Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.6017
Peer-review started: January 31, 2021
First decision: May 1, 2021
Revised: May 7, 2021
Accepted: May 26, 2021
Article in press: May 26, 2021
Published online: July 26, 2021
Processing time: 170 Days and 16.9 Hours
Myeloid sarcoma (MS) rarely occurs in acute promyelocytic leukemia (APL) at onset, but it can develop in relapse cases, especially after APL treated with all-trans retinoic acid (ATRA). Therefore little is known about the clinical features and suitable treatment for APL related MS due to the rarity of the disease, although this may be different from the treatment and prognosis of MS in the relapse stage. To our best knowledge, this is the second case report of APL initial presentation as colon MS.
A 77-year-old woman complained of intermittent right lower abdominal pain, black stool, and difficult defecation for 2 mo. Physical examination showed diffuse tenderness during deep palpation and an anemic appearance. Laboratory findings showed positivity for fecal occult blood testing; white blood cell count: 3.84 × 109/L; hemoglobin: 105 g/L; platelet count: 174 × 109/L; and negativity for tumor markers. Abdominal enhanced computed tomography showed a space occupying lesion in the colon (1.9 cm). Fibrocolonoscopy revealed a polypoid and ulcerated mass measuring 2.5 cm. The tumor was removed. To our surprise, MS was confirmed by immunohistochemistry. PML/RARα fusion gene was detected in colon specimens by fluorescent in situ hybridization and real-time reverse transcription polymerase chain reaction, which was consistent with the bone marrow. She was diagnosed as having APL related MS. A smooth and unobstructed intestinal wall was found by fibrocolonoscopy, and continuous molecular remission was confirmed in both the bone marrow and colon after four courses of ATRA + arsenic trioxide (ATO). ATRA + ATO showed a favorable therapeutic response for both APL and MS.
Early use of ATRA can benefit APL patients, regardless of whether MS is the first or recurrent manifestation.
Core Tip: Myeloid sarcoma (MS) rarely occurs in acute promyelocytic leukemia (APL) at onset. We report the second case of APL with colon MS as the initial presentation. De novo APL related MS is a very rare disease. Its clinical features and prognosis may be different from those of extramedullary disease, and the disease free survival may be shorter than MS occurring in APL relapse. To date, a total of 28 cases of APL with MS as the initial presentation have been reported worldwide. The tumor was removed. Continuous complete remission was achieved after the treatment of all-trans retinoic acid (ATRA) + arsenic trioxide (ATO). After radiotherapy or tumor resection, ATRA + ATO showed a favorable therapeutic response for both APL and MS.
- Citation: Wang L, Cai DL, Lin N. Myeloid sarcoma of the colon as initial presentation in acute promyelocytic leukemia: A case report and review of the literature. World J Clin Cases 2021; 9(21): 6017-6025
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/6017.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.6017
Myeloid sarcoma (MS) may exist as an independent tumor, or as an extramedullary disease (EMD) developing in patients with acute myeloid leukemia (AML)[1], especially M2, M4, and M5 subtypes. MS is an extramedullary tumor consisting of immature myeloid cells. It can occur in any part of the body, in particular the skin, bone, lymph nodes, soft tissue, testis, and gastrointestinal tract. MS may develop de novo or even concurrently with 3%-8% of AML patients. It may be the only initial manifestation of relapse in AML patients regardless of the examination results of blood and bone marrow. MS rarely occurs in acute promyelocytic leukemia (APL) at onset[2-4], but it can develop in relapse cases, especially after APL treated with all trans retinoic acid (ATRA)[5]. de Botton et al[3] have reported the association between treatment with ATRA and extramedullary relapses. What‘s more, de novo MS as the first manifestation of APL is really a rare event, which occurs in less than 1% of EMD cases[6,7]. Therefore, little is known about the clinical features and suitable treatment for APL related MS due to the rarity of the disease, although these may be different from those of MS in the relapse stage. To our best known, this is the second case of APL with colon MS as the initial presentation.
A 77-year-old woman complained of intermittent right lower abdominal pain, black stool, and difficult defecation for 2 mo.
The patient’s uncomfortable symptoms started 2 mo ago, which had worsened over the last week.
The patient’s medical history included diabetes and hypertension for 3 years.
The patient’s mother had a history of hypertension.
The patient’s body temperature was 36.9 °C, tachycardia was 101 bpm, respiratory rate was 20 breaths/min, and blood pressure was 145/75 mmHg. Physical examination showed muscle tension, Murphy's sign and voiced mobility was negative, but diffuse tenderness during deep palpation and bowel sounds were observed 3 times per minute, together with an anemic appearance.
Laboratory findings showed positivity for fecal occult blood testing; serum amylase: 29 U/L; serum lipase: 14.8 U/L; white blood cell (WBC) count: 3.84 × 109/L; hemoglobin: 105 g/L; platelet count: 174 × 109/L; and negativity for tumor markers.
Hepatomegaly and splenomegaly were not found by Doppler ultrasound of the abdomen. Abdominal enhanced computed tomography showed a space occupying lesion in the colon (1.9 cm), with obvious enhancement. Fibrocolonoscopy revealed a polypoid and ulcerated mass measuring 2.5 cm, with hyperemia and erosion of the ileocecal mucosa, irregular ulcer, uneven bottom, annular lesions in the mucosa, and moderate to severe inflammatory cell infiltration. Part of the tumor was removed for biopsy.
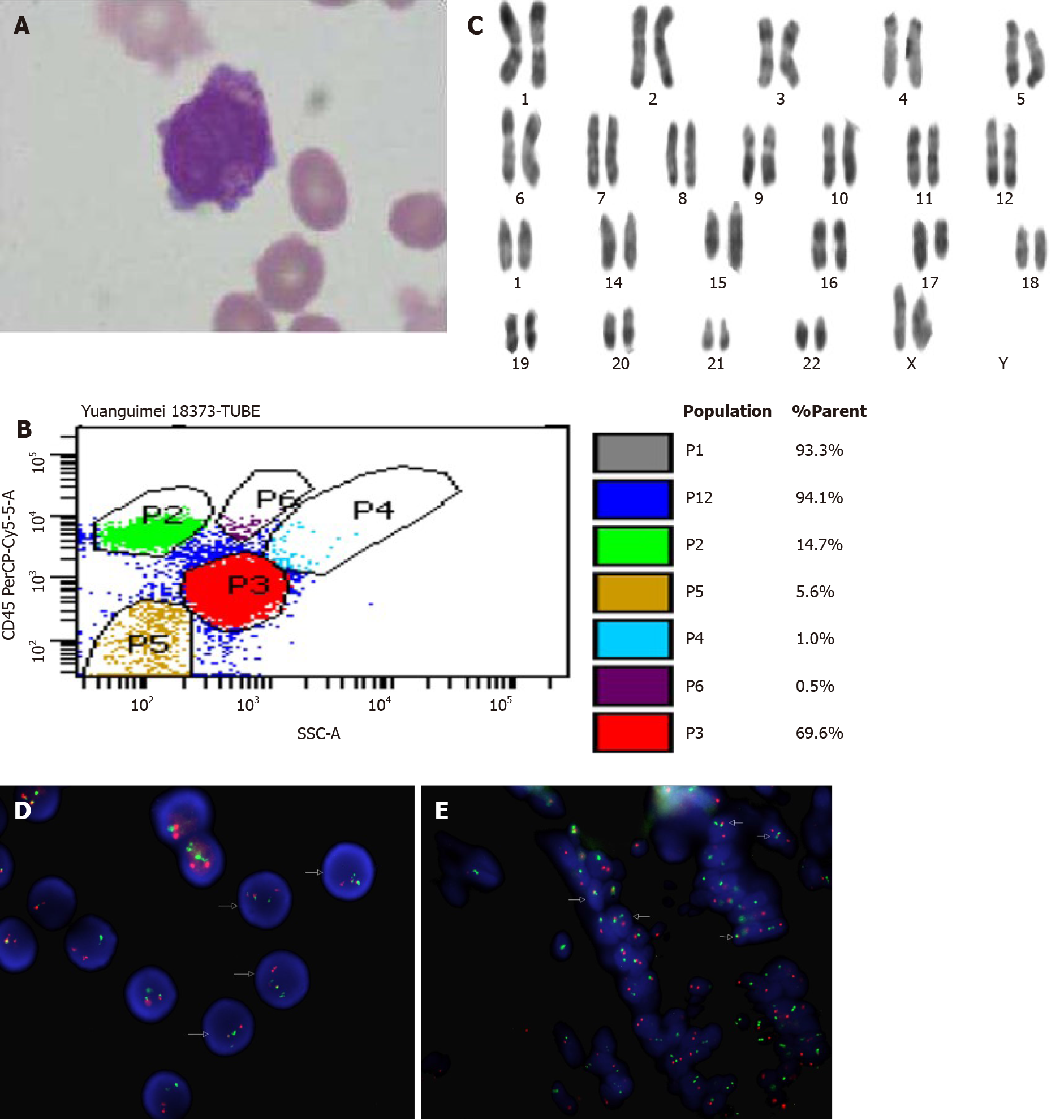
To our surprise, MS was confirmed by immunohistochemistry using a panel of myeloid cell surface marker antibodies, which revealed CK+, CD68+, CgA+, CD117+, and MPO+. The Ki67 index was 65%, and B-cell and T cell lymphomas were excluded by negative staining for CD20 and CD3. Subsequently, bone marrow aspiration revealed 68% of blasts, and the patient was diagnosed with APL based on the morphology (Figure 1A) and immunohistochemistry (CD33+, CD117+, CD13+, CD64+, MPO+, CD9+, CD34-, CD19-, CD10-, and cCD79a-) (Figure 1B). Cytogenetics revealed a karyotype with t (15; 17) (q22; q21) (Figure 1C), and a real-time reverse transcription polymerase chain reaction assay showed a typical PML/RARα fusion, which further confirmed the diagnosis. Fluorescence in situ hybridization (FISH) using a PML/RARα dual-color, dual-fusion probe showed PML/RARα fusion signals (Figure 1D). PML/RARα fusion gene was also detected in colon specimens by FISH and real-time reverse transcription polymerase chain reaction (PCR), which was consistent with the bone marrow (Figure 1E). The final diagnosis was APL related MS (MS/APL).
After two courses of ATRA + arsenic trioxide (ATO) treatment, the patient’s symptoms improved, and laboratory examination showed improvement of anemia. PCR analysis for PML/RARα transcription and FISH using a PML/RARα dual-color, dual-fusion probe on bone marrow cells confirmed molecular remission. Meanwhile, colon ulcerative lesions improved significantly, as revealed by fibrocolonoscopy.
Continuous molecular remission was confirmed in both the bone marrow and colon. A smooth and unobstructed intestinal wall was found by fibrocolonoscopy without ulcer or polypoid masses after four courses of ATRA + ATO. ATRA + ATO showed a favorable therapeutic response for both APL and MS.
MS as an initial presentation of APL is an extremely rare event, and more than 95% of cases occur at the time of relapse, especially after ATRA treatment[8]. High WBC count is suggested as a risk factor[7]. ATRA enhances the migration and adhesion of extramedullary tissues by increasing the adhesion molecules of leukemia cells in vitro, which explains why the number of patients with extramedullary relapse is increasing[9]. However, the risk of EMD after treatment with ATRA is not increased compared with chemotherapy alone in a large cohort study[10]. MS has the tendency to develop into AML, and most of the untreated MS cases transformed into acute leukemia within 6 mo. So comprehensive and careful examination of the bone marrow smear and biopsy is important for patients with MS in order to rule out bone marrow involve
De novo MS/APL is a very rare disease. Its clinical features and prognosis may be different from those of EMD, and the disease free survival may be shorter than MS occurring in APL relapse. To date, a total of 28 cases of APL with MS as the initial presentation have been reported worldwide (Table 1)[17-41]. The average age of onset was 35 (1-77) years, and sex-bias phenomenon (only 8 female) was difficult to explain due to the limited number of patients. The sites of de novo MS were widely distributed, and the most common sites were vertebra (6 cases) and extradural (6 cases), followed by the intestine, tongue, pelvis, skull, pleura, hip, mandible, spinal, humerus, tibia, femur, sternum, skin, mediastinal, thymus, cerebellum, and testicle. A single site was involved in most cases (16/28), and multiple site MS occurred in a few APL patients. Only one patient carried a complex karyotype, and PML/RARα was detected in de novo MS/APL in 22 patients. Bone marrow involvement was found in most patients (18/28), and 6 of the remainders developed bone marrow involvement within 1-16 mo. Increased/decreased WBC count was only seen in nine cases, the remainders’ (19/28) WBC count was normal at the time of onset. In addition, the coagulation dysfunction characteristic was observed in only six patients. The optimum therapy for MS/APL is also unclear. Twenty de novo MS/APL patients received ATRA with chemotherapy; 16 achieved complete remission (CR), two achieved PR, and the other two died of sepsis and cerebral hemorrhage, respectively. On the other hand, eight patients were treated only with chemotherapy without ATRA or ATO; two achieved CR, one achieved PR, and the remaining five died. The longest follow-up until now was 96 mo, and in this case, the patient was treated with radiotherapy + ATRA + chemotherapy, although radiotherapy or tumor resection together with ATRA and chemotherapy may improve the prognosis of MS/APL. MS/APL is a kind of disease with diverse clinical manifestations, molecular biology, and cytogenetics, is easily confused with stromal tumor, lymphoma, and carcinoma, and is therefore associated with a high misdiagnosis rate, poor prognosis, and high recurrence rate. Our patient complained of intermittent right lower abdominal pain, black stool, and difficult defecation, which reminded us of the possibility of gastrointestinal tumor. MS occurring in the gastrointestinal tract is relatively rare when MS occurs as the first presentation in acute promyelocytic leukemia, and only four cases have been described in the literature (Table 1, cases 25-27 and current case). Abdominal pain, change in bowel habit, and small bowel obstruction were the most common clinical presentation. In conclusion, gastro
| Case | Age/sex | Location | Single or multiple sites | Chromosome | Gene | BM involvement | Progression to APL | Coagulation abnormality | WBC | Treatment | Prognosis | Ref. |
| 1 | 55/M | Vertebra, extradural | Multiple | - | PML/RARα | No | No | No | Normal | ATRA, chemotherapy, radiation | CR (15 mo, ongoing) | Fiegl et al[17] |
| 2 | 26/M | Vertebra, extradural | Multiple | t (15; 17) | PML/RARα | Yes | - | No | Low | ATRA, chemotherapy | CR (22 wk, ongoing) | Specchia et al[10] |
| 3 | 61/F | Vertebra | Single | - | PML/RARα | No | No | - | - | Radiotherapy, ATRA, chemotherapy | CR (96 mo, ongoing) | Piñán et al[18] |
| 4 | 52/F | Vertebra | Multiple | t (15; 17) | PML/RARα | Yes | - | No | Normal | ATRA, chemotherapy | CR (54 mo, ongoing) | Cornfield et al[19] |
| 5 | 56/M | Vertebra | Single | t (15;17) | PML/RARα | Yes | - | No | Normal | ATRA, chemotherapy, ASCT, ATO | CR (24 mo, ongoing) | Shah et al[20] |
| 6 | 50/F | Vertebra | Single | t (15; 17) | PML/RARα | Yes | - | - | Low | ATRA, ATO, chemotherapy | CR (44 d, ongoing) | Mori et al[21] |
| 7 | 31/M | Extradural | Multiple | - | - | No | Yes (32 d) | Yes | Normal | Radiotherapy, ASCT | PR (23 mo, ongoing) | Zuiable et al[22] |
| 8 | 53/M | Extradural | Single | t (15; 17) | PML/RARα | Yes | - | Yes | Normal | Radiotherapy, ATRA, chemotherapy | No improvement, died of sepsis | Bittencourt et al[23] |
| 9 | 18/M | Extradural | Multiple | t (15; 17) | - | No | Yes (10 mo) | No | Normal | Chemotherapy | CR (11mo, ongoing) | Savranlar et al[24] |
| 10 | 27/M | Extradural | Single | t (15; 17) | PML/RARα | Yes | - | Yes | Normal | Tumor removed, ATRA, chemotherapy, radiotherapy | CR (293 d, ongoing) | Tosi et al[25] |
| 11 | 4/M | Pelvis | Single | - | - | Yes | - | Yes | High | Chemotherapy | CR (14 mo, ongoing) | Belasco et al[26] |
| 12 | -/M | Skull, pleura, hip | Multiple | t (15; 17) | PML/RARα | No | No | - | Normal | ATRA, chemotherapy | CR (13 mo, ongoing) | Bobbio-Pallavicini et al[27] |
| 13 | 16/F | Humerus, tibia, femur | Multiple | t (15; 17) | PML/RARα | Yes | - | No | Normal | ATRA, chemotherapy | CR (ongoing) | Fiegl et al[17] |
| 14 | 1/M | Mandible | Single | - | PML/RARα | Yes | No | - | ATRA, chemotherapy | CR (12 mo, ongoing) | Yamashita et al[28] | |
| 15 | 50/M | Spinal | Multiple | (47, XY, +8, der (11; 22) (q10; q10), add (14) (q32), der (15) t (15; 17) (q22; q12), ider (17) (q10) t (15; 17)) | PML/RARα | No | Yes (10 mo) | No | Normal | Chemotherapy | Died of a brain hemorrhage (40 mo) | Yamashita et al[29] |
| 16 | 19/M | Sternum | Single | t (15; 17) | PML/RARα | No | No | No | Normal | Tumor removed, ATRA, chemotherapy | CR (24 wk, ongoing) | Thomas et al[30] |
| 17 | 45/M | Tongue | Single | t (15; 17) | PML/RARα | Yes | - | - | High | ATRA, chemotherapy | CR (12 mo, ongoing) | Mohamedbhai et al[31] |
| 18 | 35/M | Tongue | Single | t (15; 17) | PML/RARα | Yes | - | No | High | ATRA, chemotherapy | CR (ongoing) | Ignacio-Cconchoy et al[32] |
| 19 | 34/M | Skin | Multiple | - | - | Yes | - | Yes | High | Chemotherapy | Died (1 mo) | Uematsu et al[33] |
| 20 | 23/M | Mediastinal | Single | - | - | No | Yes (2 mo) | No | Normal | Radiotherapy, chemotherapy | Died of heart failure (14 mo) | Kubonishi et al[34] |
| 21 | 21/M | Thymus | Multiple | - | - | Yes | - | No | High | Chemotherapy | Died (8 mo) | Ajarim et al[35] |
| 22 | 27/m | Testicle | Multiple | - | PML/RARα | No | Yes (16 mo) | No | Normal | Radiotherapy, ATRA, chemotherapy | PR (16 mo, ongoing) | Gopal et al[36] |
| 23 | 39/F | Cerebellum | Single | t (15; 17) | PML/RARα | Yes | - | Yes | High | Chemotherapy | Die of cerebral hemorrhage | Fukushima et al[37] |
| 24 | 26/F | Ovary | Single | - | PML/RARα | No | Yes | No | Normal | Chemotherapy, ATRA | PR (43 mo, ongoing) | Wang et al[38] |
| 25 | 17/F | Rectum | Single | t (15; 17) | PML/RARα | Yes | - | - | Normal | ATRA, chemotherapy | CR (48 mo, ongoing) | Benjazia et al[39] |
| 26 | 66/M | Small intestine | Single | t (15; 17) | PML/RARα | Yes | - | No | Normal | ATRA, chemotherapy | Died of cerebral hemorrhage | Takeh et al[40] |
| 27 | 29/M | Colon | Multiple | t (15; 17) | PML/RARα | Yes | - | No | Low | ATRA, chemotherapy | CR (2 mo, ongoing) | Damodar et al[41] |
| Current case | 77/F | Colon | Single | t (15; 17) | PML/RARα | Yes | - | No | Normal | Tumor removed, ATRA, ATO | CR (6 mo, ongoing) |
To our knowledge, this is the second case report of APL with colon MS the initial presentation. De novo MS/APL is a very rare disease. Its clinical features and prognosis may be different from those of EMD in APL relapse. Once PML/RARα fusion gene is found in de novo MS, it is recommended to use ATRA treatment and pay attention to monitoring the condition of peripheral blood and bone marrow. Early use of ATRA can benefit the APL patients, and radiotherapy or tumor resection together with ATRA and chemotherapy may improve the prognosis of MS/APL, regardless of whether MS is the first or recurrent manifestation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nagahara H S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Polyatskin IL, Artemyeva AS, Krivolapov YA. [Revised WHO classification of tumors of hematopoietic and lymphoid tissues, 2017 (4th edition):lymphoid tumors]. Arkh Patol. 2019;81:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Wiernik PH, De Bellis R, Muxi P, Dutcher JP. Extramedullary acute promyelocytic leukemia. Cancer. 1996;78:2510-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | de Botton S, Sanz MA, Chevret S, Dombret H, Martin G, Thomas X, Mediavilla JD, Recher C, Ades L, Quesnel B, Brault P, Fey M, Wandt H, Machover D, Guerci A, Maloisel F, Stoppa AM, Rayon C, Ribera JM, Chomienne C, Degos L, Fenaux P; European APL Group; PETHEMA Group. Extramedullary relapse in acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Leukemia. 2006;20:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Weiss MA, Warrell RP Jr. Two cases of extramedullary acute promyelocytic leukemia. Cytogenetics, molecular biology, and phenotypic and clinical studies. Cancer. 1994;74:1882-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Ganzel C, Douer D. Extramedullary disease in APL: a real phenomenon to contend with or not? Best Pract Res Clin Haematol. 2014;27:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Liso V, Specchia G, Pogliani EM, Palumbo G, Mininni D, Rossi V, Teruzzi E, Mestice A, Coppi MR, Biondi A. Extramedullary involvement in patients with acute promyelocytic leukemia: a report of seven cases. Cancer. 1998;83:1522-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Vega-Ruiz A, Faderl S, Estrov Z, Pierce S, Cortes J, Kantarjian H, Ravandi F. Incidence of extramedullary disease in patients with acute promyelocytic leukemia: a single-institution experience. Int J Hematol. 2009;89:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Kyaw TZ, Maniam JA, Bee PC, Chin EF, Nadarajan VS, Shanmugam H, Kadir KA. Myeloid sarcoma: an unusual presentation of acute promyelocytic leukemia causing spinal cord compression. Turk J Haematol. 2012;29:278-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Cunha De Santis G, Tamarozzi MB, Sousa RB, Moreno SE, Secco D, Garcia AB, Lima AS, Faccioli LH, Falcão RP, Cunha FQ, Rego EM. Adhesion molecules and Differentiation Syndrome: phenotypic and functional analysis of the effect of ATRA, As2O3, phenylbutyrate, and G-CSF in acute promyelocytic leukemia. Haematologica. 2007;92:1615-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Specchia G, Lo Coco F, Vignetti M, Avvisati G, Fazi P, Albano F, Di Raimondo F, Martino B, Ferrara F, Selleri C, Liso V, Mandelli F. Extramedullary involvement at relapse in acute promyelocytic leukemia patients treated or not with all-trans retinoic acid: a report by the Gruppo Italiano Malattie Ematologiche dell'Adulto. J Clin Oncol. 2001;19:4023-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S, Thomas DA, Garcia-Manero G, Ferrajoli A, Manning JT, Keating MJ, Albitar M, O'Brien S, Giles FJ. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003;17:1100-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Avni B, Rund D, Levin M, Grisariu S, Ben-Yehuda D, Bar-Cohen S, Paltiel O. Clinical implications of acute myeloid leukemia presenting as myeloid sarcoma. Hematol Oncol. 2012;30:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Shimizu H, Saitoh T, Tanaka M, Mori T, Sakura T, Kawai N, Kanda Y, Nakaseko C, Yano S, Fujita H, Fujisawa S, Miyawaki S, Kanamori H, Okamoto S. Allogeneic hematopoietic stem cell transplantation for adult AML patients with granulocytic sarcoma. Leukemia. 2012;26:2469-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Worch J, Ritter J, Frühwald MC. Presentation of acute promyelocytic leukemia as granulocytic sarcoma. Pediatr Blood Cancer. 2008;50:657-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Menasce LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology. 1999;34:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero D, Bisceglia M, Ponzoni M, Gentile A, Rinaldi P, Franco V, Vincelli D, Pileri A Jr, Gasbarra R, Falini B, Zinzani PL, Baccarani M. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 445] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 17. | Fiegl M, Rieger C, Braess J, Haferlach T, Schnittger S, Schoch C, Hiddemann W, Ostermann H. Isolated epidural chloroma with translocation t(15; 17) successfully treated with chemotherapy and all-trans-retinoic acid. Br J Haematol. 2003;122:688-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Piñán MA, Ardanaz MT, Guinea JM, García-Ruiz JC. Myeloid sarcoma preceding an acute promyelocytic leukaemia with neuromeningeal infiltration. Ann Hematol. 2014;93:339-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Cornfield D, Gheith S, Barron L. Promyelocytic sarcoma presenting with spinal cord compression and treated successfully with surgical debulking and the PETHEMA regimen for acute promyelocytic leukemia. Case Rep Clin Pathol. 2015;2:12-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Shah NN, Stonecypher M, Gopal P, Luger S, Bagg A, Perl A. Acute promyelocytic leukemia presenting as a paraspinal mass. J Community Support Oncol. 2016;14:126-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Mori, Shahram, Laloui, Lamia, Patel, Rushang D, Shen Q, Ahmed Wesam. Sternal Soft Tissue Mass as Initial Presentation in a Case of Acute Promyelocytic Leukemia (APL). Biol Blood Marrow Transplant. 2017;23:S249. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Zuiable A, Aboud H, Nandi A, Powles R, Treleaven J. Extramedullary disease initially without bone marrow involvement in acute promyelocytic leukaemia. Clin Lab Haematol. 1989;11:288-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Bittencourt H, Teixeira Junior AL, Glória AB, Ribeiro AF, Fagundes EM. Acute promyelocytic leukemia presenting as an extradural mass. Rev Bras Hematol Hemoter. 2011;33:478-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Savranlar A, Ustündag Y, Ozer T, Bayraktaroglu T, Demircan N, Ozdemir H, Borazan A. A thoracic-epidural granulocytic sarcoma case that was diagnosed preceding the onset of and that recurred co-incidental to acute promyelocytic leukemia, which developed after surgical treatment. Acta Med Okayama. 2004;58:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Tosi A, De Paoli A, Fava S, Luoni M, Sironi M, Tocci A, Assi A, Cassi E. Undifferentiated granulocytic sarcoma: a case with epidural onset preceding acute promyelocytic leukemia. Haematologica. 1995;80:44-46. [PubMed] |
| 26. | Belasco JB, Bryan JH, McMillan CW. Acute promyelocytic leukemia presenting as a pelvic mass. Med Pediatr Oncol. 1978;4:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Bobbio-Pallavicini E, Cannatelli G, Motta E, Grassi M, Bergamaschi G, Rosso R, Moroni M. Histologic diagnosis and precocious treatment in a case of isolated promyelocytic sarcoma. Leukemia. 1998;12:2035-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Yamashita Y, Isomura N, Hamasaki Y, Goto M. Case of pediatric acute promyelocytic leukemia presenting as extramedullary tumor of the mandible. Head Neck. 2013;35:E310-E313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Yamashita T, Nishijima A, Noguchi Y, Narukawa K, Oshikawa G, Takano H. Acute promyelocytic leukemia presenting as recurrent spinal myeloid sarcomas 3 years before developing leukemia: A case report with review of literature. Clin Case Rep. 2019;7:316-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Thomas X, Chelghoum Y. Promyelocytic sarcoma of the sternum: a case report and review of the literature. Korean J Hematol. 2011;46:52-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Mohamedbhai S, Pule M, Conn B, Hopper C, Ramsay A, Khwaja A. Acute promyelocytic leukaemia presenting with a myeloid sarcoma of the tongue. Br J Haematol. 2008;141:565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Ignacio-Cconchoy FL, Benites-Zapata VA, Yanac-Avila RL, Vela-Velàsquez CT. Myeloid sarcoma of the tongue as a first manifestation of acute promyelocytic leukemia: A case report. Rep Pract Oncol Radiother. 2020;25:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Uematsu I, Wataya K, Kato K, Yoshimi H, Kubori S. [Case of acute promyelocytic leukemia with leukemia cutis]. Naika. 1970;26:357-362. [PubMed] |
| 34. | Kubonishi I, Ohtsuki Y, Machida K, Agatsuma Y, Tokuoka H, Iwata K, Miyoshi I. Granulocytic sarcoma presenting as a mediastinal tumor. Report of a case and cytological and cytochemical studies of tumor cells in vivo and in vitro. Am J Clin Pathol. 1984;82:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Ajarim DS, Santhosh-Kumar CR, Higgy KE, el Saghir NS, Almomen AK, Shipkey FD. Granulocytic sarcoma of the thymus in acute promyelocytic leukaemia. Clin Lab Haematol. 1990;12:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Gopal S, Marcussen S, Dobin SM, Koss W, Donner LR. Primary myeloid sarcoma of the testicle with t(15;17). Cancer Genet Cytogenet. 2005;157:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Fukushima S, Terasaki M, Tajima Y, Shigemori M. Granulocytic sarcoma: an unusual complication of acute promyelocytic leukemia causing cerebellar hemorrhage. Case report. J Neurosurg. 2006;105:912-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Wang X, Liu H, Wu Z, Xu X, Chen X, Zhai Z, Sun Z. A case of acute promyelocytic leukemia presenting with a nonleukemic granulocytic sarcoma of the ovary, with subsequent development of acute myeloid leukemia associated with t(8;21). Leuk Res. 2009;33:580-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Benjazia E, Khalifa M, Benabdelkader A, Laatiri A, Braham A, Letaief A, Bahri F. Granulocytic sarcoma of the rectum: Report of one case that presented with rectal bleeding. World J Gastrointest Pathophysiol. 2010;1:144-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Takeh H, Farran M, Debaize JP. Granulocytic sarcoma (chloroma) of the small intestine. Acta Chir Belg. 1999;99:78-81. [PubMed] |
| 41. | Damodar S, Prashantha B, Gangoli A, Gopalakrishnan G, Jayanthi KJ. Granulocytic sarcoma of colon in a patient with acute promyelocytic leukemia. Indian J Hematol Blood Transfus. 2013;29:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |