Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.6049
Peer-review started: March 2, 2021
First decision: March 25, 2021
Revised: April 6, 2021
Accepted: May 25, 2021
Article in press: May 25, 2021
Published online: July 26, 2021
Processing time: 148 Days and 19.4 Hours
Disseminated Fusarium is rare in healthy children. Children with hematological tumors may have secondary fungal infections, including Fusarium infections, which are due to tumor bone marrow infiltration or prolonged bone marrow suppression after chemotherapy. Because of the lack of typical clinical manifestations and effective antifungal drugs, early diagnosis and treatment of the disease are difficult, and the prognosis is poor.
The patient in this case was a 13-year-old female child with rash and fever as the first symptoms. She had the characteristics of the four stages of skin that are typical of Fusarium infection. She was diagnosed with disseminated Fusarium infection through skin biopsy and blood culture and diagnosed with Fusarium solani infection based on the morphological characteristics of the blood culture. After treatment with liposome amphotericin B combined with voriconazole, the child recovered.
This case highlights that for children with secondary agranulocytosis after receiving chemotherapy for hematological malignancies, once typical abnormal skin damage is found, the possibility of Fusarium infection should be considered, and voriconazole alone or in combination with polyenes may be the most effective anti-Fusarium drugs. Amphotericin B, the traditional drug of disseminated Fusarium disease, has a high mortality rate, and it is not recommended to use it alone. Adequate neutrophil counts are essential for the treatment of disseminated Fusarium bloodstream infection.
Core Tip: We present herein the case of a Fusarium infection female child with rash and fever as the first symptoms. She had the characteristics of the four stages of skin typical of Fusarium infection. She was diagnosed with disseminated Fusarium infection through blood culture. For children with secondary agranulocytosis after receiving chemotherapy for hematological malignancies, once typical abnormal skin damage is found, the possibility of Fusarium infection should be considered, and the key to treatment is early diagnosis, the reversal of immunosuppression, and the provision of the correct antifungal treatment as soon as possible.
- Citation: Ning JJ, Li XM, Li SQ. Disseminated Fusarium bloodstream infection in a child with acute myeloid leukemia: A case report. World J Clin Cases 2021; 9(21): 6049-6055
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/6049.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.6049
Fusarium was discovered in 1958 and is a common soil mold. It was once considered to be pathogenic only in plants and to be able to cause devastating diseases in plants and crops[1]. However, Cho et al[2] first reported a case of disseminated Fusarium bloods
The patient, a 13-year-old Chinese girl, came to our hospital because she had a pale complexion for 1 mo.
One month prior to presentation, the child appeared pale, which gradually worsened. Laboratory examination indicated the following: White blood cell count (WBC) 1.54 × 109/L, N% 7.9%, hemoglobin (Hb) 47 g/L, and platelets (PLT) 64 × 109/L. The results of bone marrow cell morphology, immunology, and cellular and molecular genetics (MICM typing) confirmed acute myeloid leukemia (type M1). Then, the patient received induction DA chemotherapy (daunorubicin for 3 d and cytarabine for 7 d).
The patient had an unremarkable previous medical history.
The patient's personal and family history was nothing special.
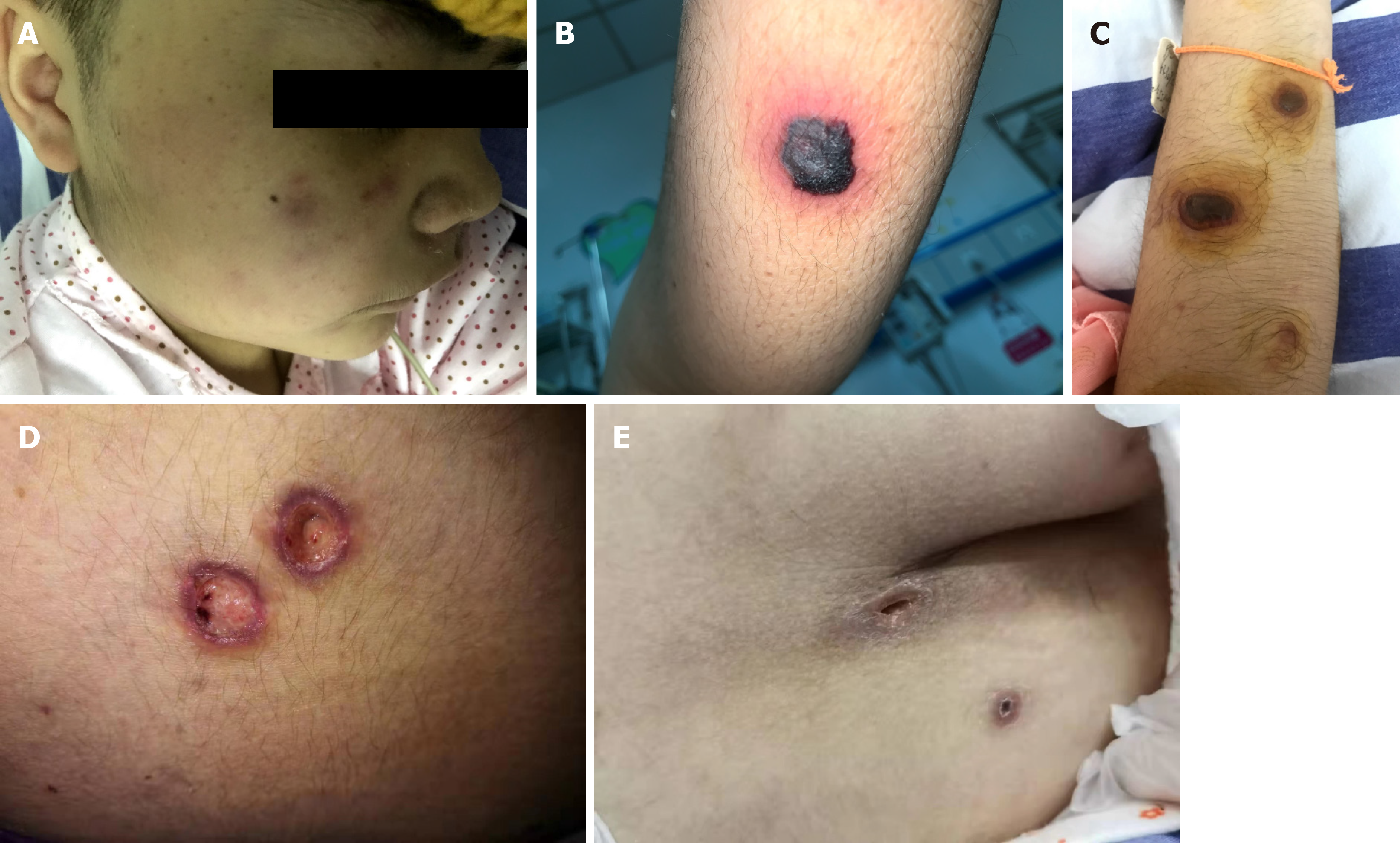
On the third day after induction chemotherapy, the child developed a high fever. Physical examination revealed maculopapules on the face, trunk, and extremities (Figure 1A). The rash was further aggravated, mainly on the trunk, with obvious tenderness, part of the rash showed central necrosis and obvious peripheral swelling (Figure 1B), and part of the rash manifested vesicular formations (Figure 1C). Subsequently, the rash further changed, and the vesicular lesions ruptured to form crater-like ulcers (Figure 1D).
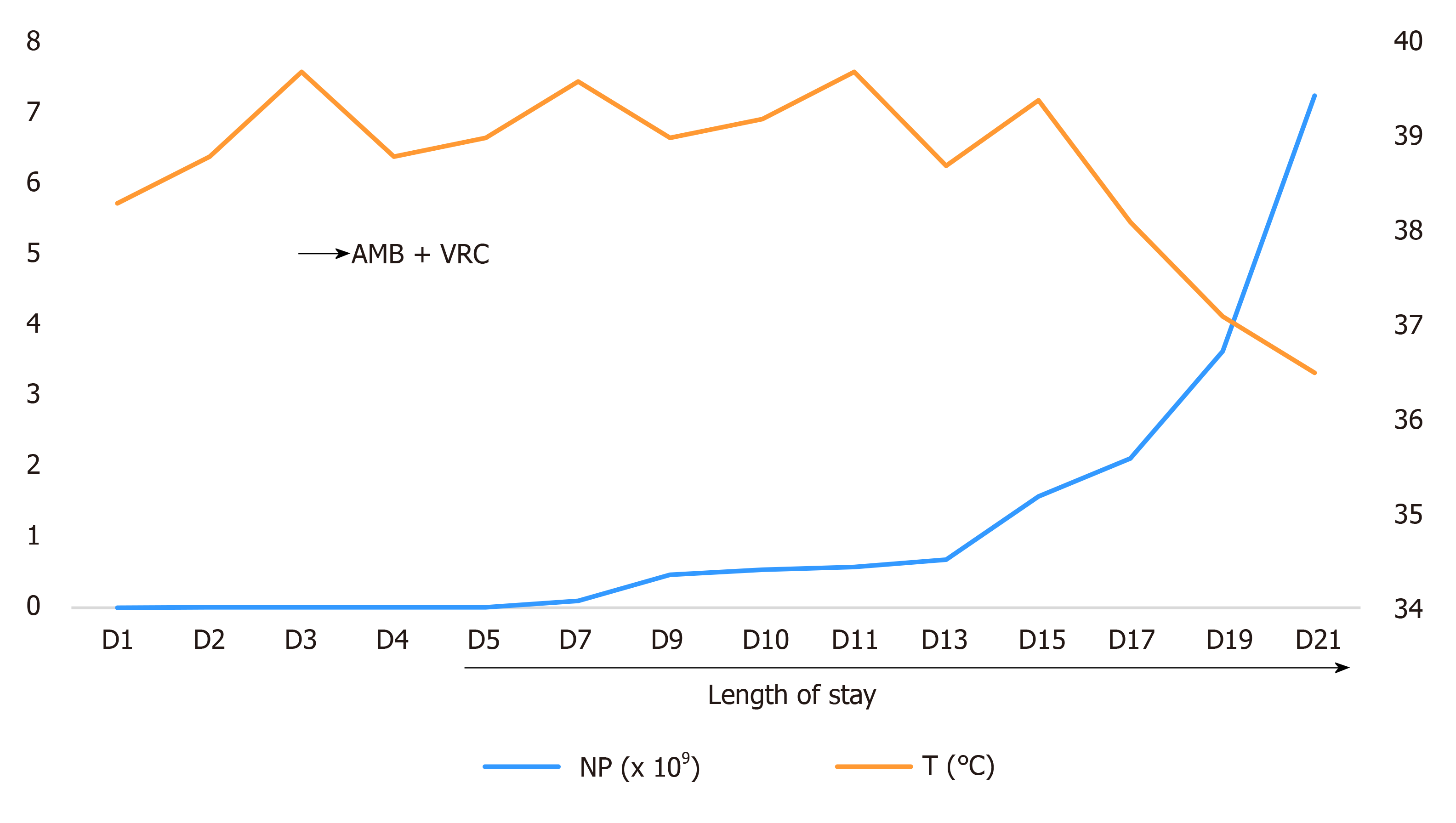
Myelosuppression occurred during chemotherapy, repeated full blood examination demonstrated persistent neutropenia (WBC 0.12 × 109/L and absolute neutrophil count 0.01 × 109/L), and C-reactive protein was 296.5 mg/L. Two repeated fungal antigen tests [galactomannan (GM) and β-D-glucan] were negative.
Computed tomography (CT) examination of the chest and abdomen showed no involvement of deep organs.
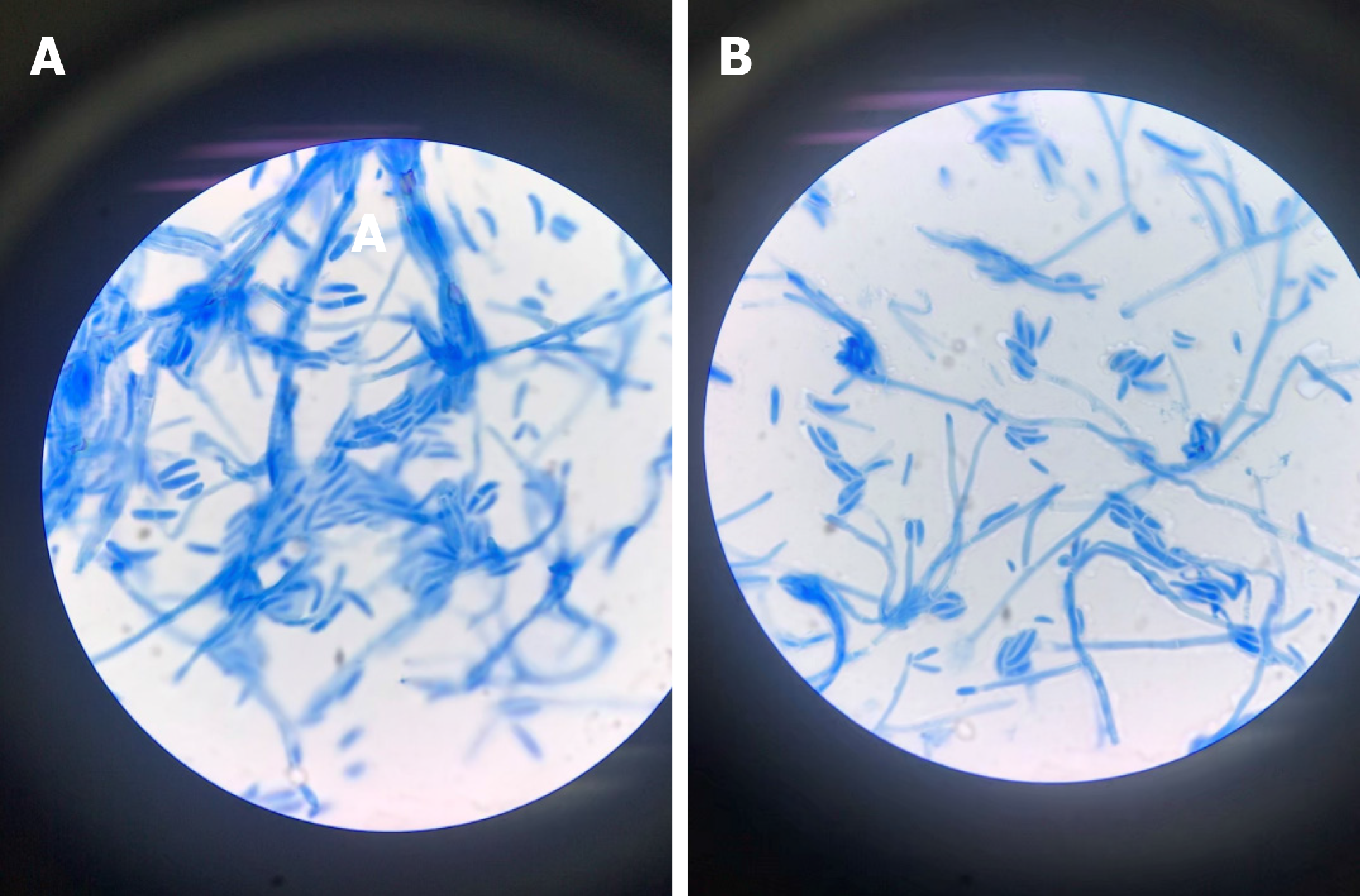
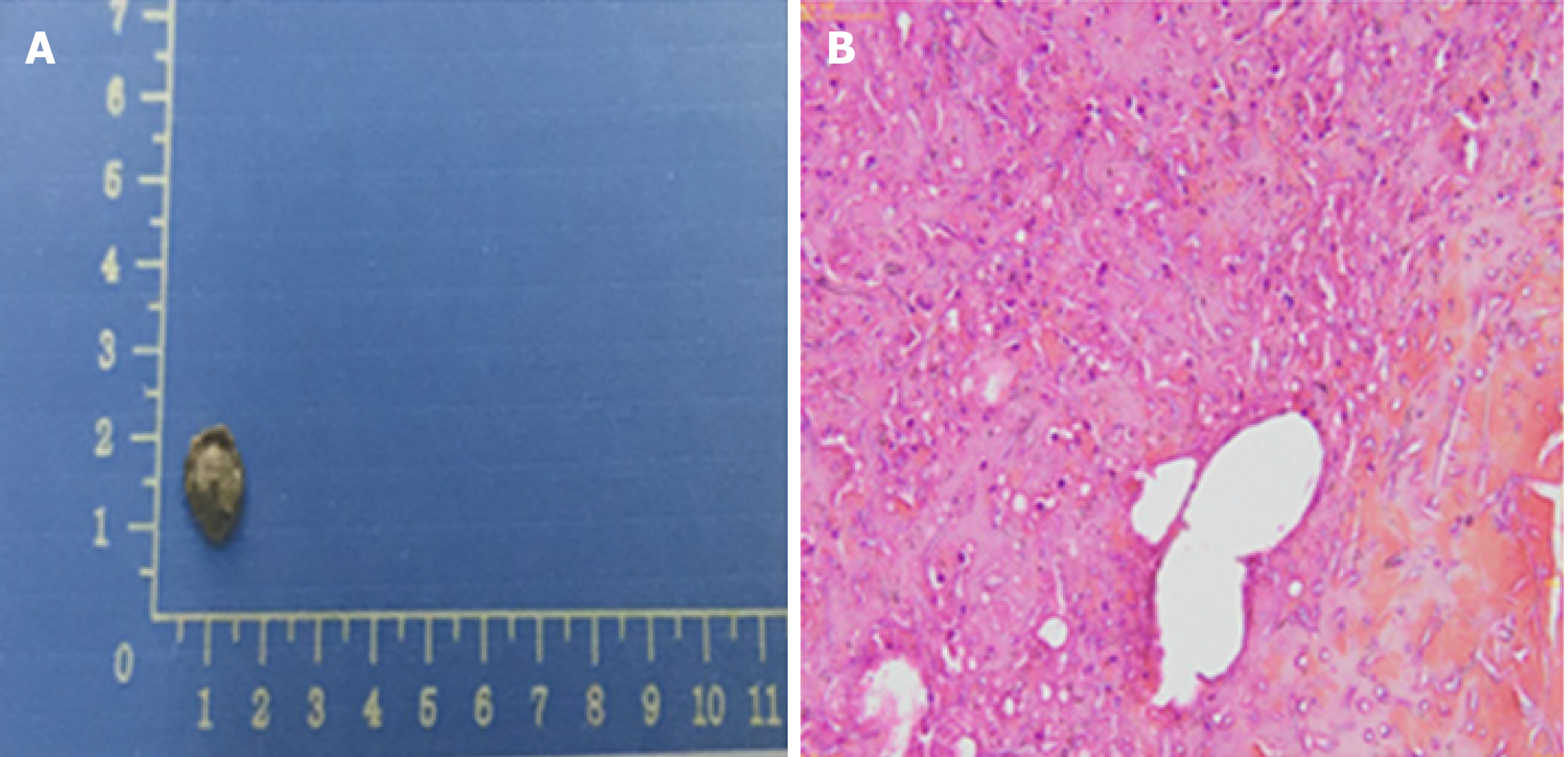
The blood culture grew a fungus, which was ultimately identified as Fusarium solani by morphological characteristics (Figure 2). Fungi were seen on the biopsy of the exfoliated scab (Figure 3).
The final diagnosis of the presented case was disseminated Fusarium bloodstream infection.
This child had febrile neutropenia, combined with skin rash as the main clinical manifestation. On the basis of imipenem and cilastatin sodium anti-infection, vancomycin was added to cover Gram-positive microorganisms. Fluconazole was used to prevent fungal infection, and granulocyte colony-stimulating factor was used to promote the recovery of hematopoietic function. However, the rash was further aggravated, and the child still suffered from repeated high fever and altered conscious state and feeding. The antibacterial drugs imipenem and cilastatin sodium and vancomycin continued to be given to treat infection. Considering the high drug-resistance rate of Fusarium and the poor prognosis of patients with low immunity and disseminated Fusarium infection, fluconazole was changed to voriconazole and liposomal amphotericin B to ensure that at least one drug was effective (Figure 4). After the body temperature stabilized, the antibiotic regimen was downgraded to cefoperazone sodium and sulbactam sodium for continued prevention of infection, and the administration of liposomal amphotericin B combined with voriconazole antifungal treatment was continued for 4 wk.
The child had a fever for 14 d. After that, the body temperature was normal, and the necrotic rash gradually healed (Figure 1E). Routine blood re-examination showed the following: WBC 8.76 × 109/L, neutrophil 7.26 × 109/L, Hb 70 g/L, PLT 35 × 109/L, and CRP 88.7 mg/L. However, before discharge, a 4 cm × 1.5 cm mass was palpable in the right calf of the child, and the tenderness was obvious. B-scan ultrasonography showed the formation of a local abscess, and CT examination of the chest and abdomen showed no involvement of deep organs. The discharge instructions were to continue posaconazole oral suspension combined with cefixime for anti-infection treatment for 2 wk. Eventually, the clinical symptoms of the child disappeared, the blood culture was negative on re-examination, and a clinical cure was achieved. There were no obvious adverse drug reactions during the treatment. However, the second cycle of chemotherapy was delayed because of the fungal infection. At present, the child is generally in good condition, and a second round of chemotherapy is planned.
Fusarium is a saprophytic fungus with strong ecological adaptability. It is widely distributed in natural soils and plants and can even live in deserts and Arctic regions. It can be parasitic on animal and plant bodies or saprophytic in animal and plant debris. Some species can produce mycotoxins under certain circumstances and threaten the health of humans and animals[4]. A multicenter retrospective study indicated a significantly increased incidence of Fusarium infection in patients with immunosuppression and acute myeloid leukemia, and this type of infection ranks second only to Aspergillus infection[5]. For people with normal immunity, Fusarium is an opportunistic pathogen, mainly causing local infections[6], but for patients with hematological malignancies, aplastic anemia, and organ transplantation or undergoing chemotherapy, Fusarium can cause invasive fungal infections. Among these patients with low immune function, disseminated Fusarium disease accounts for 70% of Fusarium infections[7].
In immunocompromised hosts, the lack of a normal immune response can lead to vascular infiltration of Fusarium, leading to necrosis and diffuse infection, with skin involvement in 70% of cases of Fusarium infection[8]. The characteristic skin lesions may provide guidance for the early diagnosis of Fusarium infection. A typical Fusarium infection-induced rash can be divided into four periods: Stage I, red maculopapular rash; stage II, necrosis in the center of the rash; stage III, vesicular bullae changes secondary to necrosis; and stage IV, after the eruption, the vesicular lesions change to cratered lesions and eventually heal. Each period of skin change is 1-2 d apart[9]. The rash in this case showed typical four-stage manifestations. This study found that the four stages of Fusarium skin lesions can coexist, and in stage III, the vesicular lesions presented as thick-walled blisters with cloudy pus, and pain was obvious during the rash. Although the rash caused by Fusarium infection is specific, it still needs to be distinguished from the rash of patients with neutropenia, including leukemic deposits, viral infections, bacterial infections (ecthyma gangrenosum), and other fungi (Aspergillus, mucormycosis, and Candida) causing skin lesions.
Fusarium infection is difficult to cure and has a poor prognosis; the overall mortality is as high as 50%-70%[10], and there is no consensus on the management of invasive disseminated Fusarium infection in the case of neutropenia. At present, the key to successful treatment is early diagnosis, the reversal of immunosuppression, and the administration of the correct antifungal treatment as soon as possible; however, Fusarium has shown widespread resistance to antifungal drugs in vitro. In vitro studies suggest that fluconazole, itraconazole, and flucytosine are not effective against Fusarium, while ketoconazole, miconazole, terbinafine, and voriconazole have limited efficacy. Amphotericin B is considered to be the most effective anti-Fusarium drug in vitro. However, Nucci et al[7] showed that the mortality rate of amphotericin B in the treatment of disseminated Fusarium was as high as 70%. The current successful treatment often comes from cases of voriconazole, so voriconazole alone or in combination with polyenes has been increasingly used for Fusarium infections. Because of the high drug resistance rate of Fusarium, the use of a single drug for the treatment of drug-resistant fungi cannot achieve a satisfactory effect, but a major concern for combination therapy is the potential antagonistic effect between antifungal agents. Some studies have shown that liposomal amphotericin B combined with voriconazole has a synergistic effect in the treatment of drug-resistant fungi[11]. Ho et al[12] also pointed out that for patients with increased skin lesions or continuous positive blood culture in monotherapy, the combination of the two drugs for treatment could be considered.
Other studies have proposed that granulocyte recovery is the most important factor for successful treatment of invasive Fusarium infection. For patients with agranulocytosis, even if systemic antifungal drugs are given, the fatality rate is still close to 100%[13]. Therefore, colony-stimulating factor (G-CSF) and/or GM-CSF should be given to promote or maintain the recovery of hematopoietic function. Another finding suggests that surgical removal of focal lesions may result in better outcomes in patients with skin or disseminated infections[3].
For children with secondary agranulocytosis after receiving chemotherapy for hematological malignancies, once the typical abnormal skin damage is found, it should be alert to the possibility of Fusarium infection, and the key to treatment is early diagnosis, reversal of immunosuppression, and correct antifungal treatment as soon as possible. Voriconazole alone or in combination with polyenes may be the most effective anti-Fusarium drugs.
All authors thank all doctors and nurses of PICU.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Niu J S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Urbaniak C, Dadwal S, Bagramyan K, Venkateswaran K. Draft Genome Sequence of a Clinical Isolate of Fusarium fujikuroi Isolated from a Male Patient with Acute Myeloid Leukemia. Genome Announc. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Cho CT, Vats TS, Lowman JT, Brandsberg JW, Tosh FE. Fusarium solani infection during treatment for acute leukemia. J Pediatr. 1973;83:1028-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Muhammed M, Anagnostou T, Desalermos A, Kourkoumpetis TK, Carneiro HA, Glavis-Bloom J, Coleman JJ, Mylonakis E. Fusarium infection: report of 26 cases and review of 97 cases from the literature. Medicine (Baltimore). 2013;92:305-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Antonissen G, Martel A, Pasmans F, Ducatelle R, Verbrugghe E, Vandenbroucke V, Li S, Haesebrouck F, Van Immerseel F, Croubels S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins (Basel). 2014;6:430-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, Pastore D, Picardi M, Bonini A, Chierichini A, Fanci R, Caramatti C, Invernizzi R, Mattei D, Mitra ME, Melillo L, Aversa F, Van Lint MT, Falcucci P, Valentini CG, Girmenia C, Nosari A. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Musa MO, Al Eisa A, Halim M, Sahovic E, Gyger M, Chaudhri N, Al Mohareb F, Seth P, Aslam M, Aljurf M. The spectrum of Fusarium infection in immunocompromised patients with haematological malignancies and in non-immunocompromised patients: a single institution experience over 10 years. Br J Haematol. 2000;108:544-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Nucci M, Marr KA, Queiroz-Telles F, Martins CA, Trabasso P, Costa S, Voltarelli JC, Colombo AL, Imhof A, Pasquini R, Maiolino A, Souza CA, Anaissie E. Fusarium infection in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2004;38:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Boutati EI, Anaissie EJ. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood. 1997;90:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35:909-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 264] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Nambiar P, Cober E, Johnson L, Brizendine KD. Fatal Fusarium infection manifesting as osteomyelitis following previous treatment with amphotericin B in a multi-visceral transplant: Case report and review of Fusarium infections in solid organ transplantation. Transpl Infect Dis. 2018;20:e12872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Martin-Vicente A, Guarro J, Capilla J. Does a triple combination have better activity than double combinations against multiresistant fungi? Experimental in vitro evaluation. 49:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Ho DY, Lee JD, Rosso F, Montoya JG. Treating disseminated fusariosis: amphotericin B, voriconazole or both? Mycoses. 2007;50:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 666] [Article Influence: 37.0] [Reference Citation Analysis (0)] |