duct stenting is an option. For type II and type III cysts, we recommend two-step sequential therapy, which can avoid the occurrence of infection, reduce cysts in a shorter time, and shorten the length of hospital stay. In terms of the treatment of pancreatic pseudocysts with the two-step sequential therapy, we recommend 7 d of naso-pancreatic duct placement, depending on the appearance of the drainage fluid (clear) in the naso-pancreatic duct and reduction in the amount of drainage fluid. In addition to that described earlier, a repeat CT examination also allows observation of the patient's abdomen, such as smaller abdominal bulge, disappeared or significantly reduced abdominal mass, and relief of abdominal pain. The active and low drainage in the sequential method will shrink the cyst faster than the pancreatic duct stent alone. For cysts with a CT cycle threshold above 13 HU, active aspiration and dilution with normal saline via the naso-biliary duct are recommended.
The advantages of sequential therapy with trans-papillary ENPD combined with ERPD for pancreatic pseudocysts can be summarized as follows: (1) Compared with trans-gastric drainage, it meets the physiological requirements and avoids complications, such as infection and bleeding of the cyst, due to the connection between the stomach and the cyst; (2) Compared with pancreatic duct stenting, it facilitates postoperative observation and makes rinsing and unblocking easy after duct blockage, which greatly reduces the risk of infection and shortens the treatment time of pancreatic pseudocysts with a low recurrence rate, effectively reducing the total treatment costs; and (3) In recent years, good results have been reported in the literature with the use of fully covered self-expanding metal stents for the treatment of pancreatic pseudocysts through endoscopic trans-gastric ultrasonography-guided puncture drainage. If the diameter of the stent is large, the drainage effect is good and infection due to the use of plastic stents which are susceptible to blockage and need frequent replacement is avoided[15-17,23]. However, the treatment costs are high, and treatment is challenging especially in underdeveloped areas where endoscopic ultrasonography technicians are not qualified and devices are not of good quality. Our clinical results showed that a large proportion of such patients had co-infection or co-bleeding (data not shown). The two-step procedure can be widely used in clinical practice with low requirements for devices; thus, sequential therapy is particularly suitable for hospitals at all levels.
Based on our current experience, the application of this approach still has the following drawbacks: (1) This study was retrospective and has inherent limitations; (2) The sample size was small; (3) It was impossible to accurately determine whether the pancreatic pseudocyst was connected to the pancreatic duct before surgery; and (4) Some patients may be at risk of infection or fever.
Sequential therapy with trans-papillary ENPD combined with ERPD is safe and effective for pancreatic pseudocysts and can be carried out by experienced endoscopists. Future prospective and randomized clinical trials are needed to validate the present findings.
Endoscopic retrograde pancreatic drainage (ERPD) and stent implantation is associated with a high recurrence rate and infection rate.
A two-step trans-papillary procedure involving endoscopic naso-pancreatic drainage (ENPD) and ERPD sequential therapy for pancreatic pseudocysts may reduce the infection-related complications seen with single stent implantation, address concerns related to tube blockage, and reduce patient discomfort due to long-term single naso-pancreatic duct implantation.
To manage pancreatic pseudocysts by sequential therapy with ENPD combined with ERPD and evaluate the treatment outcome.
One hundred and fifty-two cases of pancreatic pseudocysts were intubated via the duodenal papilla, and 92 cases involved pancreatic duct stent implantation and 60 cases involved sequential therapy with ENPD combined with ERPD. The success rate of the procedure, incidence of complications (infection, bleeding, etc.), recurrence, and length and cost of hospitalization were compared between the two groups.
The incidence of infection was significantly higher in the ERPD group (12 cases) than in the two-step procedure group (2 cases). The reoperation rate was also significantly higher in the ERPD group (7 cases) than in the two-step procedure group (0 cases). Similarly, the recurrence rate was significantly higher in the ERPD group (19 cases) than in the two-step procedure group (0 cases).
Two-step sequential therapy with ENPD combined with ERPD is safe and effective in patients with pancreatic pseudocysts.
The sequential therapywith trans-papillary ENPD combined with ERPD for pancreatic pseudocysts meets the physiological requirements and avoids complications, such as infection and bleeding of the cyst. Compared with pancreatic duct stenting, it facilitates postoperative observation and makes rinsing and unblocking easier after duct blockage, which greatly reduces the risk of infection and shortens the treatment time of pancreatic pseudocysts with a low recurrence rate, effectively reducing the total treatment costs. The two-step procedure can be widely used in clinical practice with low requirements for devices; thus, this sequential therapy is particularly suitable for hospitals at all levels.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Thandassery RB S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (1)] |
| 2. | Andalib I, Dawod E, Kahaleh M. Modern Management of Pancreatic Fluid Collections. J Clin Gastroenterol. 2018;52:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Ge PS, Weizmann M, Watson RR. Pancreatic Pseudocysts: Advances in Endoscopic Management. Gastroenterol Clin North Am. 2016;45:9-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Zerem E, Imamović G, Omerović S, Ljuca F, Haracić B. Percutaneous treatment for symptomatic pancreatic pseudocysts: Long-term results in a single center. Eur J Intern Med. 2010;21:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Gambitta P, Maffioli A, Spiropoulos J, Armellino A, Vertemati M, Aseni P. Endoscopic ultrasound-guided drainage of pancreatic fluid collections: The impact of evolving experience and new technologies in diagnosis and treatment over the last two decades. Hepatobiliary Pancreat Dis Int. 2020;19:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Grace PA, Williamson RC. Modern management of pancreatic pseudocysts. Br J Surg. 1993;80:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 102] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB, Yeaton P. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Siddiqui AA, Dewitt JM, Strongin A, Singh H, Jordan S, Loren DE, Kowalski T, Eloubeidi MA. Outcomes of EUS-guided drainage of debris-containing pancreatic pseudocysts by using combined endoprosthesis and a nasocystic drain. Gastrointest Endosc. 2013;78:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Szakó L, Mátrai P, Hegyi P, Pécsi D, Gyöngyi Z, Csupor D, Bajor J, Erőss B, Mikó A, Szakács Z, Dobszai D, Meczker Á, Márta K, Rostás I, Vincze Á. Endoscopic and surgical drainage for pancreatic fluid collections are better than percutaneous drainage: Meta-analysis. Pancreatology. 2020;20:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Keane MG, Sze SF, Cieplik N, Murray S, Johnson GJ, Webster GJ, Thorburn D, Pereira SP. Endoscopic versus percutaneous drainage of symptomatic pancreatic fluid collections: a 14-year experience from a tertiary hepatobiliary centre. Surg Endosc. 2016;30:3730-3740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 11. | Melman L, Azar R, Beddow K, Brunt LM, Halpin VJ, Eagon JC, Frisella MM, Edmundowicz S, Jonnalagadda S, Matthews BD. Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg Endosc. 2009;23:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Law R, Baron TH. Endoscopic management of pancreatic pseudocysts and necrosis. Expert Rev Gastroenterol Hepatol. 2015;9:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Giovannini M. What is the best endoscopic treatment for pancreatic pseudocysts? Gastrointest Endosc. 2007;65:620-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | vanSonnenberg E, Wittich GR, Casola G, Brannigan TC, Karnel F, Stabile BE, Varney RR, Christensen RR. Percutaneous drainage of infected and noninfected pancreatic pseudocysts: experience in 101 cases. Radiology. 1989;170:757-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 169] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Ahn JY, Seo DW, Eum J, Song TJ, Moon SH, Park DH, Lee SS, Lee SK, Kim MH. Single-Step EUS-Guided Transmural Drainage of Pancreatic Pseudocysts: Analysis of Technical Feasibility, Efficacy, and Safety. Gut Liver. 2010;4:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Arvanitakis M, Delhaye M, Bali MA, Matos C, De Maertelaer V, Le Moine O, Devière J. Pancreatic-fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007;65:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Weckman L, Kylänpää ML, Puolakkainen P, Halttunen J. Endoscopic treatment of pancreatic pseudocysts. Surg Endosc. 2006;20:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Bhasin DK, Rana SS, Nanda M, Chandail VS, Gupta R, Kang M, Nagi B, Sinha SK, Singh K. Comparative evaluation of transpapillary drainage with nasopancreatic drain and stent in patients with large pseudocysts located near tail of pancreas. J Gastrointest Surg. 2011;15:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Behrns KE, Ben-David K. Surgical therapy of pancreatic pseudocysts. J Gastrointest Surg. 2008;12:2231-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Shinozuka N, Okada K, Torii T, Hirooka E, Tabuchi S, Aikawa K, Tawara H, Ozawa S, Ogawa N, Miyazawa M, Takeda A, Otani Y, Koyama I. Endoscopic pancreatic duct drainage and stenting for acute pancreatitis and pancreatic cyst and abscess. J Hepatobiliary Pancreat Surg. 2007;14:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Bhasin DK, Rana SS, Udawat HP, Thapa BR, Sinha SK, Nagi B. Management of multiple and large pancreatic pseudocysts by endoscopic transpapillary nasopancreatic drainage alone. Am J Gastroenterol. 2006;101:1780-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Akshintala VS, Saxena P, Zaheer A, Rana U, Hutfless SM, Lennon AM, Canto MI, Kalloo AN, Khashab MA, Singh VK. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc. 2014;79:921-8; quiz 983.e2, 983.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Fugazza A, Sethi A, Trindade AJ, Troncone E, Devlin J, Khashab MA, Vleggaar FP, Bogte A, Tarantino I, Deprez PH, Fabbri C, Aparicio JR, Fockens P, Voermans RP, Uwe W, Vanbiervliet G, Charachon A, Packey CD, Benias PC, El-Sherif Y, Paiji C, Ligresti D, Binda C, Martínez B, Correale L, Adler DG, Repici A, Anderloni A. International multicenter comprehensive analysis of adverse events associated with lumen-apposing metal stent placement for pancreatic fluid collection drainage. Gastrointest Endosc. 2020;91:574-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 24. | Beckingham IJ, Krige JE, Bornman PC, Terblanche J. Endoscopic management of pancreatic pseudocysts. Br J Surg. 1997;84:1638-1645. [PubMed] |
| 25. | Negm AA, Poos H, Kruck E, Vonberg RP, Domagk D, Madisch A, Voigtländer T, Manns MP, Wedemeyer J, Lankisch TO. Microbiologic analysis of peri-pancreatic fluid collected during EUS in patients with pancreatitis: impact on antibiotic therapy. Gastrointest Endosc. 2013;78:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Zerem E, Hauser G, Loga-Zec S, Kunosić S, Jovanović P, Crnkić D. Minimally invasive treatment of pancreatic pseudocysts. World J Gastroenterol. 2015;21:6850-6860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Singh S. Surgical management of complications associated with percutaneous and/or endoscopic management of pseudocyst of the pancreas. Ann Surg. 2006;244:630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Nealon WH, Walser E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage). Ann Surg. 2002;235:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Lin H, Zhan XB, Jin ZD, Zou DW, Li ZS. Prognostic factors for successful endoscopic transpapillary drainage of pancreatic pseudocysts. Dig Dis Sci. 2014;59:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |