Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.5812
Peer-review started: February 24, 2021
First decision: April 18, 2021
Revised: May 2, 2021
Accepted: May 26, 2021
Article in press: May 26, 2021
Published online: July 26, 2021
Processing time: 146 Days and 23.7 Hours
Hepatitis B surface antigen (HBsAg) loss, a functional cure in patients with chronic hepatitis B (CHB) undergoing antiviral therapy, might be an ideal endpoint of antiviral treatment in clinical practice. The factors that contribute to the functional cure remain unclear, and the predictors of functional cure are worth exploring. The concentration and kinetics of soluble programmed death-1 (sPD-1) in patients with CHB may play an important role in elucidating the immune response associated with functional cure after nucleos(t)ide analogs therapy.
To investigate the factors associated with HBsAg loss and explore the influence of sPD-1 Levels.
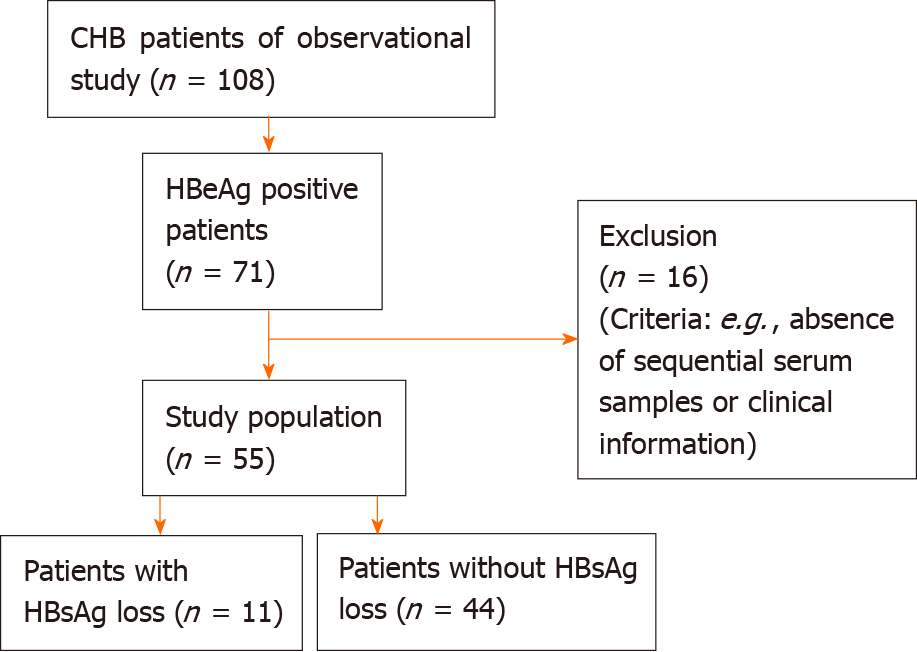
This study analyzed the data and samples from patients with CHB who underwent antiviral treatment in a non-interventional observational study conducted at Peking University First Hospital in Beijing (between 2007 and 2019). All patients were followed up: Serum samples were collected every 3 mo during the first year of antiviral treatment and every 6 mo thereafter. Patients with positive hepatitis B e antigen levels at baseline and with available sequential samples who achieved HBsAg loss during antiviral treatment served as the case group. This case group (n = 11) was further matched to 44 positive hepatitis B e anti patients without HBsAg loss as controls. The Spearman’s rank correlation test and receiver operating characteristic curves analysis were performed.
The sPD-1 Levels were higher in patients with HBsAg loss than in those without HBsAg loss from baseline to month 96, and the differences were significant between the groups at baseline (P = 0.0136), months 6 (P = 0.0003), 12 (P < 0.0001), 24 (P = 0.0007), 48 (P < 0.0001), and 96 (P = 0.0142). After 6 mo of antiviral treatment, the sPD-1 levels were positively correlated with alanine transaminase (ALT) levels (r = 0.5103, P = 0.0017), and the sPD-1 levels showed apparent correlation with ALT (r = 0.6883, P = 0.0192) and HBV DNA (r = 0.5601, P = 0.0703) levels in patients with HBsAg loss. After 12 mo of antiviral treatment, the sPD-1 levels also showed apparent correlation with ALT (r = 0.8134, P = 0.0042) and HBV DNA (r = 0.6832, P = 0.0205) levels in patients with HBsAg loss. The sPD-1 levels were negatively correlated with HBsAg levels in all patients after 12 mo of antiviral treatment, especially at 24 (r = -0.356, P = 0.0497) and 48 (r = -0.4783, P = 0.0037) mo. After 6 mo of antiviral treatment, the AUC of sPD-1 for HBsAg loss was 0.898 (P = 0.000), whereas that of HBsAg was 0.617 (P = 0.419). The cut-off value of sPD-1 was set at 2.34 log pg/mL; the sensitivity and specificity were 100% and 66.7%, respectively.
The sPD-1 levels at 6 mo can predict HBsAg loss after 144 mo of antiviral treatment.
Core Tip: This study analyzed the data and samples from patients with chronic hepatitis B who underwent antiviral treatment and were followed up for 12 years to investigate the factors associated with hepatitis B surface antigen (HBsAg) loss and explore the impact of soluble programmed death-1 (sPD-1) levels. The sPD-1 levels were significantly different between patients with and without HBsAg loss during antiviral treatment. The sPD-1 levels at 6 mo can predict HBsAg loss after 12 years of antiviral treatment.
- Citation: Tan N, Luo H, Kang Q, Pan JL, Cheng R, Xi HL, Chen HY, Han YF, yang YP, Xu XY. Soluble programmed death-1 is predictive of hepatitis B surface antigen loss in chronic hepatitis B patients after antiviral treatment. World J Clin Cases 2021; 9(21): 5812-5821
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/5812.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.5812
More than 240 million individuals worldwide are infected with chronic hepatitis B (CHB)[1]. Among them, 15%-40% of untreated CHB cases progress to cirrhosis that may result in liver failure and even liver cancer[2]. Nucleos(t)ide analogues (NAs) were widely used for antiviral treatment because of their none contraindications and high tolerability in patients with CHB[2]. Hepatitis B surface antigen (HBsAg) levels and other virological indicators such as hepatitis B virus (HBV) DNA and hepatitis B e antigen (HBeAg) are measured to monitor the effects of antiviral treatment during the follow-up of patients with CHB[3]. Since HBsAg levels reflect the transcriptional activity of covalently closed circular DNA, especially in HBeAg-positive patients, HBsAg loss, a functional cure in CHB patients undergoing antiviral therapy, might be an ideal endpoint of antiviral treatment in clinical practice[4]. However, only 1%-12% of CHB patients are cured in response to NA therapy within 5-7 years[5]. The factors that contribute to the cure remain unclear, and the predictors of functional cure are worth exploring.
Functional HBV-specific T cells (with helper or cytotoxic effects) are considered as crucial factors that influence the prognosis of HBV-infected patients[6]. However, HBV-specific T cell function is progressively impaired by exposure to chronic inflammation and high viral load[7]. Programmed death-1 (PD-1) is considered a critical factor for T cell exhaustion in chronic infections[8]. Studies have shown that HBV-specific T cells express PD-1, and the blockade of PD-1 and programmed death-ligand 1 (PD-L1) partially restores the function of T cells[8].
Recent studies measured serum PD-1 levels and considered whether serum PD-1 might compete with PD-L1 expressed on the cell surface and impair the inhibitory effects of PD-1/PD-L1 interactions on T cells[9]. Nevertheless, the potential functional effects of serum PD-1 are not clear owing to the lack of relevant data. The concentration and kinetics of soluble PD-1 (sPD-1) in patients with CHB may play an important role in elucidating the immune response associated with functional cure after NA therapy. This study analyzed the data and samples from patients with CHB who underwent antiviral treatment and were followed up for 12 years to investigate the factors associated with HBsAg loss and explore the influence of sPD-1 Levels.
Patients with CHB (HBsAg positive ≥ 6 mo) who initiated NA therapy in a non-interventional observational study conducted at Peking University First Hospital in Beijing (between 2007 and 2019) were enrolled in this study. All patients were followed up: Serum samples were collected every 3 mo during the first year of antiviral treatment and every 6 mo thereafter. Patients with human immunodeficiency virus, hepatitis C virus, hepatitis D virus, hepatitis E virus, other liver diseases, hepatocellular carcinoma, cardiovascular disease, diabetes, pregnancy, or autoimmune disease were excluded. Patients who consumed alcoholic beverages were excluded. Patients with steatosis were also excluded according to ultrasound[10]. HBeAg-positive patients before NA treatment with available sequential samples and HBsAg loss during antiviral treatment served as the case group. This case group (n = 11) was further matched to 44 positive HBeAg patients without HBsAg loss (Figure 1). These 55 patients were treated with NAs: Lamivudine 100 mg per day (n = 3), adefovir dipivoxil 10 mg per day (n = 17), or entecavir 0.5 mg per day (n = 35) as monotherapy for 144 mo. All participants provided written informed consent, and the study was approved by the ethics committee of Peking University First Hospital. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Shanghai Jing’an District Central Hospital (Approval No. 090f51e6809a26e1 v1.0).
HBsAg [hepatitis surface antibody (anti-HBs)] and HBeAg (anti-HBe) levels were measured using chemiluminescence assay kits (Roche Diagnostics GmbH, Mannheim, Germany). The COBAS TaqMan assay (Roche Diagnostics, Basel, Switzerland) was used to quantify serum HBV DNA levels (lower limit of detection, 100 IU/mL). Serum sPD-1 levels in CHB patients were measured using an enzyme-linked immunosorbent assay (ELISA) kit (DuoSet Human PD-1, R&D Systems, Minneapolis, MN, United States) according to the manufacturer’s instructions, and absorbance was measured at 450 nm. Serum alanine transaminase (ALT) levels were assessed at the Peking University First Hospital using a standard procedure. The upper limit of normal ALT level was 50 U/L in men and 40 U/L in women.
Baseline was defined as the time at which NA therapy was initiated. Continuous variables were expressed as median (interquartile range, IQR). Categorical variables were expressed as values. Continuous variables were compared using a two-tailed Student’s t-test or Mann-Whitney U test depending on the distribution. Correlations between sPD-1 and detection markers were assessed using the Spearman’s rank correlation test. The predictive value of different biomarkers for HBsAg loss during treatment was summarized using receiver operating characteristic (ROC) curves. Data were analyzed using the SPSS software, version 25 [SPSS Inc. (IBM Corp., Armonk, NY, United States]. Statistical tests were two-sided; P < 0.05 was considered statistically significant.
Neither the patients nor the public were involved in the design, conduct, report, or dissemination of our research.
Before treatment, the patient's baseline period was defined. The baseline characteristics of the 55 patients are presented in Table 1. The median age of patients with HBsAg loss was 35 years (IQR: 23.00–38.00) and that of patients without HBsAg loss was 36.5 (IQR: 28.00–42.75). In the patients with HBsAg loss, 10 were male and 1 was female. In the patients without HBsAg loss, 37 were male and 7 were female. The median values of ALT, HBV DNA, HBsAg, and sPD-1 in patients with HBsAg loss were 140 IU/mL, 8.447 log IU/mL, 3.331 log IU/mL, and 2.766 Log IU/mL, respectively. There were no statistical differences in age, sex, ALT, HBV DNA, HBsAg levels, and response to antiviral therapy between the two groups at baseline.
| Patients characteristics | HBsAg loss (n = 11) | w/o HBsAg loss (n = 44) | P value |
| Age | 35.00 (23.00-38.00) | 36.5 (28-42.75) | 0.3243 |
| Gender | 10/1 | 7/37 | 0.515 |
| ALT (IU/mL) | 140 (77-245) | 116 (75-219) | 0.9590 |
| HBV DNA (log IU/mL) | 8.447 (6.923-8.614) | 8.024 (6.909-8.934) | 0.8844 |
| Antiviral therapy (first-line/second-line)1 | 6/5 | 29/15 | 0.488 |
| ADV/LAM | 4/1 | 13/2 | 0.400 |
| HBsAg (log IU/mL) | 3.331 (2.826-4.582) | 3.856 (3.526-4.347) | 0.1274 |
| sPD-1 (log pg/mL) | 2.766 (2.622-2.903) | 2.657 (2.540-2.765) | 0.0136 |
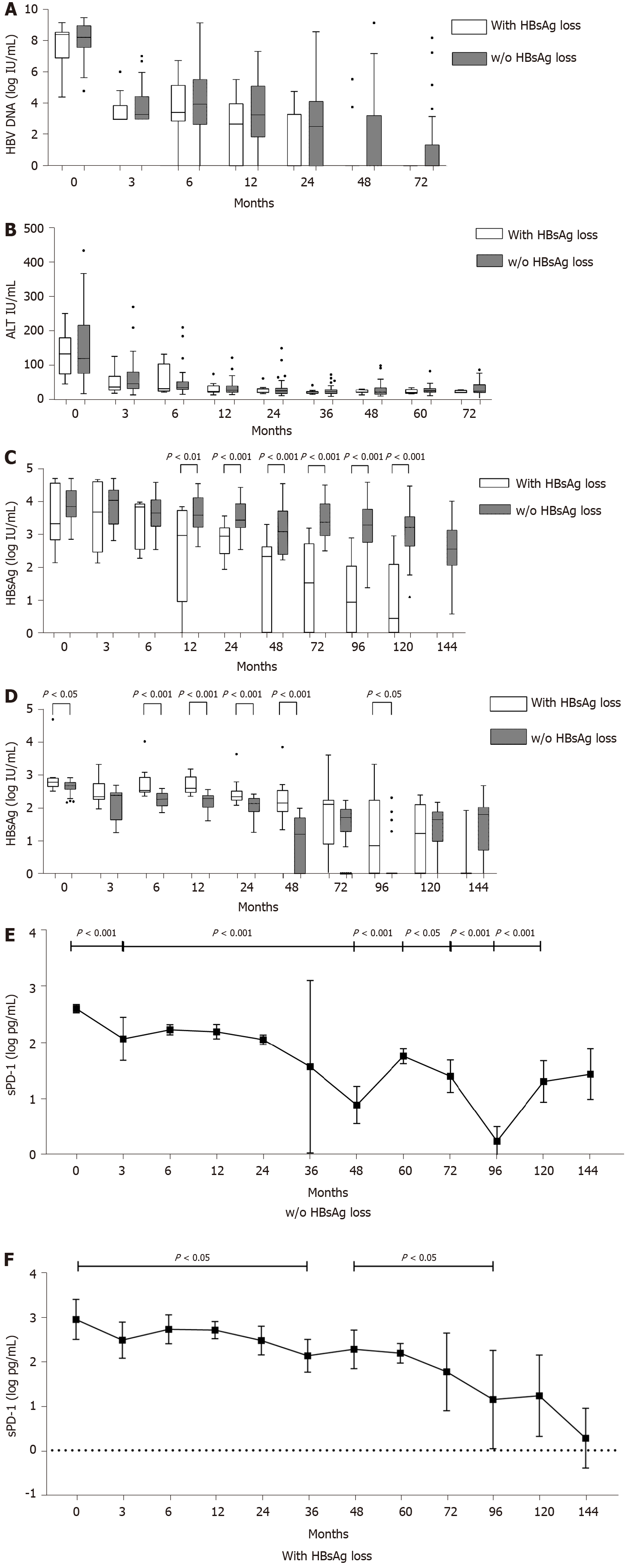
During NA treatment, HBV DNA levels decreased in all patients with similar kinetics, and there were no statistical differences at different time points between the two groups (Figure 2A). HBV DNA was undetectable in patients with HBsAg loss after 60 mo of antiviral treatment. After 132 mo of antiviral treatment, HBV DNA levels were undetectable in all patients. The time to HBV DNA undetectable of each patient from baseline was calculated, and there was no statistical difference between two groups of patients (P = 0.0805).
ALT levels decreased after antiviral treatment in the two groups of patients, and there was no statistical difference between two groups at different time points in ALT levels (Figure 2B). The time to the normalization of ALT levels from baseline of each patient showed no statistical difference between the two groups of patients (P = 0.3678). ALT levels in patients with HBsAg loss normalized after an average of 7.91 mo, whereas that in patients without HBsAg loss normalized after an average of 13.77 mo.
The median HBsAg level (log10 IU/mL) in patients with or without HBsAg loss was similar between the two groups before month 12 (Figure 2C). HBsAg levels were statistically different between the two groups after 12 mo of antiviral treatment (P = 0.0022). In patients with HBsAg loss, HBsAg level (log10 IU/mL) showed a significant decline from 3.33 (2.83-4.58) log10 IU/mL before treatment to 2.973 (0.9415–3.748) log10 IU/mL after 12 mo (Figure 2C), and it decreased gradually thereafter. HBsAg in patients without HBsAg loss remained high during NA treatment.
All patients with HBsAg loss achieved HBeAg seroconversion after 120 mo. Among patients without subsequent HBsAg loss, 18 did not achieve HBeAg seroconversion during 144 mo of follow-up. Among patients with HBeAg seroconversion, the average time of HBeAg seroconversion was 43.09 mo in patients with HBsAg loss and 48.596 mo in patients without HBsAg loss and showed no statistical difference between the two groups.
The sPD-1 levels were higher in patients with HBsAg loss than in those without HBsAg loss from baseline to month 96 (Figure 2D), and the differences were significant between the two groups at baseline (P = 0.0136), months 6 (P = 0.0003), 12 (P < 0.0001), 24 (P = 0.0007), 48 (P < 0.0001), and 96 (P = 0.0142).
After antiviral treatment, the sPD-1 levels of all patients decreased, especially in patients without HBsAg loss, showing a significant decline in sPD-1 levels from baseline to month 3 (P < 0.0001, Figure 2E). The sPD-1 levels in patients without HBsAg loss decreased significantly from 2.12 (1.87–2.29) at month 24 to 1.19 (0–1.70) at month 48, whereas that in patients with HBsAg loss stayed above 2 logs until month 72 (Figure 2F).
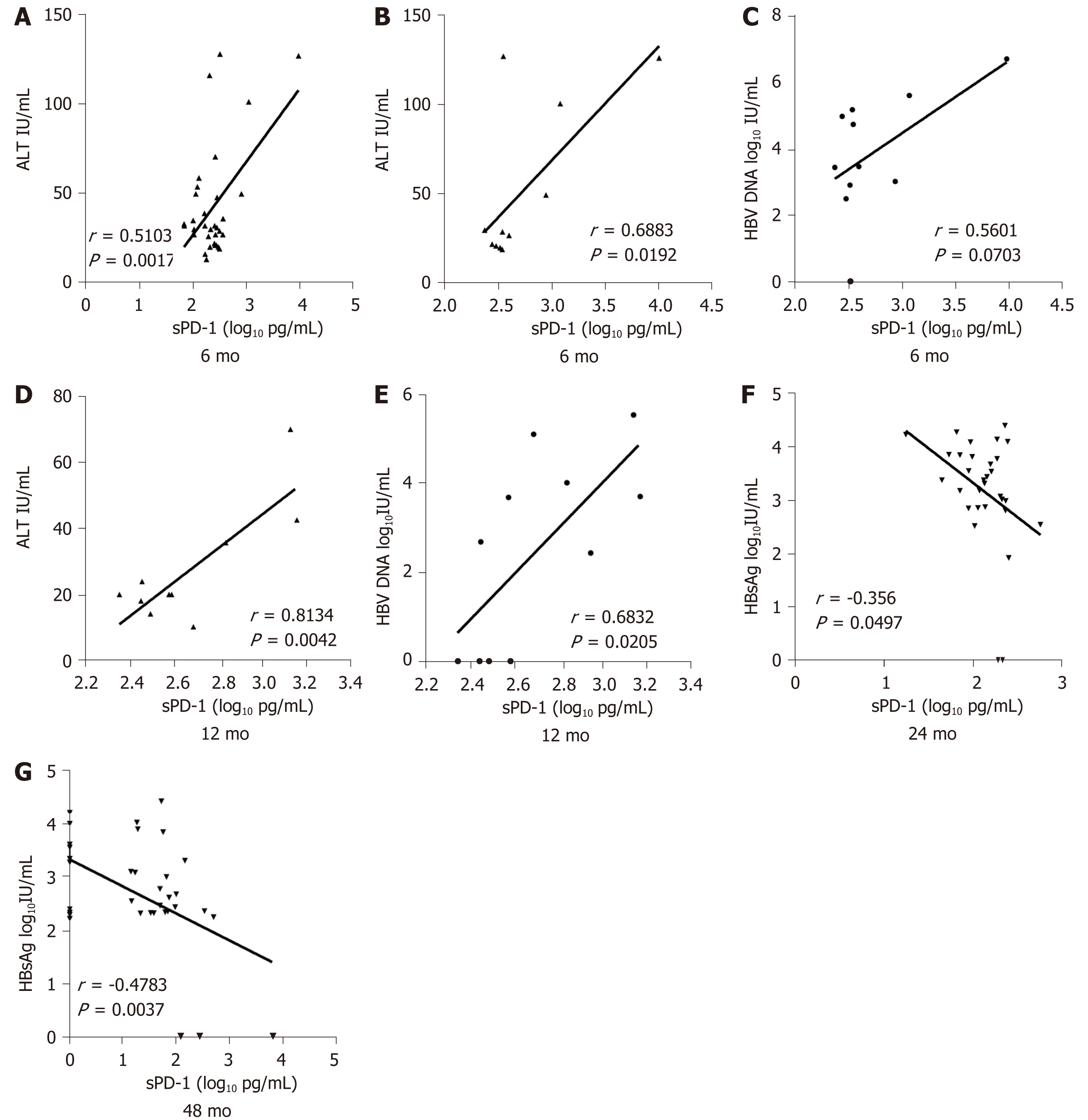
The sPD-1 levels showed no significant correlation with HBsAg, serum HBV DNA, or ALT levels at baseline. After 6 mo of antiviral treatment, the sPD-1 levels were positively correlated with ALT levels (r = 0.5103, P = 0.0017, Figure 3A) and showed weak correlation with HBsAg and HBV DNA levels. For further study, correlation analysis was conducted in patients with or without subsequent HBsAg loss after 144 mo of follow-up, respectively. The sPD-1 levels showed apparent correlation with ALT (r = 0.6883, P = 0.0192, Figure 3B) and HBV DNA (r = 0.5601, P = 0.0703, Figure 3C) levels in patients with HBsAg loss. After 12 mo of antiviral treatment, the sPD-1 levels also showed apparent correlation with ALT (r = 0.8134, P = 0.0042, Figure 3D) and HBV DNA (r = 0.6832, P = 0.0205, Figure 3E) levels in patients with HBsAg loss.
However, the number of patients with a normalization of ALT levels or undetectable HBV DNA increased over time. After 12 mo of antiviral treatment, the sPD-1 levels were negatively correlated with HBsAg levels in all patients, especially at 24 (r = -0.356, P = 0.0497, Figure 3F) and 48 (r = -0.4783, P = 0.0037, Figure 3G) mo.
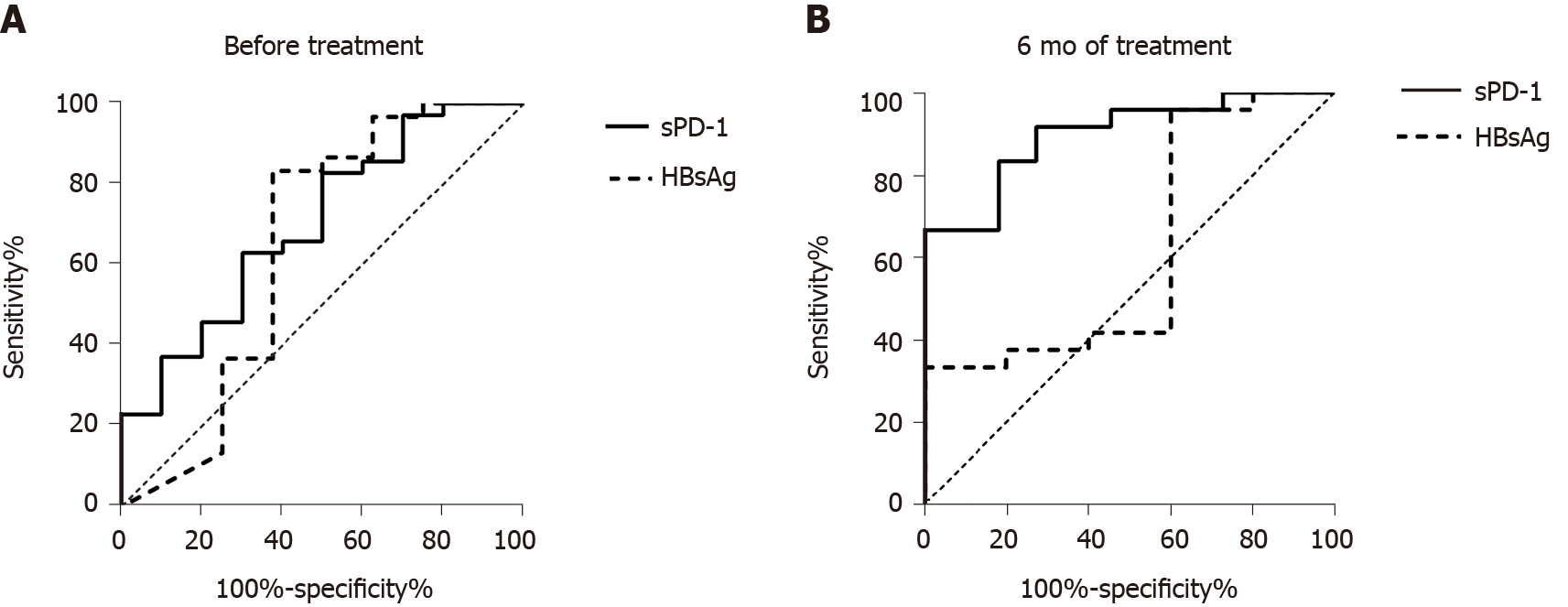
To analyze the predictive value of the different available virologic biomarkers at baseline for HBsAg loss, ROC curve analysis was performed. Before NA treatment, the strongest predictor was sPD-1 [area under the curve (AUC) = 0.700, Figure 4A), whereas HBsAg (AUC = 0.6458) and HBV DNA (AUC = 0.5589) showed no significant prediction of response to NA therapy.
The above results suggested that statistical differences were only observed in sPD-1 levels between the two groups of patients within 12 mo of NA treatment. To explore the predictive value of sPD-1 for HBsAg loss, ROC curve analysis was performed. After 6 mo of antiviral treatment, the AUC of sPD-1 for HBsAg loss was 0.898 (P = 0.000, Figure 4B), whereas that of HBsAg was 0.617 (P = 0.419). The cut-off value of sPD-1 was set at 2.34 log pg/mL; the sensitivity and specificity were 100% and 66.7%, respectively.
Patients with HBsAg loss after antiviral treatment were not different from patients without HBsAg loss in terms of HBV DNA, ALT, and HBsAg levels at baseline. The sPD-1 levels were significantly different between the two groups of patients during antiviral treatment, especially after 6 mo of antiviral treatment. This study examined the predictive value of sPD-1 levels for HBsAg loss in patients with CHB under NA treatment and found that sPD-1 levels at 6 mo had higher AUC values than HBsAg associated with HBsAg loss. The sPD-1 levels at 6 mo can better predict HBsAg loss during antiviral treatment than HBsAg levels.
Different ELISA kits could contribute to discrepant results. Xia et al[11] used different ELISA kits for dosing sPD-1 Levels. There was a significant correlation between the sPD-1 levels and ALT levels at baseline in Zhou et al[9], but a weak correlation in Xia et al[11]. However, their research showed similar results that patients with CHB had higher levels of sPD-1 than healthy controls. Consistent with Xia et al[11], our results showed that the sPD-1 levels were positively correlated with ALT and HBV DNA levels at 6 and 12 mo. The sPD-1 levels decreased in all patients after baseline, which might be associated with lower viral load and lower expression of viral antigen after antiviral treatment. Meanwhile, patients with subsequent HBsAg loss had higher sPD-1 levels until month 96 than patients without HBsAg loss. The sPD-1 levels in patients with subsequent HBsAg loss remained at high levels of two logs from baseline to month 72, whereas those in patients without HBsAg loss decreased quickly from baseline to month 48. These results suggested that patients with higher sPD-1 levels during antiviral treatment were more likely to develop HBsAg loss.
We found that sPD-1 levels were negatively correlated with HBsAg levels after 24 and 48 mo of antiviral treatment, and other studies have not explored sPD-1 levels for this long time period. Other virological and biochemical biomarkers, such as HBV DNA and ALT levels, showed no significant correlation with sPD-1 levels. This might associate with the increasing number of patients with the normalization of ALT levels or undetectable HBV DNA over time. And HBsAg levels were obviously different between patients with or without HBsAg loss after 12 mo of antiviral treatment.
Zhou et al[9] found that the sPD-1 levels in patients in the immune-active phase were the highest among all CHB disease stages. Therefore, the high sPD-1 levels were associated with immune activation[12,13]. The sPD-1 could interact with the membrane bound PD-L1 and counteract the inhibition of PD-1/PD-L1 signal path on virus-specific T cells[14]. The sPD-1 levels at 6 mo might reflect the immune activity, and high levels of sPD-1 at that time might benefit the HBV-specific immunity. HBsAg loss could reflect the suppression of HBV covalently closed circular DNA expression[15,16]. Therefore, the sPD-1 levels at 6 mo could predict HBsAg loss, and the cut-off value of sPD-1 was set at 2.34 Log pg/mL; the sensitivity and specificity were 100% and 66.7%, respectively.
In consequence, patients with higher sPD-1 levels before and during the early stage of antiviral treatment could develop more effective immune response to HBV infection.
There were some limitations in this study. The correlation between sPD-1 levels and HBsAg loss depended on the immune response of functional HBV-specific T cells to HBV infection. The T cell function of patients from this study needed to be detected. In addition, this study was done on a smaller number of cases. Next, we plan to set up a cohort of 300 patients with CHB and divide them into different groups by sPD-1 levels at baseline to explore the prognosis of two groups of patients.
The sPD-1 levels were significantly different between the two groups of patients during antiviral treatment, especially within 12 mo after baseline. We found that sPD-1 levels at 6 mo had higher AUC values than HBsAg associated with HBsAg loss.
Recent studies analyzed serum soluble programmed death-1 (sPD-1) levels and indicated that sPD-1 might play an important role in virus-specific immunity in chronic viral infection.
The factors that contribute to the functional cure in patients with chronic hepatitis B remain unclear, and the predictors of functional cure are worth exploring.
To investigate the factors associated with hepatitis B surface antigen (HBsAg) loss and explore the impact of sPD-1 Levels.
Patients with positive hepatitis B e antigen (HBeAg) levels at baseline and with available sequential samples who achieved HBsAg loss during antiviral treatment served as the case group. This case group (n = 11) was further matched to 44 positive HBeAg patients without HBsAg loss as controls.
Patients with HBsAg loss had higher levels of sPD-1 than patients without HBsAg loss from baseline to month 96, and the differences were significant between the groups at baseline (P = 0.0136), months 6 (P = 0.0003), 12 (P < 0.0001), 24 (P = 0.0007), 48 (P < 0.0001), and 96 (P = 0.0142). The sPD-1 levels were positively correlated with ALT and HBV DNA levels in patients with HBsAg loss within 12 mo of antiviral treatment. After 12 mo of antiviral treatment, the sPD-1 levels were negatively correlated with HBsAg levels in all patients, especially at 24 (r = -0.356, P = 0.0497) and 48 (r = -0.4783, P = 0.0037) mo. The AUC of sPD-1 levels at 6 mo for HBsAg loss was 0.898 (P = 0.000), whereas that of HBsAg was 0.617 (P = 0.419). The cut-off value of sPD-1 was set at 2.34 log pg/mL; the sensitivity and specificity were 100% and 66.7%, respectively.
The sPD-1 levels were significantly different between the two groups of patients during antiviral treatment, especially within 12 mo after baseline. The sPD-1 levels at 6 mo can predict HBsAg loss after 144 mo of antiviral treatment.
The correlation between sPD-1 levels and HBsAg loss depended on the immune response of functional HBV-specific T cells to HBV infection. The T cell function of patients from this study needed to be detected.
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ding Y, Fraga RS S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Tang LSY, Covert E, Wilson E, Kottilil S. Chronic Hepatitis B Infection: A Review. JAMA. 2018;319:1802-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 484] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3773] [Article Influence: 471.6] [Reference Citation Analysis (1)] |
| 3. | Höner Zu Siederdissen C, Maasoumy B, Cornberg M. What is new on HBsAg and other diagnostic markers in HBV infection? Best Pract Res Clin Gastroenterol. 2017;31:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Zeisel MB, Lucifora J, Mason WS, Sureau C, Beck J, Levrero M, Kann M, Knolle PA, Benkirane M, Durantel D, Michel ML, Autran B, Cosset FL, Strick-Marchand H, Trépo C, Kao JH, Carrat F, Lacombe K, Schinazi RF, Barré-Sinoussi F, Delfraissy JF, Zoulim F. Towards an HBV cure: state-of-the-art and unresolved questions--report of the ANRS workshop on HBV cure. Gut. 2015;64:1314-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 5. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2817] [Article Influence: 402.4] [Reference Citation Analysis (0)] |
| 6. | Rivino L, Le Bert N, Gill US, Kunasegaran K, Cheng Y, Tan DZ, Becht E, Hansi NK, Foster GR, Su TH, Tseng TC, Lim SG, Kao JH, Newell EW, Kennedy PT, Bertoletti A. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128:668-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 7. | Park JJ, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, Khalili M, Sterling RK, Kowdley KV, Bzowej N, Lau DT, Kim WR, Smith C, Carithers RL, Torrey KW, Keith JW, Levine DL, Traum D, Ho S, Valiga ME, Johnson GS, Doo E, Lok AS, Chang KM; Hepatitis B Research Network. Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B.. Gastroenterology. 2016;150:684-695.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol. 2014;61:1212-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 9. | Zhou L, Li X, Huang X, Chen L, Gu L, Huang Y. Soluble programmed death-1 is a useful indicator for inflammatory and fibrosis severity in chronic hepatitis B. J Viral Hepat. 2019;26:795-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3168] [Article Influence: 352.0] [Reference Citation Analysis (4)] |
| 11. | Xia J, Huang R, Chen Y, Liu Y, Wang J, Yan X, Zhang Z, Wu C. Profiles of serum soluble programmed death-1 and programmed death-ligand 1 Levels in chronic hepatitis B virus-infected patients with different disease phases and after anti-viral treatment. Aliment Pharmacol Ther. 2020;51:1180-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;64:S71-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 358] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 13. | Rehermann B, Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology. 2015;61:712-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Shi W, Yang B, Sun Q, Meng J, Zhao X, Du S, Li X, Jiao S. PD-1 regulates CXCR5+ CD4 T cell-mediated proinflammatory functions in non-small cell lung cancer patients. Int Immunopharmacol. 2020;82:106295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 689] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 16. | Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HLA, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |