Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.5988
Peer-review started: January 14, 2021
First decision: February 10, 2021
Revised: February 15, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: July 26, 2021
Processing time: 187 Days and 18.2 Hours
Recurrent hepatocellular carcinoma (HCC) with inferior vena cava tumor thrombus is a great challenge for oncologists and has a poor prognosis. To date, the safety and efficacy of programmed cell death ligand 1 (PD-L1) inhibitors are still unknown.
A 59-year-old male was identified as having a tumor thrombus in the inferior vena cava 3 years after surgery. The patient underwent a second surgery and adjuvant chemotherapy. However, the level of alpha-fetoprotein was elevated after 2 mo, and lung metastases and mediastinal lymph node metastases were identified. The expression of PD-L1 in HCC and inferior vena cava tumor thrombus tissues was analyzed by immunohistochemistry. Then, the patient received atezolizumab immunotherapy. The level of alpha-fetoprotein dropped to normal, the mediastinal lymph node metastases decreased in size and the lung metastases disappeared after 3 mo of immunotherapy. The patient had no signs of recurrence at 21 mo of follow-up. A 60-year-old male underwent left hepatic tumor resection, inferior vena cava incision and thrombus removal, followed by regular chemotherapy. The patient developed lung and splenic metastases after surgery. Pembrolizumab was used for six courses, and the splenic metastasis shrank, after which splenectomy was performed. The patient continued to receive pembrolizumab for thirteen courses, and the lung metastases showed no progression. A 34-year-old male was diagnosed with liver cancer with inferior vena cava tumor thrombus. The patient underwent right hepatectomy and received tislelizumab for three courses. He is still receiving immunotherapy and in good condition.
Anti-PD-L1 therapy in HCC patients with inferior vena cava tumor thrombus and metastasis is associated with relatively good patient outcomes.
Core Tip: Recurrent hepatocellular carcinoma (HCC) with inferior vena cava tumor thrombus (IVCTT) is a great challenge for oncologists and has a poor prognosis. The safety and efficacy of programmed cell death ligand 1 inhibitors are still unknown. To the best of our knowledge, the first case is the first report of successful treatment of recurrent and metastatic HCC with inferior vena cava tumor thrombus with atezolizumab. We reported two other cases of HCC with IVCTT at the first diagnosis, for which patients were treated with a programmed cell death protein 1 inhibitor after surgery or recurrence, and both patients achieved a good outcome. Anti-programmed cell death ligand 1 therapy in HCC patients with IVCTT and metastasis is associated with relatively good patient outcomes.
- Citation: Liu SR, Yan Q, Lin HM, Shi GZ, Cao Y, Zeng H, Liu C, Zhang R. Anti-programmed cell death ligand 1-based immunotherapy in recurrent hepatocellular carcinoma with inferior vena cava tumor thrombus and metastasis: Three case reports. World J Clin Cases 2021; 9(21): 5988-5998
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/5988.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.5988
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death worldwide[1]. Surgical resection and liver transplantation are curative methods for HCC, but the recurrence rate at 5 years after surgery is as high as 70%[2]. Additionally, only some patients are eligible for surgical resection or liver transplantation. HCC with inferior vena cava tumor thrombus (IVCTT) shows a poor prognosis with a median survival time of 2-4 mo without treatment[3]. IVCTT may cause sudden death due to complications, such as heart failure and pulmonary embolism. IVCTT can also increase the risk of metastasis as tumor cells directly enter the circulation[4]. The HCC guidelines do not recommend surgery for these patients because of poor survival and high surgical risks[5]. HCC with metastasis also indicates that the tumor is at an advanced stage, and patients in this stage have poor survival. There is almost no cure for unresectable advanced HCC patients.
Atezolizumab is a programmed cell death ligand 1 (PD-L1)-binding immunoglobulin G4 antibody that selectively targets PD-L1 expressed on tumor cells to block its interaction with programmed cell death protein 1 (PD-1), which induces T cell suppression. PD-L1 overexpression has been found in many solid tumors, such as gastric cancer, pancreatic cancer, renal cell carcinoma, ovarian cancer and HCC[6]. PD-L1 plays an important role in the immune escape and microenvironment of tumor cells, which makes blockade of the PD-1/PD-L1 pathway a promising therapy[7]. Data show that PD-1 or PD-L1 inhibitors might provide a new therapeutic option for a substantial proportion of HCC patients.
In this article, we reported the case of a 59-year-old Asian male with recurrent and metastatic HCC with IVCTT who received atezolizumab immunotherapy. The patient showed an excellent symptomatic and radiological response to this anti-PD-L1 therapy, and his lung and mediastinum metastases ceased to deteriorate after 3 mo of therapy. We reported two other cases of HCC with IVCTT at the first diagnosis, for which patients were treated with a PD-1 inhibitor after surgery or recurrence, and both patients achieved a good outcome. In addition, we reviewed and analyzed the available literature to elucidate the role of PD-1/PD-L1 inhibitors in the treatment of HCC as well as the safety and efficacy of PD-1/PD-L1 inhibitors in various solid tumors.
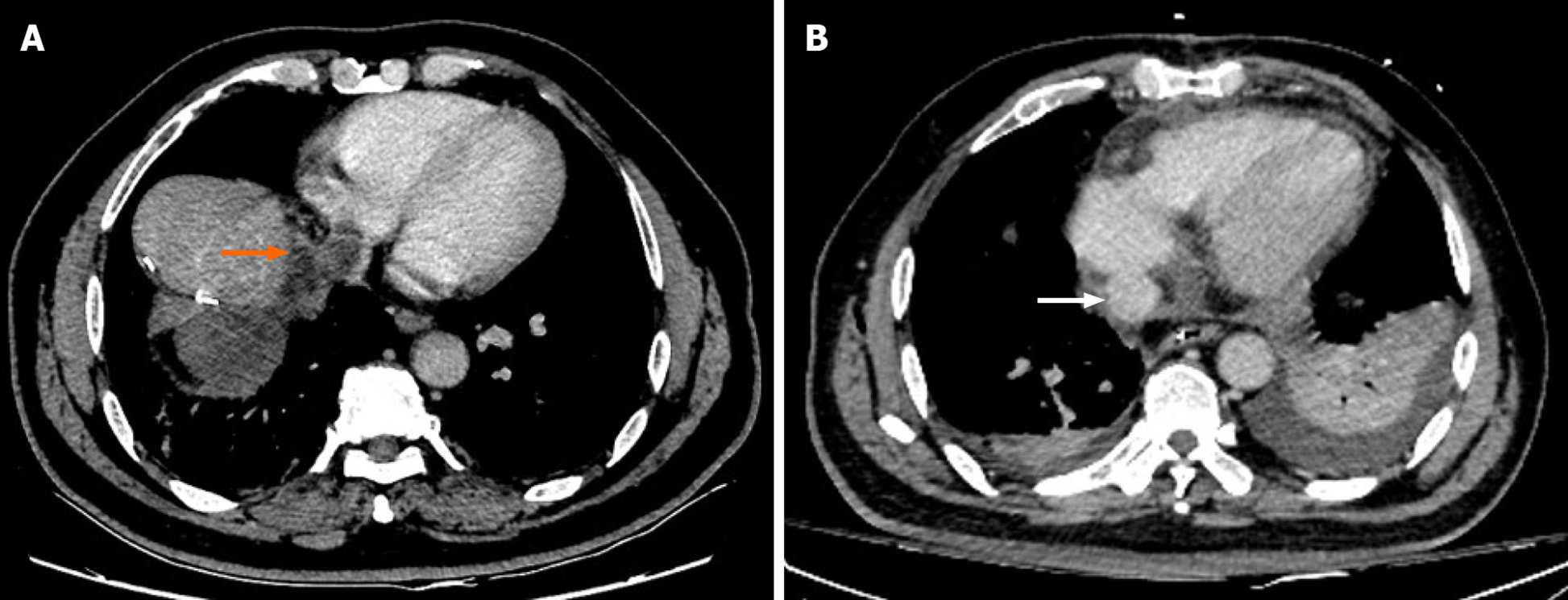
Case 1: A 59-year-old male patient who had underwent right hepatectomy was referred to our hospital because a computed tomography (CT) scan showed recurrence of HCC with IVCTT (Figure 1A).
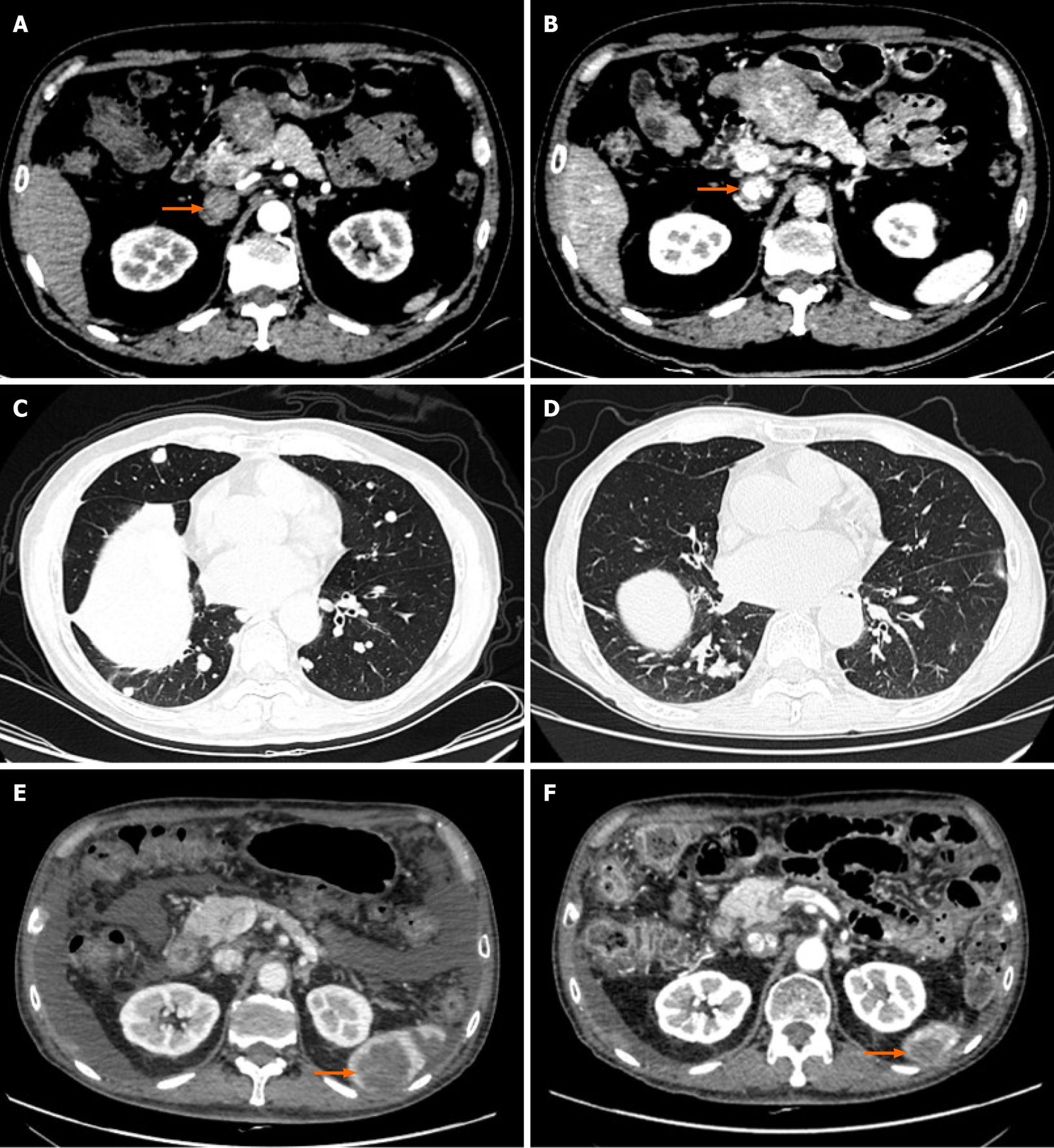
Case 2: A 60-year-old male patient underwent left hepatic massive tumor resection, inferior vena cava incision and thrombus removal due to the existence of IVCTT (Figure 2A and B) before the surgery and was reviewed regularly thereafter. A lung CT scan on June 16, 2017 showed the possibility of metastatic cancer in both lungs. The patient was treated with oxaliplatin 150 mg + calcium levofolinate 200 mg + S-1 60 mg bid × 14 d q30d for two courses and transcatheter arterial chemoembolization (TACE) twice, and he was maintained on S-1 60 mg bid × 14 d q21d after the operation. Re-examination of the patient on January 29, 2018 showed more metastases in both lungs (Figure 2C). The patient was given oxaliplatin 150 mg + calcium levofolinate 200 mg + S-1 60 mg bid × 14 d q30d for one course. From March 9, 2018 to April 19, 2019, the patient received S-1 combined with regorafenib, anlotinib or thymalfasin for treatment. The patient was re-examined on June 14, 2019, and a metastatic tumor was found at the lower pole of the spleen (Figure 2E). The patient came to our department for further treatment.
Case 3: A 34-year-old male had been diagnosed as a hepatitis B virus (HBV) carrier for more than 10 years. In the past month, he lost 10 kg in weight, which was accom
Case 1: Three years ago, the patient was referred to our hospital because of abdominal pain for 1 mo. He was diagnosed with HCC according to an imaging examination and elevated alpha-fetoprotein (AFP) level (491.30 ng/mL). The patient underwent right hepatectomy in our hospital and was followed up regularly. His pathological results were consistent with the preoperative diagnosis.
Case 2: The patient presented to our hospital on July 7, 2016 due to upper abdominal pain for more than 10 d. A CT scan showed a round mass in the left lobe of the liver with a size of approximately 88 mm × 79 mm. The left hepatic vein and the left branch of the portal vein were invaded, and the proximal inferior vena cava had a filling defect. The mass was considered a massive liver tumor with IVCTT (Figure 2A and B). The patient underwent left hepatic massive tumor resection, inferior vena cava incision and thrombus removal. His postoperative diagnosis was HCC of cT3bN0M0, stage IIIB and stage C according to Barcelona Clinic Liver Cancer staging. After TACE, the patient began four courses of oxaliplatin 150 mg + calcium levofolinate 200 mg + S-1 (tegafur, gimeracil and oteracil potassium capsules) 40 mg bid and sorafenib 0.4 g bid targeted therapy. The chemotherapy regimen was well tolerated, and after the second TACE procedure, the patient accepted maintenance treatment with S-1 60 mg bid × 14 d.
Case 3: The patient had not previously shown abnormal liver function or liver occupancy.
Case 1: The patient was in good health, without a history of HBV infection, long-term alcohol abuse or liver fluke infection.
Case 2: The patient had a history of HBV infection and did not regularly receive antiviral treatment. The patient had no history of long-term alcohol abuse or liver fluke infection. The patient had a history of hypertension and took nifedipine 10 mg qd to control his blood pressure.
Case 3: The patient had a 10-year history of HBV infection and did not regularly receive antiviral treatment. The patient had no history of long-term alcohol abuse or liver fluke infection.
There were no other patients with liver cancer or other tumors in any of the families.
Case 1: The abdomen of the patient was flat. The scars from the first surgery were visible, and the wounds had healed well. There was no palpable mass or tenderness.
Case 2: The patient had arrhythmia, with a split-second heart sound in the pulmonary valve auscultation area. The abdomen of the patient was flat. The scars from the first surgery were visible, and the wounds had healed well. There was no palpable mass or tenderness.
Case 3: The abdomen of the patient was flat. No varicose veins were observed in the abdominal skin. There was no palpable mass or tenderness. The patient did not have percussion pain in the liver area.
Case 1: The results of laboratory tests were mostly in the corresponding normal range except for tumor biomarkers, including AFP (1632.00 ng/mL, normal value: < 25 U/mL) and carcinoembryonic antigen (CEA; 5.9 U/mL, normal value: < 5 U/mL). His Child–Pugh classification was grade A (5 points).
Case 2: Before the first operation, the aspartate aminotransferase level was 207 U/mL, and the alanine aminotransferase level was 169 U/mL. The AFP level was 43.26 ng/mL, and carbohydrate antigen 19-9 level was 87.1 U/mL. The parameters HBV surface antigen, anti-HBV e antibody, anti-HBV core antibody and HBV pre-S1 antigen were positive. The HBV load was 1.65 × 106 IU/mL.
Case 3: The AFP level of the patient was more than 121000 ng/mL, and the carbo
Case 1: An enhanced CT scan showed a 2.3 cm × 2.1 cm mass in the resection margin adjacent to the second hilar, with a tumor thrombus measuring 33 mm in the inferior vena cava (IVC) and partially protruding into the right atrium (Figure 1A).
Case 2: A lung CT scan on June 16, 2017 showed the possibility of metastatic cancer in both lungs. The patient's re-examination on January 29, 2018 showed more metastases in both lungs (Figure 2C). The patient was re-examined on June 14, 2019, and a metastatic tumor was found at the lower pole of the spleen (Figure 2E). Color Doppler ultrasound of the heart showed slight regurgitation of the tricuspid valve and pulmonary valve caused by hypertension.
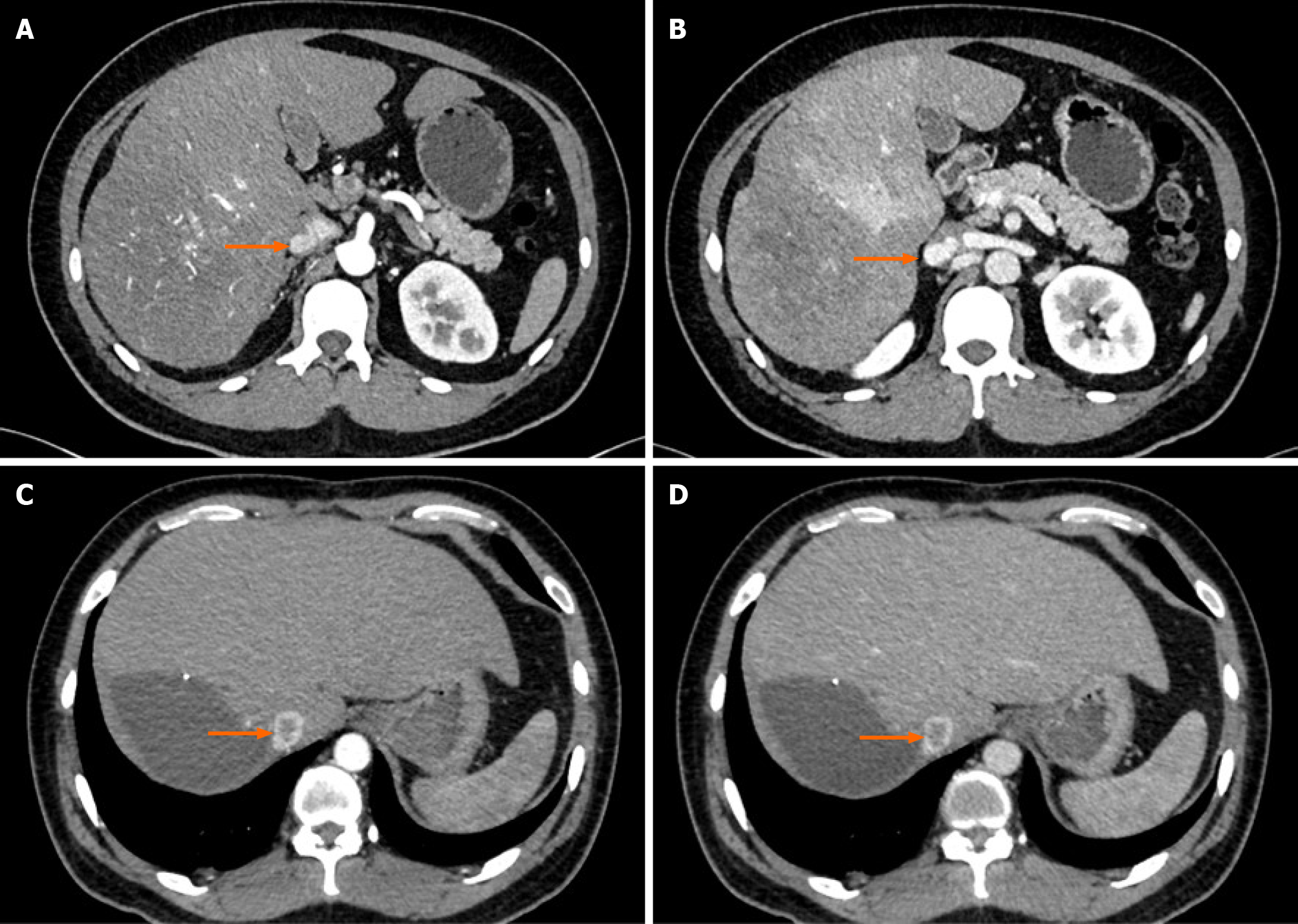
Case 3: Imaging examination revealed a substantial mass of 19.3 cm × 12.5 cm × 15.7 cm in the right lobe of the liver. This mass was considered a malignant tumor and invaded the IVC (Figure 3A and B), right hepatic vein and right anterior and right posterior branches of the portal vein with tumor thrombosis present.
The patient was diagnosed with recurrent HCC with IVCTT.
The patient was diagnosed with metastatic HCC with IVCTT, chronic hepatitis B, hypertension and hypertensive heart disease.
The patient was diagnosed with HCC with IVCTT and chronic hepatitis B.
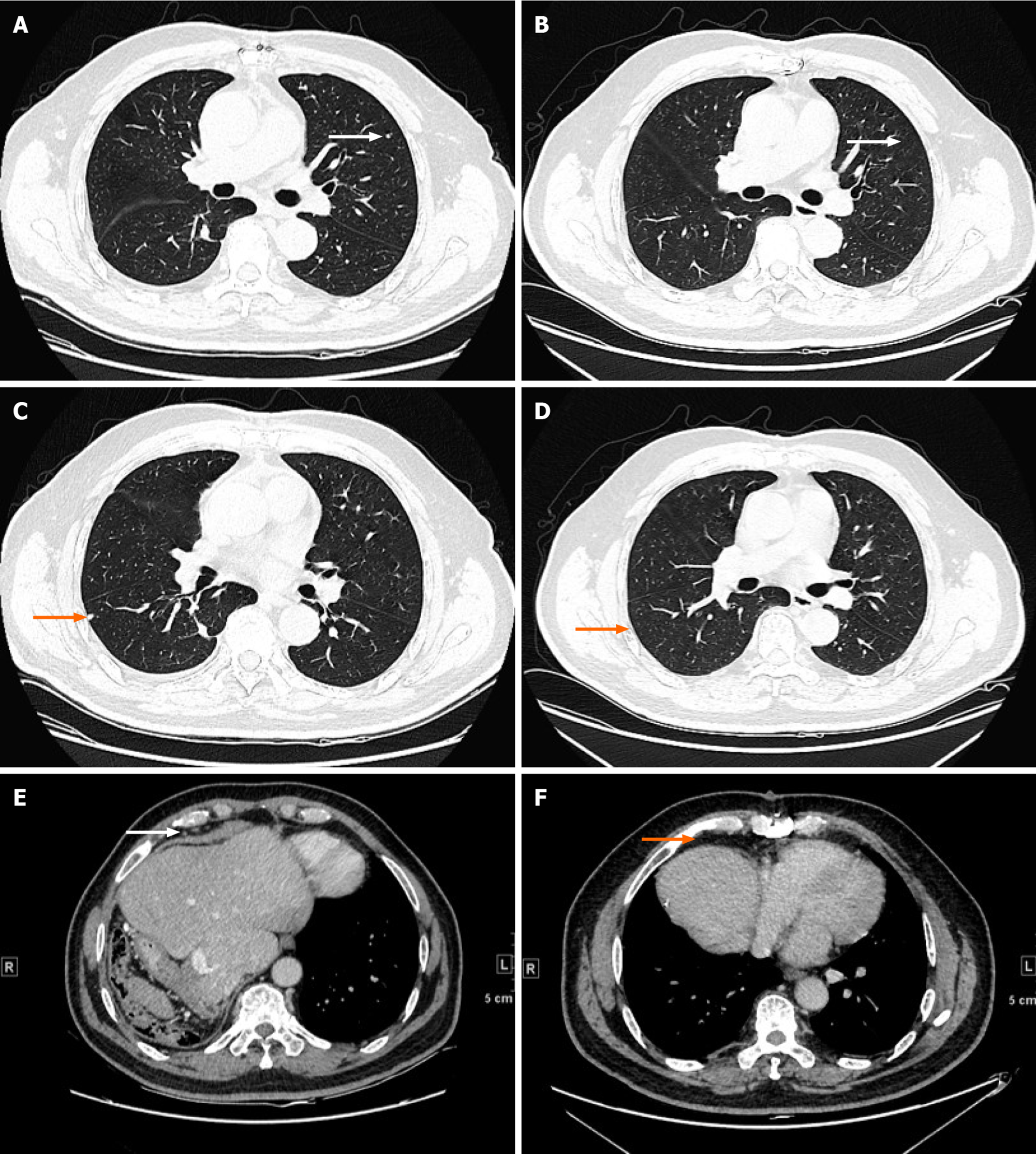
Thoracoabdominal surgery was performed, and we removed the IVCTT (Figure 1B) and tumor in the liver. The immunohistochemistry staining results were GPC3(+), cytokeratin (CK) 19(+), hepatocyte(+), caudal type homeobox transcription factor 2 (+), CK7(-), CK20(-) and CD34(+). The patient recovered well and started to receive chemotherapy (oral tegafur 60 mg qd from day 1 to day 14). After two courses of chemotherapy, an elevated serum AFP level was found, and CT examination showed lung metastases (Figure 4A and C) and mediastinal lymph node metastases (Figure 4E). The expression of PD-L1 and tumor-related gene mutations in HCC tissues was analyzed by immunohistochemistry and gene sequencing. The tumor proportion score of PD-L1 expression was 1%. The tumor mutational burden was 10.48 mutations/Mb, which ranked at 11% among HCC patients. Moreover, clinically actionable mutations in TP53 and microsatellite stability were identified. No other mutations were detected, namely, there were no mutations in CD274, programmed cell death 1 ligand 2, MutL homolog 1, MutS homolog 2, MutS homolog 6, PMS1 homolog 2, polymerase delta 1, DNA polymerase epsilon, mouse double minute 2 homolog or mouse double minute 4 homolog. Then, the patient received atezolizumab immunotherapy (1200 mg/mo).
The patient received 120 mg pembrolizumab immunotherapy for the first time on July 3, 2019 and then received pembrolizumab combined with S-1 or thymalfasin for four courses. On November 12, 2019, CT examination revealed that the lower pole of the splenic metastasis was smaller than before (Figure 2F), and splenectomy was per
Postoperatively, the patient received pembrolizumab 200 mg + lenvatinib 12 mg qd targeted therapy + S-1 60 mg bid × 14 d q30d on December 25, 2019 and February 19, 2020, March 10, April 1, April 22, May 13, June 3, June 24, July 15, August 5, August 26, Sept 16, October 9, October 30, November 20 and December 12 for a total of thirteen courses.
The patient underwent right hepatectomy. The pathological results showed that the tumor was HCC with moderate to poor differentiation. The immunohistochemistry staining results were hepatocyte(+), GPC3(+), CK19(+), AFP minority(+), heat shock protein 70 minority(+), Ki67 approximately 70%(+), CK7(-), CD10(-) and arginase-1(-), and CD34 staining showed capillary blood sinusoids. The patient received tislelizumab (PD-1 inhibitor, 200 mg q3w) combined with lenvatinib (8 mg qd) targeted therapy after surgery. During the third course of treatment, a chest CT scan showed that the hilar IVC had a tumor thrombus (Figure 3C and D), and intrahepatic lesions and IVCTT were treated with TACE.
Additionally, the lung metastasis disappeared (Figure 4B and D), and the metastases in the mediastinal lymph nodes were smaller (Figure 4F) after 3 mo of immunotherapy. The serum level of AFP was also normal after 3 mo of immunotherapy. The patient received a total of seven courses of atezolizumab immunotherapy without other adjuvant therapy. During the process of atezolizumab immunotherapy, he had no severe treatment-related adverse events. He was regularly followed up and underwent imaging examinations and blood tests. After 27 mo of follow-up, the patient had recovered well and showed no signs of recurrence or metastasis. Atezolizumab immunotherapy proved effective in the patient, who has since enjoyed a high quality of life.
The patient has been receiving immunotherapy regularly for thirteen courses and is now in a good living condition without recurrence (Figure 2D). The level of AFP fluctuated between 97.70 and 244.30.
The patient has now received three courses of tislelizumab immunotherapy (September 29, 2020, October 23 and December 15) and is still receiving treatment.
HCC is one of the leading causes of cancer morbidity and cancer-related death worldwide[8]. The 5-year overall survival rate of HCC patients was reported to be between 12% and 18%[9,10]. Surgical resection and liver transplantation are possible curative treatments, but the recurrence rate is high[11,12]. Moreover, only 30% of HCC patients can benefit from surgery at the time of diagnosis because many patients are at an advanced stage at the time of their first diagnosis[13]. There is almost no cure for these patients. The occurrence of IVCTT is relatively rare, and HCC patients with IVCTT are considered to be at an advanced stage[3]. HCC accompanied by IVCTT or distal metastasis indicates poor survival[14,15].
Surgical removal of IVCTT is not recommended due to the poor prognosis and high surgical risks. However, there have been some reports on radical surgery for HCC with IVCTT, which achieved better survival than systemic therapy[16,17]. Sorafenib is the only recommended option for patients with unresectable disease[18]. However, the curative effect is unsatisfactory, and better therapies are urgently needed.
Immunotherapy is a revolutionary therapy and has achieved certain progress in the treatment of many solid tumors[19]. Blockade of the PD-1/PD-L1 pathway is one of the most important immunotherapeutic strategies being studied. The PD-1 inhibitors pembrolizumab and nivolumab have produced promising clinical responses as second-line treatments for advanced HCC in phase 1/2 studies[20,21]. Moreover, there are many ongoing phase III clinical trials comparing PD-1/PD-L1 inhibitors with sorafenib or a placebo[22].
Atezolizumab is a PD-L1-binding immunoglobulin G4 antibody that acts as an immune checkpoint inhibitor by selectively blocking the interaction between PD-1 expressed on activated T cells and its ligand PD-L1 expressed on immune cells or tumor cells. It has been shown to improve significantly survival in many solid tumors, such as breast cancer and non-small cell lung cancer[23,24].
Recently, a phase III clinical trial reported that atezolizumab combined with bevacizumab could achieve better overall and progression-free survival than sorafenib in patients with unresectable HCC[25]. The overall survival rate at 12 mo in the atezolizumab-bevacizumab group (n = 336) was higher than that in the sorafenib group (n = 165). The median progression-free survival times were 6.8 mo and 4.3 mo for the respective groups. This was the first article to suggest the value of immunotherapy in HCC and has brought hope to patients with advanced HCC.
In this article, we presented three cases of recurrent and metastatic HCC with IVCTT treated with PD-1/PD-L1 inhibitors that responded excellently to anti-PD-1/PD-L1 immunotherapy. The first patient underwent surgery for HCC and had recurrence of HCC with IVCTT 3 years after his first surgery. We performed a second surgery and removed the recurrent tumor and IVCTT. However, 2 mo later, imaging results and the level of a serum tumor marker showed metastases of the tumor into the lungs and mediastinum. After gene sequencing and immunohistochemistry, we suggested the application of a PD-L1 inhibitor to treat the patient. After 3 mo of immunotherapy, the level of AFP decreased to normal, and imaging examination showed the disappearance of metastatic lesions. The patient was followed up regularly and had no signs of recurrence or severe immune-related adverse events. We reported two other cases of HCC with IVCTT at the first diagnosis; these patients were treated with a PD-1 inhibitor after surgery or recurrence, and both achieved a good outcome.
To the best of our knowledge, the first case represents the first report of successful treatment of recurrent and metastatic HCC with IVCTT with atezolizumab. Pro
Anti-PD-L1 therapy in HCC patients with IVCTT and metastasis is associated with relatively good patient outcomes.
Manuscript source: Unsolicited manuscript
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang H S-Editor: Ma YJ L-Editor: Filiopdia P-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55647] [Article Influence: 7949.6] [Reference Citation Analysis (131)] |
| 2. | Kluger MD, Salceda JA, Laurent A, Tayar C, Duvoux C, Decaens T, Luciani A, Van Nhieu JT, Azoulay D, Cherqui D. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol. 2015;62:1131-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Kokudo T, Hasegawa K, Yamamoto S, Shindoh J, Takemura N, Aoki T, Sakamoto Y, Makuuchi M, Sugawara Y, Kokudo N. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol. 2014;61:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Sung AD, Cheng S, Moslehi J, Scully EP, Prior JM, Loscalzo J. Hepatocellular carcinoma with intracavitary cardiac involvement: a case report and review of the literature. Am J Cardiol. 2008;102:643-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Bai Y, Wu J, Zeng Y, Chen J, Wang S, Chen S, Qiu F, Zhou S, You S, Tian Y, Wang Y, Yan M. Nomogram for Predicting Long-Term Survival after Synchronous Resection for Hepatocellular Carcinoma and Inferior Vena Cava Tumor Thrombosis: A Multicenter Retrospective Study. J Oncol. 2020;2020:3264079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023-5039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 594] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 7. | Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell. 2019;76:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 8. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3142] [Article Influence: 523.7] [Reference Citation Analysis (37)] |
| 9. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 955] [Article Influence: 136.4] [Reference Citation Analysis (2)] |
| 10. | Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 1107] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 11. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 647] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 12. | Mazzola A, Costantino A, Petta S, Bartolotta TV, Raineri M, Sacco R, Brancatelli G, Cammà C, Cabibbo G. Recurrence of hepatocellular carcinoma after liver transplantation: an update. Future Oncol. 2015;11:2923-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Abdel-Rahman OM, Elsayed Z. Yttrium-90 microsphere radioembolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2016;2:CD011313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O'Neil B, Poon R, Schwartz L, Tepper J, Yao F, Haller D, Mooney M, Venook A. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994-4005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 15. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4512] [Article Influence: 347.1] [Reference Citation Analysis (2)] |
| 16. | Li AJ, Zhou WP, Lin C, Lang XL, Wang ZG, Yang XY, Tang QH, Tao R, Wu MC. Surgical treatment of hepatocellular carcinoma with inferior vena cava tumor thrombus: a new classification for surgical guidance. Hepatobiliary Pancreat Dis Int. 2013;12:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Kasai Y, Hatano E, Seo S, Taura K, Yasuchika K, Okajima H, Kaido T, Uemoto S. Proposal of selection criteria for operative resection of hepatocellular carcinoma with inferior vena cava tumor thrombus incorporating hepatic arterial infusion chemotherapy. Surgery. 2017;162:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1373] [Article Influence: 196.1] [Reference Citation Analysis (0)] |
| 19. | Iñarrairaegui M, Melero I, Sangro B. Immunotherapy of Hepatocellular Carcinoma: Facts and Hopes. Clin Cancer Res. 2018;24:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 20. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1880] [Article Influence: 268.6] [Reference Citation Analysis (0)] |
| 21. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3285] [Article Influence: 410.6] [Reference Citation Analysis (1)] |
| 22. | Johnston MP, Khakoo SI. Immunotherapy for hepatocellular carcinoma: Current and future. World J Gastroenterol. 2019;25:2977-2989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 23. | Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M; IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2747] [Article Influence: 392.4] [Reference Citation Analysis (0)] |
| 24. | Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA; IMpassion130 Trial Investigators. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379:2108-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2345] [Cited by in RCA: 2987] [Article Influence: 426.7] [Reference Citation Analysis (0)] |
| 25. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4607] [Article Influence: 921.4] [Reference Citation Analysis (2)] |