Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.6145
Peer-review started: March 28, 2021
First decision: May 7, 2021
Revised: May 13, 2021
Accepted: May 26, 2021
Article in press: May 26, 2021
Published online: July 26, 2021
Processing time: 114 Days and 13.1 Hours
Madelung’s disease, also known as multiple symmetrical lipomatosis, is a rare, underrecognized disorder of fat metabolism that results in unusual accumulation of subcutaneous fat deposits around the neck, shoulders, upper arms, trunk, hips, and upper thighs. Our case demonstrates the importance of differential diagnosis and the value of a superb microvascular imaging technique for suspecting and confirming Madelung’s disease. Timely diagnosis and alcohol abstinence could prevent the progression of growing fatty masses and prevent surgery.
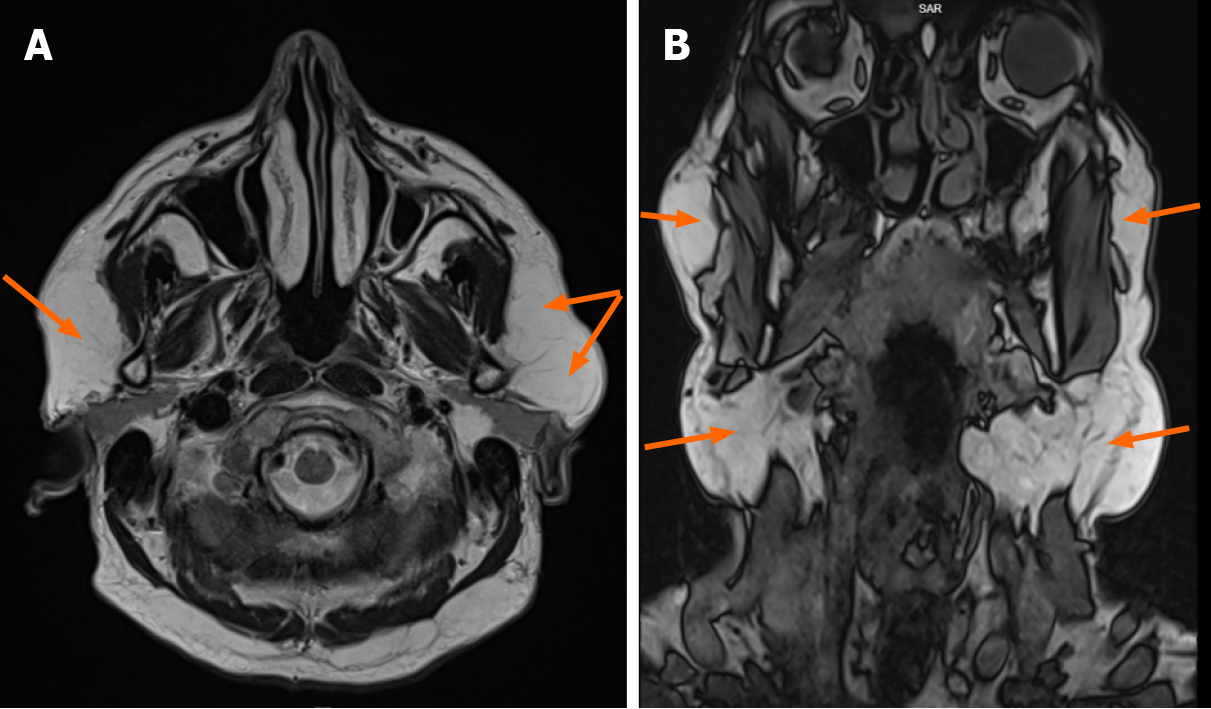
A 62-year-old male was admitted to the Rheumatology center complaining of symmetric subcutaneous tumors in the area of the parotid and submandibular salivary glands, small soft masses in the occiput and upper third of the forearm, rashes on calves. A high titer of rheumatoid factor and low concentrations of serum complements were detected. The high-end ultrasound and magnetic reso
Madelung’s disease consists of many co-occurring disorders imitating and overlapping with other conditions. Ultrasonography is the first choice for sus
Core Tip: Madelung’s disease, also known as benign symmetrical lipomatosis, is a rare disorder of fat metabolism resulting in unusual accumulation of subcutaneous fat deposits in different areas of the body and is mostly predisposed by alcohol abuse. The disease mimics and overlaps with other pathologies such as obesity, and oncological and connective tissue diseases and is associated with cirrhosis. High-end ultrasonography techniques have an important role in evaluating fatty tumors, and its importance for confirming diagnosis and follow-up for malignancy is unquestionable. A comprehensive approach to the patient and innovative ultrasound techniques are the key components for making an accurate diagnosis.
- Citation: Seskute G, Dapkute A, Kausaite D, Strainiene S, Talijunas A, Butrimiene I. Multidisciplinary diagnostic dilemma in differentiating Madelung’s disease — the value of superb microvascular imaging technique: A case report. World J Clin Cases 2021; 9(21): 6145-6154
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/6145.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.6145
Madelung’s disease (rare disease code ORPHA:2398) is also known as familial benign symmetrical lipomatosis, multiple symmetric lipomatosis (MSL), cephalothoracic lipodystrophy, or Launois-Bensaude syndrome[1]. It is characterized by symmetrical accumulation of fatty tissue, usually around the head and neck, the trunk, upper arms, and other less common places according to the type of disease. Patients with a history of chronic alcoholism comprise 60%-90% of cases and suffer from secondary liver cirrhosis[2,3]. Mediterranean men from 30-year-old to 60-year-old appear to be at highest risk[1,4]. The exact prevalence and incidence are unknown, but estimates are available for certain countries (e.g., the prevalence for males in Italy is 1/25.000)[1,2]. A vast complex of life-threatening disorders often occurs with Madelung’s disease including liver cirrhosis, diabetes, dyslipidemia, hyperuricemia, gynecomastia, neuropathy, hypothyroidism, chronic obstructive pulmonary disease, and adrenal dysfunction[1,5].
There are no specific tests for diagnosing this disorder, and the diagnosis is mostly based on history, clinical appearance, and the results of imaging examinations. Due to the slow growth of fat masses and alcohol-influenced decreased self-care, the disease is often recognized late. The changes in a patient’s facial appearance due to fat accumulation are often mistaken with the widely known chronic alcoholism-induced pseudo-Cushing syndrome (also known as “facies alcoholica”)[6]. Early diagnosis and alcohol cessation might prevent the progression of growing fatty masses and avoid surgery.
We report a case of a patient diagnosed with liver cirrhosis and progressive sym
A 62-year-old male was admitted to the Vilnius University Hospital Santaros Clinics Rheumatology department presenting with growing symmetric subcutaneous tumors in the area of the parotid and submandibular salivary glands, small soft masses in the occiput, and the upper third of the forearm. The patient also complained about bilateral small hand joint pain and morning stiffness for 6 mo, dryness of the mouth, rash on calves, and nail deformations for several years.
The subcutaneous tumors in the area of the parotid and submandibular salivary glands were present for 2 years and grew more rapidly in the last 7 mo. The patient had a history of moderate alcohol consumption (mostly beer) and was diagnosed with metabolic liver cirrhosis (Child-Turcotte-Pugh [CTP] B class; Model for the End-Stage Liver Disease [MELD] score equal to 9), portal hypertension (splenomegaly, hypersplenism, paraumbilical shunt), and secondary megaloblastic anemia in 2019. Since then, he was followed up by a gastroenterologist. In 2019, the ultrasound examination of the neck and salivary glands’ soft tissues discovered only small, lush adipose tissue around the neck without enlarged lymph nodes. At first, facial changes were asso
The patient was previously diagnosed with primary arterial hypertension and poly
The patient had a history of moderate alcohol consumption, but he abstained from consuming alcohol for approximately > 6 mo. He claimed to have no other hazardous habits, and no allergies to food or medicines. There was no history of surgical treatment and congenital or similar disorders in his family.
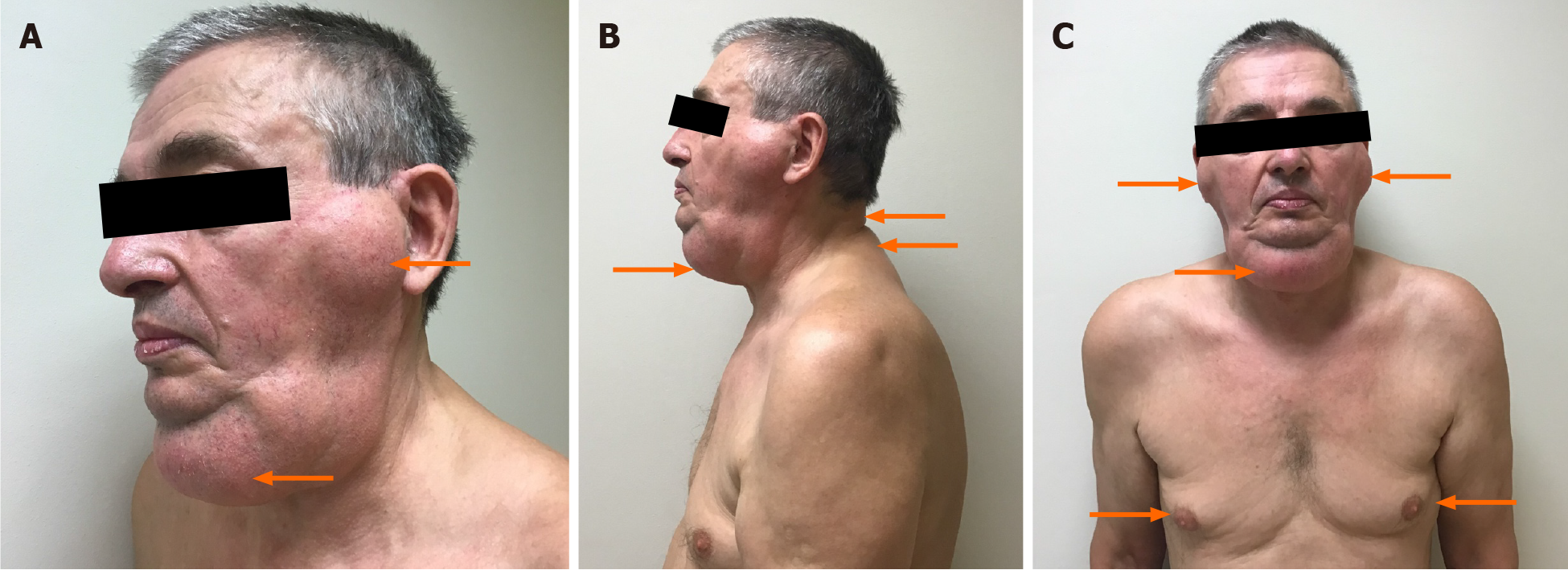
During the examination, egg-sized hard and painless subcutaneous tumors in the area of the parotid and submandibular salivary glands, soft and small masses in the occiput, hump, the upper third of the forearm. Occipital and neck area masses were difficult to notice without a meticulous inspection. Minor gynecomastia (elastic masses, which could be moved without causing the patient any pain and had no definite margins) was suspected (Figure 1A-C). The palpation of the small joints of both hand was painful. Onychodystrophy, rashes on calves — red patches of skin covered with thick, silvery scales, and dry tongue were observed. The patient was a bit overweight (body mass index 25.5 kg/m2).
Changes in the blood test results showed elevated erythrocyte sedimentation rate (ESR) by 26 mm/h, a high titer of rheumatoid factor (RF) 235.0 U/mL, low levels of complement C4, normal immunoglobulin (Ig) G4 1.26 g/L, and a normal cyclic citrullinated peptide antibody test. Extractable nuclear antigen antibodies and anti-neutrophil cytoplasmic antibodies were negative. Schirmer’s test was positive (right eye: 6 mm/5 min, left eye: 5 mm/5 min). Laboratory test results were also compatible with the diagnosis of alcohol-related liver cirrhosis (aspartate aminotransferase > 2× alanine aminotransferase, slightly elevated γ-glutamyl transferase, mild coagulopathy, and megaloblastic anemia). Calculated alcoholic liver disease/nonalcoholic fatty liver disease index was 14.2, meaning 100% probability of alcohol-related liver disease. Viral hepatitis markers were negative. Hyperglycemia and hyperuricemia were also noted (Table 1).
| Main laboratory findings | Value | Normal range |
| Hemoglobin (g/L) | 109 | 128-160 |
| MCV (fl) | 102 | 78-96 |
| MCH (pg) | 34.1 | 26-31 |
| Platelet count (/L) | 70 | 130-400 × 109 |
| C reactive protein (mg/L) | 4.22 | < 5 |
| Erythrocyte sedimentation rate (mm/h) | 26 | ≤ 10 |
| Glucose (mmol/L) | 6.70 | 4.2-6.1 |
| Aspartate aminotransferase (U/L) | 57 | < 40 |
| Alanine aminotransferase (U/L) | 22 | < 40 |
| γ-glutamyl transferase (U/L) | 123 | ≤ 36 |
| Alkaline phosphatase (U/L) | 181 | 40-150 |
| Total bilirubin (mol) | 17.5 | < 21 |
| Albumin (g/L) | 37 | 36-52 |
| SPA (%) | 62 | 70-130 |
| Creatinine (µmol/L) | 68 | 62-110 |
| Uric acid (µmol/L) | 510 | 208-428 |
| INR by Owren | 1.24 | 0.90-1.19 |
| Complement C4 (g/L) | 0.14 | 0.15-0.57 |
| Complement C3c (g/L) | 0.7 | 0.9-1.8 |
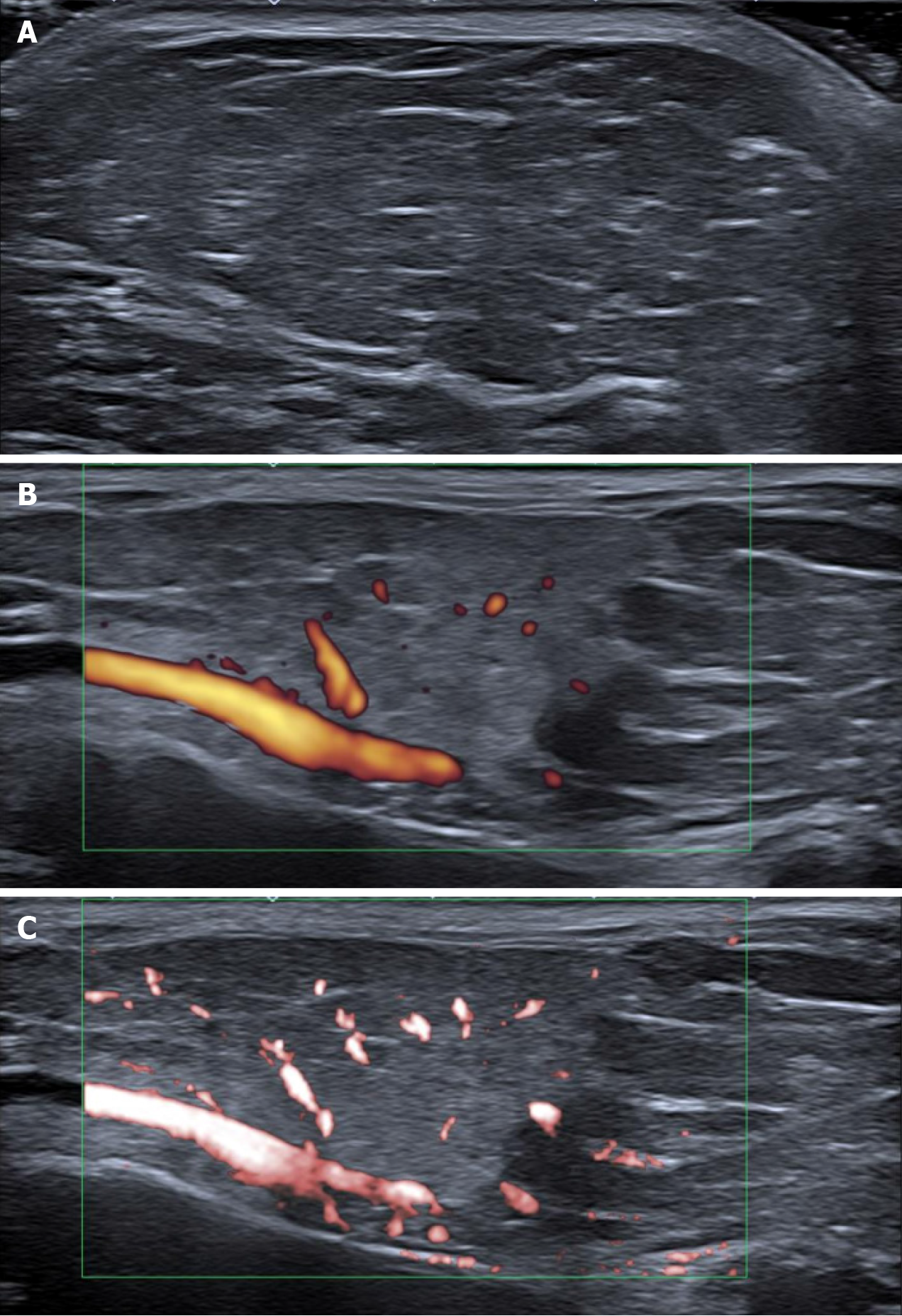
Ultrasound examination of the soft tissues of the parotid glands and neck area showed fatty solid masses close to parotid glands and thickening of the subcutaneous adipose tissue layer above the chin, right and left part of the neck in II-III vertebrae zone. Face and neck lipomatosis was suspected. However, small vascularity in the parotid gland tumor area was alleged. Figure 2 shows high-resolution ultrasound images (using Canon Aplio i800 14 MHz linear probe with power Doppler [PD] and color superb microvascular imaging [SMI] techniques) of the fatty tumor close to the left parotid gland area. Grayscale ultrasound showed typical superficial lipoma well-circumscribed with parallel linear and thin echogenic lines (Figure 2A). Features that suggest malignancy include: the presence of thick septa (> 2 mm), the presence of nodular and/or globular or non-adipose mass-like areas, and decreased percentage of fat composition (< 75% fat)[7]. Lipomatous soft tissue lesions with only thin septa that do not enhance at magnetic resonance imaging (MRI) can be confidently confirmed as a lipoma[8]. PD detected several small internal dots — more than minimal flow/vascu
Other possible comorbidities were excluded: Spirometry for chronic pulmonary disease and electroneuromyography for polyneuropathy were without changes. A small salivary gland biopsy was performed and showed no histological signs of Sjogren’s syndrome or amyloid deposition.
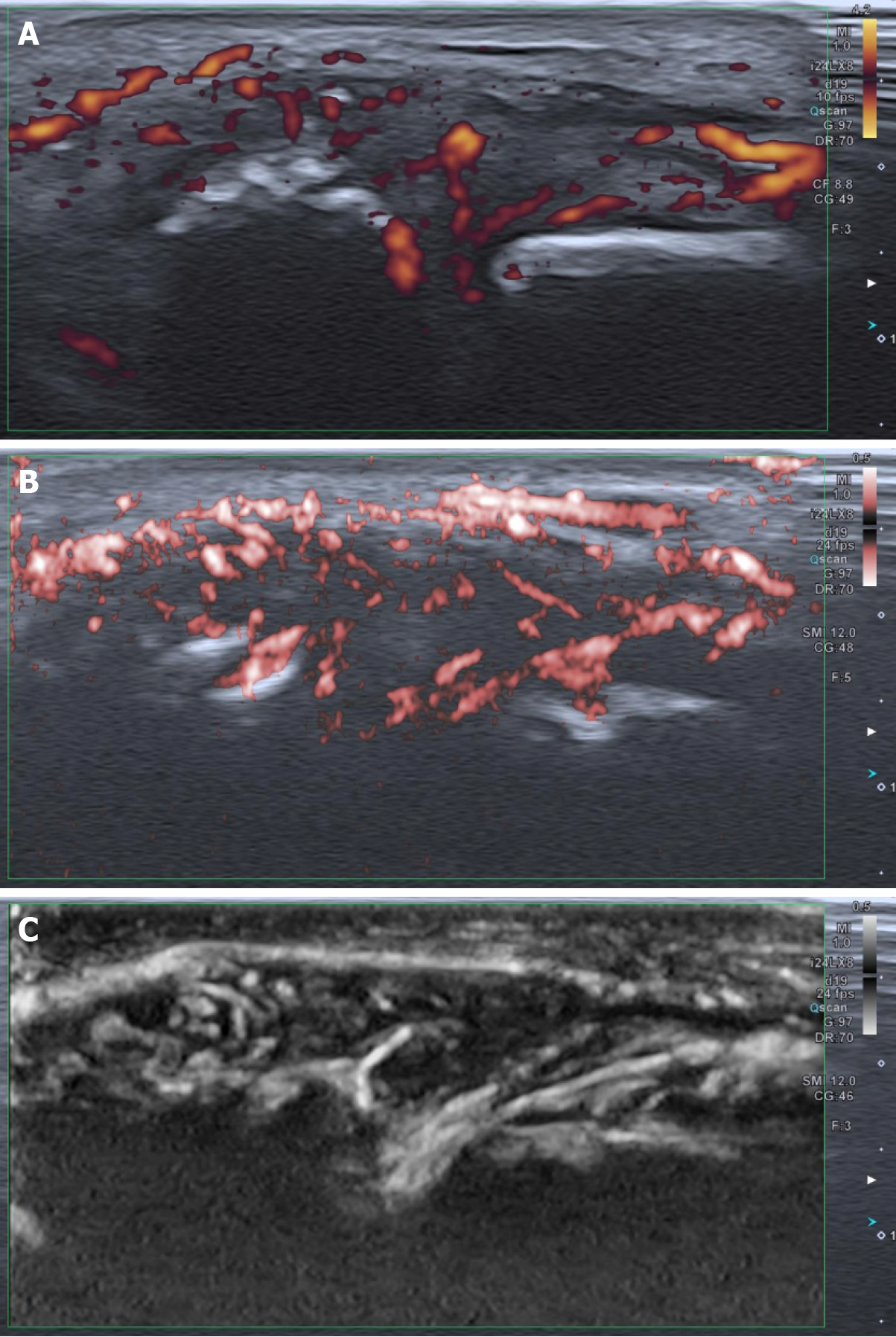
Psoriasis was confirmed by a dermatologist, and high-resolution ultrasound imaging (using Canon Aplio i800 24 MHz linear probe) showed active synovial arthritis in the metacarpophalangeal joints (Figure 4A-C).
Benign symmetrical lipomatosis (Madelung’s disease), type I, metabolic liver cirrhosis (CTP B, MELD 9), megaloblastic anemia, toxic peripheral polyneuropathy, hyperuri
The patient was informed about the importance of alcohol abstinence and weight control. The symptomatic treatment for comorbidities was recommended: hepatoprotection for cirrhosis, alpha-lipoic acid for previously diagnosed polyneuropathy, allopurinol for hyperuricemia, small doses of methylprednisolone 6 mg per day for psoriatic arthritis.
Although the patient did not consume alcohol for > 6 mo, and his liver enzymes normalized, he noticed that fatty masses continued to grow. He felt tremendous and socially isolating cosmetic discomfort due to the changed physical appearance around the neck. Therefore, he was referred to a maxillofacial surgeon for surgical treatment, and a lipectomy was performed. A histological examination of the removed tissue confirmed the diagnosis of benign symmetrical lipomatosis.
The patient was referred to an endocrinologist due to hyperglycemia. We also recommended getting an appointment with a dietologist to manage the diet. As the patient doesn’t have dyslipidemia yet, prevention and a healthy lifestyle must be the priority. The gastroenterologist should also observe the patient every 3-6 mo due to cirrhosis and its complications. The follow-up visit to the rheumatologist was set after a month. Repeated check-ups and ultrasound imaging are needed to observe possible disease regression and evaluate the progression of other fatty masses.
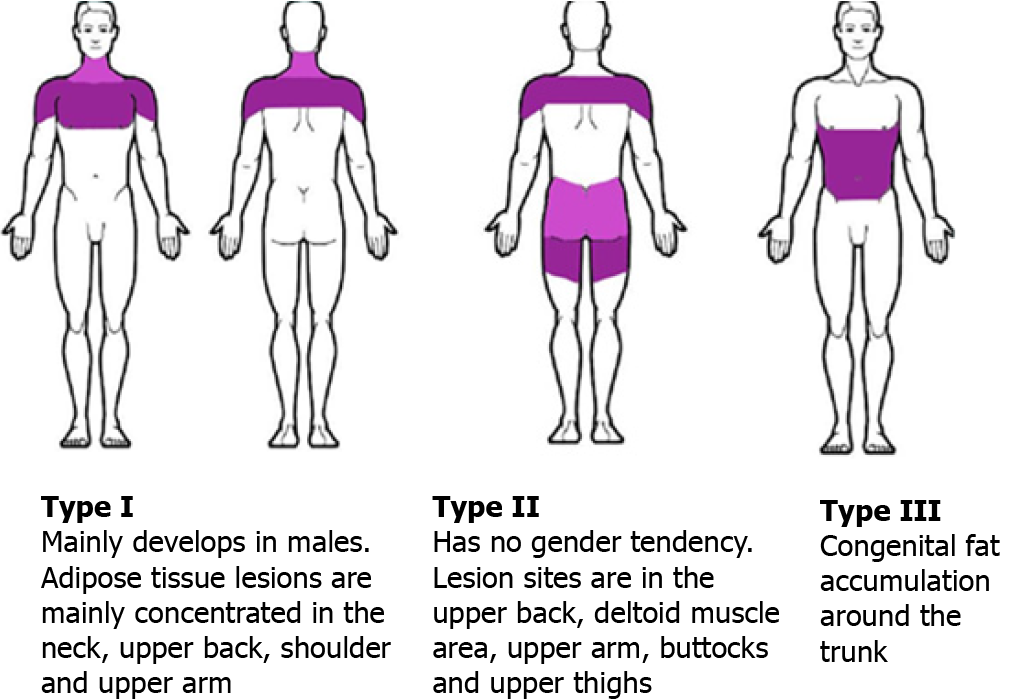
MSL is a very rare lipid metabolism disorder described by the localization of fat masses in different combinations of distribution. Donhauser et al[10] classified it into three types: first type — dominant cervical localization, second — pseudo athletic appearance, third — changes are located mostly in the abdomen area (Figure 5). The pathogenesis of the disease is unclear. It is thought that the disease might develop as a result of defects in mitochondrial function of adipose tissues, decreases in cytochrome C oxidase activity, and catecholamine-induced fat deposition. Alcohol consumption influences a decreased number and activity of β-adrenergic receptors and promotes fat synthesis[11]. It can also directly affect mitochondrial activity and cause premature oxidation of mitochondrial DNAs or mitochondrial DNA mutation, resulting in fat accumulation throughout the body[12]. Systemic diseases, such as primary hypothy
Precise physical examination of the whole body is essential for suspected symme
The differential diagnosis of systemic rheumatic diseases, such as Sjogren’s syndrome and IgG4-related disease, was performed due to salivary glands’ masses, elevated ESR and RF, low complements, positive Shirmer’s test, and arthritis. According to the classification criteria, Sjogren’s syndrome was excluded: immunological and histopathological tests were negative[15]. Common features of IgG4 syndrome, like IgG4-related autoimmune pancreatitis, swelling of or within an organ system (an inflammatory pseudotumor), salivary gland disease (material from salivary glands didn’t show lymphoplasmacytic infiltrate enriched in IgG4-positive plasma cells and fibrosis), lymphadenopathy, skin manifestations (only psoriasis was con
Ultrasound imaging is usually the first step in the investigation, differentiation, and confirmation of lipomas. SMI is an innovative ultrasound Doppler technique that provides visualization of low velocity and microvascular flow never seen before with the ultrasound[17]. SMI can suppress noise caused by motion artifacts with an intelligent filter system without eliminating the weak signal arising from small vessel blood flow and using any contrast agent. Hence it achieves a greater sensitivity than the conventional PD technique. There are two modes: color (cSMI, which demonstrates B-mode and color information simultaneously) and monochrome (SMI, which focuses only on the vasculature)[18]. Both modes demonstrate the value in differentiating a wide variety of clinical situations: benign and malign tumors (density and shape of tumor vessels), the therapeutic effect of the treatment (chemotherapy or immunosuppressive treatment), inflammatory diseases (synovial vascularity in arthritis, colitis) many other medical conditions[19,20]. The main disadvantage is that there are no studies and standards on the application of SMI for liposarcoma. The main tips for evaluation lipomatous tissue are comparing SMI and PD images with focusing on mSMI mode, which is more sensitive for vasculature, adjusting gain, and other settings.
In this case, SMI was used for better visualization of vascularity in differentiating lipomas and arthritis. If pathological vascularity and heterogeneous echotexture are suspected, then MRI is preferred over computed tomography for excluding liposar
Comorbidities, such as dyslipidemia, hypertension, chronic obstructive pulmonary disease, hypothyroidism, and diabetes mellitus, often co-occur with Madelung’s disease[1,13]. Diseases related to alcohol abuse, such as chronic liver disease, ma
Madelung‘s disease is difficult to diagnose at the early stages, as patients mostly seek medical help due to obvious cosmetic disorders or when liver cirrhosis and other comorbidities have already been developed. The main challenge for the doctors is to combine all the co-occurring symptoms into one disorder. In our case, predominant masses looked like solid tumors in the area of parotid glands imitating Sjogren’s syndrome and IgG4-related disease (Mikulicz disease). Ultrasound confirmed that tumors in the parotid glands area were not related to salivary glands and were closely located in front of them.
The primary treatment of Madelung’s disease is symptomatic. Alcohol withdrawal and weight loss are essential to control MSL. However, it does not guarantee the inhibition or reversion of the disease[5,23]. There is no effective pharmacotherapy in treating Madelung’s disease to date[5]. Lipectomy or liposuction is the only available effective treatment[23,24]. However, there is no consensus on the optimal surgical approach, and the overall recurrence rate is up to 63%[23,25]. Despite the high recurrence rate, the surgical approach must be considered as MSL also affects social life and worsens overall quality of life[26]. The histological investigation of the surgical material is essential, as there is always a small risk of liposarcoma. If the patient refuses surgery, ultrasound imaging control is strongly recommended, especially in cases of rapid mass growth.
Patients with Madelung’s disease need long-term observation and multidisciplinary systemic management of comorbidities[27]. It is also important to observe these patients due to disease relapse, the need for re-operation, and possible liposarcoma by ultrasonography. There are no established intervals for the patient’s surveillance.
Ultrasonography has an important role in differentiating fatty tumors, particularly when evaluating tissue homogeneity and vascularity. Nevertheless, high-end ultra
We wish to thank the patient and Vilnius University Hospital Santaros Clinics for giving the consent and providing the data to report this case.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: The Emerging European League Against Rheumatism Network; and Lithuanian Rheumathologists Association.
Specialty type: Rheumatology
Country/Territory of origin: Lithuania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali SM, Zhou S S-Editor: Gao CC L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Vantyghem MC. Multiple symmetric lipomatosis. Orphanet encyclopedia 2019. [cited 14 March 2021]. In: orpha.net [Internet]. Available from: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Expert=2398&lng=EN. [Cited in This Article: ] |
| 2. | González-García R, Rodríguez-Campo FJ, Sastre-Pérez J, Muñoz-Guerra MF. Benign symmetric lipomatosis (Madelung's disease): case reports and current management. Aesthetic Plast Surg. 2004;28:108-112; discussion 113. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Meningaud JP, Pitak-Arnnop P, Bertrand JC. Multiple symmetric lipomatosis: case report and review of the literature. J Oral Maxillofac Surg. 2007;65:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Chuang CC, Cheng YF, Chang HP, Lin CZ. Madelung's disease. J Chin Med Assoc. 2004;67:591-594. [PubMed] [Cited in This Article: ] |
| 5. | Szewc M, Sitarz R, Moroz N, Maciejewski R, Wierzbicki R. Madelung's disease - progressive, excessive, and symmetrical deposition of adipose tissue in the subcutaneous layer: case report and literature review. Diabetes Metab Syndr Obes. 2018;11:819-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Besemer F, Pereira AM, Smit JW. Alcohol-induced Cushing syndrome. Hypercortisolism caused by alcohol abuse. Neth J Med. 2011;69:318-323. [PubMed] [Cited in This Article: ] |
| 7. | Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24:1433-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in RCA: 364] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Shimamori N, Kishino T, Morii T, Okabe N, Motohashi M, Matsushima S, Yamasaki S, Ohtsuka K, Shibahara J, Ichimura S, Ohnishi H, Watanabe T. Sonographic Appearances of Liposarcoma: Correlations with Pathologic Subtypes. Ultrasound Med Biol. 2019;45:2568-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Donhauser G, Vieluf D, Ruzicka T, Braun-Falco O. [Benign symmetric Launois-Bensaude type III lipomatosis and Bureau-Barrière syndrome]. Hautarzt. 1991;42:311-314. [PubMed] [Cited in This Article: ] |
| 11. | Gao H, Xin ZY, Yin X, Zhang Y, Jin QL, Wen XY. Madelung disease: A case report. Medicine (Baltimore). 2019;98:e14116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Gámez J, Playán A, Andreu AL, Bruno C, Navarro C, Cervera C, Arbós MA, Schwartz S, Enriquez JA, Montoya J. Familial multiple symmetric lipomatosis associated with the A8344G mutation of mitochondrial DNA. Neurology. 1998;51:258-260. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Brea-García B, Cameselle-Teijeiro J, Couto-González I, Taboada-Suárez A, González-Álvarez E. Madelung's disease: comorbidities, fatty mass distribution, and response to treatment of 22 patients. Aesthetic Plast Surg. 2013;37:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Jang JH, Lee A, Han SA, Ryu JK, Song JY. Multiple Symmetric Lipomatosis (Madelung's Disease) Presenting as Bilateral Huge Gynecomastia. J Breast Cancer. 2014;17:397-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Rasmussen A, Ice JA, Li H, Grundahl K, Kelly JA, Radfar L, Stone DU, Hefner KS, Anaya JM, Rohrer M, Gopalakrishnan R, Houston GD, Lewis DM, Chodosh J, Harley JB, Hughes P, Maier-Moore JS, Montgomery CG, Rhodus NL, Farris AD, Segal BM, Jonsson R, Lessard CJ, Scofield RH, Sivils KL. Comparison of the American-European Consensus Group Sjogren's syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann Rheum Dis. 2014;73:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Wallace ZS, Naden RP, Chari S, Choi HK, Della-Torre E, Dicaire JF, Hart PA, Inoue D, Kawano M, Khosroshahi A, Lanzillotta M, Okazaki K, Perugino CA, Sharma A, Saeki T, Schleinitz N, Takahashi N, Umehara H, Zen Y, Stone JH; Members of the ACR/EULAR IgG4-RD Classification Criteria Working Group. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. 2020;79:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in RCA: 374] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 17. | Jiang ZZ, Huang YH, Shen HL, Liu XT. Clinical Applications of Superb Microvascular Imaging in the Liver, Breast, Thyroid, Skeletal Muscle, and Carotid Plaques. J Ultrasound Med. 2019;38:2811-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Hata J. Seeing the Unseen. New Techniques in Vascular Imaging 2014. [cited 14 March 2021]. In: Toshiba Medical Systems Corporation [Internet]. Available from: https://us.medical.canon/download/aplio-500-wp-smi-seeing-the-unseen. [Cited in This Article: ] |
| 19. | Artul S, Nseir W, Armaly Z, Soudack M. Superb Microvascular Imaging: Added Value and Novel Applications. J Clin Imaging Sci. 2017;7:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | He MN, Lv K, Jiang YX, Jiang TA. Application of superb microvascular imaging in focal liver lesions. World J Gastroenterol. 2017;23:7765-7775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Borriello M, Lucidi A, Carbone A, Iannone V, Ferrandina G. Malignant transformation of Madelung's disease in a patient with a coincidental diagnosis of breast cancer: a case report. Diagn Pathol. 2012;7:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Tizian C, Berger A, Vykoupil KF. Malignant degeneration in Madelung's disease (benign lipomatosis of the neck): case report. Br J Plast Surg. 1983;36:187-189. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Sharma N, Hunter-Smith DJ, Rizzitelli A, Rozen WM. A surgical view on the treatment of Madelung's disease. Clin Obes. 2015;5:288-290. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 24. | Pinto CI, Carvalho PJ, Correia MM. Madelung's Disease: Revision of 59 Surgical Cases. Aesthetic Plast Surg. 2017;41:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Enzi G, Busetto L, Ceschin E, Coin A, Digito M, Pigozzo S. Multiple symmetric lipomatosis: clinical aspects and outcome in a long-term longitudinal study. Int J Obes Relat Metab Disord. 2002;26:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Maximiano LF, Gaspar MT, Nakahira ES. Madelung disease (multiple symmetric lipomatosis). Autops Case Rep. 2018;8:e2018030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Suito M, Kitazawa T, Takashimizu I, Ikeda T. Madelung's disease: long-term follow-up. J Surg Case Rep. 2019;2019:rjy356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |