Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.5782
Peer-review started: February 10, 2021
First decision: March 30, 2021
Revised: April 13, 2021
Accepted: May 25, 2021
Article in press: May 25, 2021
Published online: July 26, 2021
Processing time: 160 Days and 20.2 Hours
The breakthrough of immune checkpoint inhibitor (ICI) therapy has created extensive opportunities for cancer immunotherapy. Especially, the block of programmed death-1/programmed death ligand 1 (PD-L1) axis using ICIs has become a new therapeutic strategy to treat advanced gastric cancer (GC). However, in the past decade, single-arm and randomized trials for single-drug ICI therapy showed that the therapeutic effect was not satisfactory, including clinical trials for advanced GC. However, after selecting suitable predictive biomarkers and developing a combination of anti-angiogenic targeted drugs and other chemotherapeutic drugs, the objective response rate and progression-free survival of patients with gastric cancer were improved significantly. The United States Food and Drug Administration has approved treatment with pembrolizumab for patients with advanced GC with PD-L1 expression or microsatellite instability-high/mismatch repair deficiency. In this review, the updated data from the latest trial results of combination immunotherapy for GC are presented. Based on the outcome of combination therapy, we discuss its possible molecular mechanism and summarize effective predictive biomarkers. We also discuss possible problems stemming from results of other clinical trials of ICI treatment and propose other directions for ICI therapy.
Core Tip: Immune checkpoint inhibitors gigantically expand the methods of immunotherapy and bring a glimmer of hopefulness for patients with advanced gastric cancer (GC). Ongoing clinical trials show that the effect of monotherapy was not satisfactory, while the combination therapy manifested a better response rate. The most recent clinical trial results of GC immunotherapy are reviewed to suggest the reasons and mechanisms of the high response rate. Additionally, we propose the potential problems of these trials and speculate on the benefits of immune checkpoint inhibitors in neoadjuvant therapy.
- Citation: Liang C, Wu HM, Yu WM, Chen W. Research status on immunotherapy trials of gastric cancer. World J Clin Cases 2021; 9(21): 5782-5793
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/5782.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.5782
Gastric cancer (GC) is the fifth most common malignancy diagnosed worldwide and the third dominant cause of death from cancer[1]. The mild symptoms of early GC and lack of awareness of physical examination mean that most patients with GC are diagnosed at advanced stages, frequently presenting infiltration or metastasis. Cytotoxic chemotherapy for advanced GC is a first-line standard of care, which comprises treatment with fluoropyrimidine plus a platinum agent, and the decision on whether to administer a combination comprising trastuzumab depends on the presence of human epidermal growth factor receptor 2 (HER2) gene expression; however, the 5-year survival rate remains poor[2-4].
According to medicine development for oncology in the past decade, immune checkpoint inhibitors (ICIs), such as an anti-cytotoxic T-lymphocyte antigen 4 (CTLA4) monoclonal antibody and anti-programmed death-1/programmed death ligand-1 (PD-1/PD-L1) monoclonal antibodies, represent a significant breakthrough[5,6]. Several studies have demonstrated that PD-L1 is constitutively expressed in various kinds of malignant tumors, including GC[7]. A meta-analysis by Chen et al[8] revealed that ICI treatment could enhance moderate survival benefits for patients with advanced gastric or gastroesophageal junction (G/GEJ) cancer. In particular, anti-PD-1/PD-L1 drugs could raise the 12-mo and 18-mo overall survival (OS) rates and manifested a long effective time of the therapeutic response[8]. Meanwhile, the patient’s response showed that anti-PD-1/PD-L1 drugs are more efficacious in molecular subtypes that are PD-L1 positive, microsatellite instability (MSI)-high, or Epstein-Barr virus (EBV) positive, or those with a high mutation burden[8]. However, most patients with advanced GC are not sensitive to ICI monotherapies, according to the results of recent randomized trials[9-12], thus for patients with refractory advanced GC, it is critical to select the appropriate combination therapy to improve their responses to anti-PD-1 therapy or other ICIs[13]. Consequently, the development of safe and effective ICI combination strategies and predictive biomarkers has become an urgent requirement to enhance their therapeutic effect in advanced GC.
In this review, the lately reported trials of combined immunotherapy for advanced GC are summarized, focusing on the combination strategies that have a better overall therapeutic effect, analyzing the possible molecules and their mechanisms of action, and exploring their use as predictive biomarkers. The shortcomings of other cancer types in clinical trials are discussed to indicate the potential problem of developing combined immunotherapy for GC. We also discuss whether ICI could be employed as a neoadjuvant therapy.
Since the advent of ICI-related drugs, numerous clinical trials are being carried out worldwide. As of September 2018, there were a total of 2250 ongoing clinical trials, of which 1716 trials tested regimens that combined anti-PD1/PD-L1 drugs with other cancer treatments[14]. In these trials, lung cancer (254 trials), melanoma (139 trials), breast cancer (106 trials), lymphoma (99 trials), and head and neck cancer (72 trials) were the most studied, while research on GC was relatively limited. In this section, we list the latest clinical trial research progress of ICI drugs for advanced GC in the past two years[9,10,15-18] (Table 1). Compared with the results of previous clinical trials of ICI monotherapy, these trials of ICI combinations showed better objective response rates (ORRs) and median progression free survival (PFS) commonly. In the open-label phase 2 trial EPOC1706, patients with advanced GC were given Lenvatinib (a multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors and other receptor tyrosine kinases) plus pembrolizumab as anti-tumor therapy in the first-line or second-line settings[15]. This trial enrolled 29 patients with metastatic or recurrent adenocarcinoma of G/GEJ and measured disease using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. The primary endpoint of the trial was the ORR by RECIST. The results report showed that 20 [69%, 95% confidence interval (CI): 49-85] of 29 patients had an objective response at data cutoff. This indicated that Lenvatinib plus pembrolizumab has promising anti-tumor activity in patients with advanced GC.
| Ref. | Trial phase | Cancer type | n | Drug(s) tested | Key outcome | Trial name (NCT) |
| Kawazoe et al[15], 2020 | 2 | GC | 29 | Pembrolizumab + lenvatinib | ORR 69% (95%CI: 49-85) mPFS 7.1 mo (95%CI: 5.4-13.7) | EPOC1706 (NCT03609359) |
| Kawazoe et al[16], 2020 | 2b | GC/GEJC | 54 | Pembrolizumab + SOX | ORR 72.2% (95%CI: 58.4-83.5) mPFS 6.9 mo (95%CI: 5.6-8.3) | KEYNOTE-659 (NCT03382600) |
| Catenacci et al[17], 2020 | 1b-2 | GEJC | 92 | Margetuximab + pembrolizumab | ORR 18% (95%CI: 11-28) mPFS 2.73 mo | CP-MGAH22-05 (NCT02689284) |
| Bang et al[9], 2019 | 2 | GC/GEJC | 25 | Pembrolizumab + SOC | ORR 60.0% (95%CI: 38.7-78.9) mPFS 6.6 mo (95%CI: 5.9-10.6) | KEYNOTE-059 (NCT02335411) |
| 31 | Pembrolizumab | ORR 25.8% (95%CI: 11.9–44.6) mPFS 3.3 mo (95%CI: 2.0-6.0) | ||||
| Tabernero et al[10], 2019 | 3 | GC/GEJC | 256 | Pembrolizumab | ORR 14.5% (95%CI: 10.4-19.4) mPFS 2.0 mo (95%CI: 1.5-2.8) | KEYNOTE-062 (NCT02494583) |
| 257 | Pembrolizumab + SOC | ORR 48.6% (95%CI: 42.4-54.9) mPFS 6.9 mo (95%CI: 5.7-7.3) | ||||
| 250 | Placebo + SOC | ORR 36.8% (95%CI: 30.8-43.1) mPFS 6.4 mo (95%CI: 5.7-7.0) | ||||
| Boku et al[18], 2019 | 2 | G/GEJ | 21 | Nivolumab + SOX | ORR 57.1% (95%CI: 34.0-78.2) mPFS 9.7 mo (95%CI: 5.8-NR) | ATTRACTION-4 (NCT02746796) |
| 18 | Nivolumab + CapeOX | ORR 76.5% (95%CI: 50.1-93.2) mPFS 10.6 m0 (95%CI: 5.6-12.5) |
Another non-randomized, multicenter, open-label phase IIb study, KEYNOTE-659, evaluated the efficacy and safety of pembrolizumab in combination with S-1 plus oxaliplatin (SOX) as first-line treatment in Japanese patients with G/GEJ cancer[16]. At data cutoff, 54 patients were evaluated and the median follow-up was 10.1 mo. The ORR and disease control rate by blinded independent central review were 72.2% (95%CI: 58.4-83.5) and 96.3% (95%CI: 87.3-99.5), respectively. Median duration of response, time to response, PFS, and OS were as follows: Not reached, 1.5 mo, 9.4 mo, and not reached. This result demonstrated encouraging efficacy for SOX plus pembrolizumab treatment of advanced G/GEJ cancer.
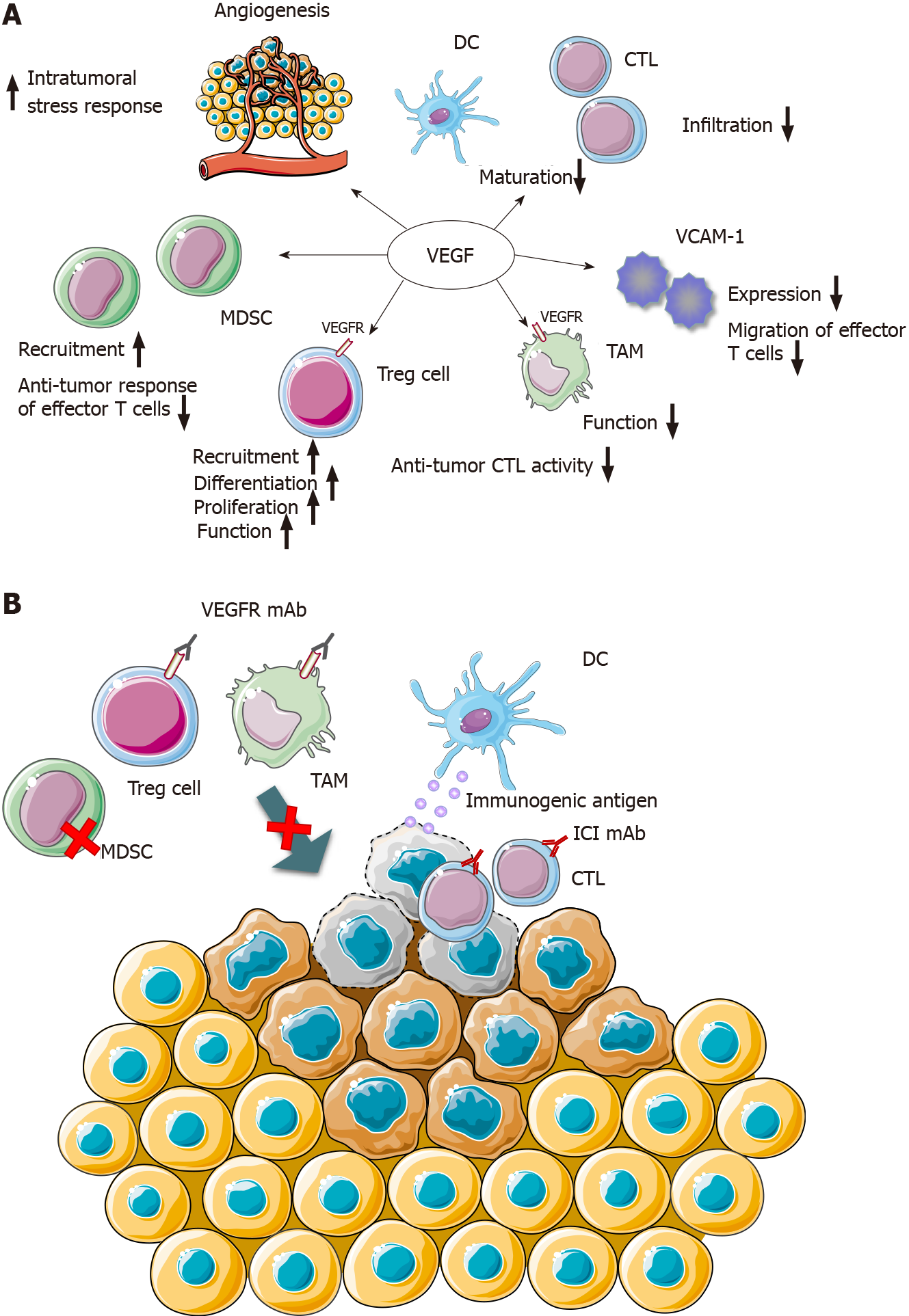
The EPOC1706 trial of combination therapy trial of ICI and antivascular targeted therapy indicated that the VEGF pathway can regulate the antitumor immune response to promote better efficacy of ICIs. Previous research proved that tumor-induced angiogenic factors, including VEGF, could stimulate tumor neovascularization and produce functional and structural abnormalities, leading to an increased intratumoral stress response and hindering of the infiltration of effector T cells[19,20]. Anti-tumor cytotoxic T lymphocytes (CTLs) fail to penetrate into the tumor microenvironment (including GC). Other factors, including downregulation of vascular endothelial cell adhesion molecule-1, prevent the migration of effector T cells into the tumor stroma[21]. In addition, the accumulation of myeloidderived inhibitor cells further suppresses the anti-tumor response of effector T cells[22]. Preventing the maturation of effector T cells and dendritic cells also inhibits the function of CTLs[22]. Therefore, the normalization of tumor blood vessels after anti-VGFR therapy can reduce the intratumoral pressure, restore anti-CTL infiltration, and enhance the anti-tumor effect of ICI[23,24]. In addition, several types of immunosuppressive cells, including regulatory T cells (Tregs) and tumor-associated macrophages (TAMs), are present in the tumor microenvironment of GC. Previous studies have reported that the activity of Tregs and TAMs depends in part on the VEGF/VEGF receptor (VEGFR) axis, and overexpression of VEGF promotes the recruitment, differentiation, and proliferation of Tregs in tumors[25]. Likewise, tumor-induced angiogenic factors or a hypoxic state can further enhance the function of Tregs and TAMs via the VEGF/VEGFR axis, resulting in downregulation of anti-tumor CTL activity[26]. These above-mentioned mechanisms provide a conceivable theoretical basis for the combined therapy of anti-vascular targeted drugs and ICIs (Figure 1). Kato et al[27] certified that the antivascular targeted drug lenvatinib, via decreasing TAMs and enhancing the activation of the interferon (IFN) signaling pathway, upregulated the anti-tumor activity of PD-1 inhibitors in vivo[27].
In the past decade, researchers have explored the hypothesis that traditional chemotherapy drugs might interact with the immune mechanism. For instance, chemotherapy drugs such as cyclophosphamide, gemcitabine, platinum, and paclitaxel have been reported to improve the antigenicity of tumor cells by increasing the expression of major histocompatibility complex class I molecules[28,29] and enhancing the sensitivity of tumor cells to immune therapy by upregulation of the mannose-6-phosphate receptor (e.g., paclitaxel, cisplatin, and doxorubicin)[30]. Vacchelli et al[31] stated that chemotherapy drugs (paclitaxel, doxorubicin, and cisplatin) could activate the immune system via direct effects on cytotoxic lymphocytes and the elimination of immunosuppressive cells via clinical trial results of certain chemotherapy drugs (paclitaxel, gemcitabine, and 5Fu)[31]. Traditional chemotherapy drugs are able to enhance the patients' antitumor immune response, and ICIs could further eliminate tumor foci that are resistant to chemotherapy, correspondingly. Thus, the combination of ICI and chemotherapeutics for the treatment of advanced refractory tumors presents clinical benefits, and numerous clinical trials seem to have verified this hypothesis (KEYNOTE-659, KEYNOTE-059, and KEYNOTE-062).
Moreover, additional combination treatment options are worth exploring. The combined use of two ICI drug treatments, such as the combination of CTLA4 and PD-1/PD-L1 blocking antibodies, can kill T lymphocytes in the immune initial and effector stages[32]. For example, the Checkmate032 trial aims to assess the efficacy of the combination treatment of nivolumab (anti-PD-1 antibody) and ipilimumab (anti-CTL4 antibody) in patients with advanced solid tumors, including advanced GC. As a result, the combined scheme of nivolumab (1 mg/kg) + ipilimumab (3 mg/kg) had a sustained antitumor effect in the treatment of advanced GC, thereby prolonging the OS[33,34]. In addition, radiotherapy is certified to cause immunogenic cell death and has a synergistic effect on anti-tumor CTLs[35]. This principle revealed the possibility of the combined application of radiotherapy and ICI, although there has been no corresponding clinical trial for GC at present. In a novel case report, a patient with HER2-negative advanced GC (stage IV, T4N3M1) received 4 mo of treatment with a combination of concurrent SOX regimen chemotherapy, radiotherapy, and ICI immunotherapy, which showed a satisfactory complete response for tumor lesions, even metastatic lesions (almost complete response, disappearance of all target lesions)[36]. This example demonstrated that the continuous exploration and advances of combined immunotherapy will improve the survival benefits of patients with GC significantly.
The Cancer Genome Atlas has proposed a molecular classification dividing GC into four subtypes: EBV positive (9%), genomically stable (20%), MSI-high (22%), and chromosomal instability (50%)[37]. Among the four molecular subtypes of GC, EBV-positive tumors and MSI-high tumors show better responses to ICIs[38]. The Food and Drug Administration (FDA) has approved treatment with pembrolizumab for patients with PD-L1 positive and MSI-high/DNA mismatch repair deficiency (dMMR) advanced GC in the second- or third-line setting[39,40]. Accordingly, PD-L1 positive and MSI-high/dMMR advanced GC patients are currently the most widely applied for ICI therapy.
PD-L1 expression in tumor cells is determined by immunohistochemistry using formalin-fixed paraffin-embedded sections, and the proportion of PD-L1-stained tumor cells and immune cells is calculated to obtain a clinical prediction score (CPS). The result of KEYNOTE-059 trial shows that patients with PD-L1 positive tumors (CPS ≥ 1) achieved 22.7% ORR and 2.7% complete response via anti-PD-1 treatment, compared with 8.6% ORR and 3.4% complete response of patients with PD-L1 negative tumors (CPS < 1)[39]. Another randomized phase III trial, KEYNOTE-062[10], enrolled 763 patients with HER2-negativity and CPS ≥ 1. The results showed that 281 (37% of the enrollees) had a CPS score of ≥ 10. The patients were divided into three groups by their treatment options as initial therapy: Intravenous pembrolizumab, pembrolizumab plus chemotherapy, or chemotherapy plus placebo. The primary endpoint of this trial showed that for patients with HER2-negative, PD-L1-positive (CPS ≥ 1), advanced GC, combination treatment with pembrolizumab plus chemo
Microsatellites are repetitive sequences of small DNA fragments that exist in the genome, and false microsatellites are produced and accumulated during replication, which is called MSI-high status. Mismatched DNA is frequently repaired by the mismatch repair system, and therefore MSI-high status is usually related to dMMR. Once MSI-high/dMMR status occurs in tumor cells, immune mechanism could handle hypermutation generation and the formation of immunogenic neoantigens. Consequently, numerous immune cells accumulate in tumors with MSI-high/dMMR characteristics, and ICIs are more effective for such tumors[41]. In the report of the phase II KEYNOTE-059 trial, the ORR of ICI therapy of advanced GC patients in the MSI high group was significantly higher than that of the non-MSI high group (57.1% vs 9.0%). Meanwhile, the authors of KEYNOTE-062 trial presented a directional analysis of 50 patients with MSI-high tumors, indicating that the ORRs of patients treated with pembrolizumab monotherapy and chemotherapy were 57.1% and 36.8%, respectively, and those of patients treated with pembrolizumab plus chemotherapy vs chemotherapy was 64.7% vs 36.8%[10].
Furthermore, high tumor mutational burden (TMB), EBVpositivity, immune-related gene expression, IFNG (IFN-γ) gene activation, and circulating tumor DNA (ctDNA) have been explored as other biomarkers to predict the clinical endpoints of immunotherapy using ICIs[42,43]. TMB represents the total number of mutations in each coding region of a tumor genome, and it has been found that ICI-treated patients with high TMB exhibit a considerable ORR and PFS in multiple cancers[42]. Derks et al[38] showed that tumor or tumor infiltrating immune cells with PD-L1 expression are also a general phenomenon in EBV-positive GC, as well as enrichment of an IFNG gene expression signature[38]. Interestingly, in the KEYNOTE-059 trial, Fuchs et al[39] reported that six patients with EBV-positive tumors achieved a partial or complete remission using pembrolizumab treatment[39], suggesting that EBV-positive tumors show an effective response to ICI treatment. In the same report, the expression profile of 18 immune-related genes [CCL5, CD27, CD274 (PD-L1), CD276 (B7-H3), CD8A, CXCL9, CMKLR1, CXCR6, HLADQA1, HLA-DRB1, HLAE, NKG7, IDO1, LAG3, PDCD1 -KLG2 (PD-L2), PSMB10, STAT1, and TGIT] illustrated that the statistical score of responders among patients with GC was significantly higher than that of non-responders. Similarly, six IFN-γ-related gene signatures were remarkably associated with the improvement of PFS in ICI-treated patients with GC[43]. Thus, the proposed association with IFN-γ signal transduction and T cell activation of immune-related gene biological signatures, can be used as a biomarker to predict the efficacy of ICI treatment. ctDNA is released into the circulatory system from apoptotic or necrotic tumor cells, and could be used for early diagnosis of some cancers. Moreover, ctDNA mutational burden score is related to the clinical response of patients with GC to pembrolizumab treatment, indicating that ctDNA can be used to screen those patients with GC that are sensitive to ICI treatment[44].
To explore the best schemes of immune combination treatments, it is essential to determine the best endpoints in early phase clinical trials to better opt the correct schemes for confirmatory randomized trials[45]. The development and ratification of single-agent ICIs depended largely on the FDA accelerated approval, and ORR is typically adopted as the primary endpoint in the ICI clinical trials leading to accelerated approval[46]. Table 1 lists these clinical trials that employed ORR as the primary endpoint, and the use of the ORR seemed to prove the excellent effect of ICI treatment. However, traditional clinical trial endpoints, like ORR and PFS, might not be able to predict the long-term survival of patients receiving ICI treatments because the mechanisms of action and reaction patterns for ICI agents differ substantially from those of conventional chemotherapy. Equally, conventional RECIST criteria might underestimate the benefit from ICI agents[47,48]. For instance, pseudoprogression was initially described in advanced melanoma treated with ipilimumab and was incapable of being assessed by the conventional RECIST criteria[49,50]. Chiou et al[50] reported that ORR is correlated insufficiently with PFS and OS in the single-arm and randomized trials of ICI treatment (the r correlation coefficients of ORR with 6-mo PFS and 12-mo OS were 0.37 and 0.08, respectively). By contrast, a conspicuous correlation between 6-mo PFS and 12-mo OS was discovered (the r correlation coefficient was 0.74)[51]. Thus, in single-arm trials, ICI efficacy analysis by selecting the PFS rate at particular time points (e.g., 6 or 12 mo) might be considered as a better surrogate endpoint than ORR, thereby also improving the evaluation criteria of solid tumors under ICI treatment.
ICI-related trials with multiple primary endpoints require rigorous methods to control the overall type I error rate, yet the generally recognized clinical benefits of ICI therapy in patients with advanced cancer might not be "statistically significant"[46], as demonstrated by the IMvigor211 trial of atezolizumab for the treatment of urothelial carcinoma and the KEYNOTE-240 trial of pembrolizumab for the treatment of advanced hepatocellular carcinoma[52,53]. The demand for volunteers has greatly increased with the development of more and more clinical trials, especially in past few years, and ICI trials have been recruiting patients faster than other interventional oncology trials. However, the recruitment rate has recently declined significantly, from 1.15 patients per site every month in 2014 to 0.35 patients per site every month in 2018, indicating that combination trials may face recruitment challenges in the near future[14]. Furthermore, the low recruitment rate not only affects the progress of clinical trials, but also incurs greater costs. Formulating innovative, efficient, and rational trial schemes to allocate patient volunteers reasonably is required.
Neoadjuvant chemotherapy with FLOT (5-FU, leucovorin, oxaliplatin, and docetaxel) regimen is the standard treatment in the West. In contrast, SOX regimen is the preferred neoadjuvant chemotherapy regimen in the East. The phase II randomized clinical trial NCT03636893 reported 74 patients with locally advanced resectable GC, and the primary outcomes did not show statistically significant differences between neoadjuvant FLOT and SOX regimens[54]. The FLOT and SOX groups showed desired therapeutic effect, yet several hematological grade 3–4 adverse events were observed (29.0% and 16.1%, respectively). It is necessary to explore more treatment methods to expand the scope of medication, and to design the best combined treatment plan for patients to maximize anti-tumor effects and reduce adverse events.
The neoadjuvant treatment of ICI is applied presurgically, playing a pivotal role in radical treatment for tumors, such as anti–CTLA-4 treatment for bladder cancer and melanoma[55,56]. Mechanistically, compared with adjuvant therapy directed only against micrometastatic foci after resection, neoadjuvant ICI makes full use of higher tumor antigen load in vivo before surgery, resulting in more tumor-specific T cells being present in the systemic circulation[57]. Anti-PD-L1/anti-PD-1 monoclonal antibody restores the activity of tumor-specific cytotoxic T cells that already exist in the tumor microenvironment and promotes their activation, proliferation, and transfer to micrometastases. Afterwards, the focus of anti-PD-(L)1 activity might be in the tumor draining lymph nodes, where dendritic cells present tumor antigens to T cells, and then these tumor-specific T cells enter the bloodstream and migrate to the tumor sites[57,58]. The aforementioned hypothetical mechanism was verified in a spontaneously metastatic transplantable mouse breast cancer model, showing that survival after neoadjuvant immunotherapy was significantly better than that after adjuvant immunotherapy[59]. Topalian et al[57] proposed several potential clinical advantages of neoadjuvant therapy, including preoperative tumor shrinkage and the capability to assess the pathological response as an early surrogate indicator for relapse-free survival and OS, offering sufficient tissue availability therapies for in-depth scientific research to explore mechanism of drug action and predictive biomarkers[57]. Currently, clinical trials of neoadjuvant immunotherapy based on ICIs have started, such as the first trial report of neoadjuvant anti–PD-1 therapy, which is a phase II trial of nivolumab in 21 patients with high-risk (stage I, II, or IIIA) non–small-cell lung cancer, which has indicated that 45% of resected tumors have a major pathological response[60]. However, few clinical trials have been carried out on the application of ICI as neoadjuvant treatment in GC, and only one clinical trial (NCT03064490) is registered in ClinicalTrials.gov. Jin et al[61] submitted a notable case report, in which a patient with PD-L1 positive and MSI-high advanced GC (cT4aN+M0 state) received a single dose of anti-PD-1 therapy in combination with chemotherapy and radical gastrectomy, resulting in a pathological complete response (pT0N0M0)[61]. This case report suggested that a combined ICI scheme might be feasible as neoadjuvant treatment of GC.
Through the continuous exploration of clinical trials, the combined scheme of immunotherapy for GC is being implemented. Based on previous feedback, identifying the most valuable predictive biomarkers and trial schemes to promote the further development of clinical trials is required. In terms of prolonging the survival of patients with GC, ICI might possess merit as a neoadjuvant treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mao X, Yao K S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20483] [Article Influence: 2048.3] [Reference Citation Analysis (19)] |
| 2. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1112] [Article Influence: 123.6] [Reference Citation Analysis (0)] |
| 3. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5285] [Article Influence: 352.3] [Reference Citation Analysis (3)] |
| 4. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1904] [Article Influence: 238.0] [Reference Citation Analysis (1)] |
| 5. | Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1782] [Article Influence: 178.2] [Reference Citation Analysis (0)] |
| 6. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2870] [Cited by in RCA: 3555] [Article Influence: 355.5] [Reference Citation Analysis (0)] |
| 7. | Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer--response. Clin Cancer Res. 2013;19:5542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Chen C, Zhang F, Zhou N, Gu YM, Zhang YT, He YD, Wang L, Yang LX, Zhao Y, Li YM. Efficacy and safety of immune checkpoint inhibitors in advanced gastric or gastroesophageal junction cancer: a systematic review and meta-analysis. Oncoimmunology. 2019;8:e1581547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, Borg C, Doi T, Yoon HH, Savage MJ, Wang J, Dalal RP, Shah S, Wainberg ZA, Chung HC. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22:828-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 10. | Tabernero J, Van Cutsem E, Bang YJ, Fuchs CS, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Salguero HRC, Mansoor W, Braghiroli M, Goekkurt E, Chao J, Wainberg ZA, Kher U, Shah S, Kang SP, Shitara K. Pembrolizumab with or without chemotherapy vs chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. J Clin Oncol. 2019;37:2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 11. | Satoh T, Kang YK, Chao Y, Ryu MH, Kato K, Cheol Chung H, Chen JS, Muro K, Ki Kang W, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Tanimoto M, Chen LT, Boku N. Exploratory subgroup analysis of patients with prior trastuzumab use in the ATTRACTION-2 trial: a randomized phase III clinical trial investigating the efficacy and safety of nivolumab in patients with advanced gastric/gastroesophageal junction cancer. Gastric Cancer. 2020;23:143-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS; KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 991] [Article Influence: 141.6] [Reference Citation Analysis (0)] |
| 13. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F. Gastric cancer. The Lancet. 2020;396:635-648. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2792] [Article Influence: 558.4] [Reference Citation Analysis (5)] |
| 14. | Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. 2018;17:854-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 300] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 15. | Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, Mikamoto Y, Shima H, Fujishiro N, Higuchi T, Sato A, Kuwata T, Shitara K. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 16. | Kawazoe A, Yamaguchi K, Yasui H, Negoro Y, Azuma M, Amagai K, Hara H, Baba H, Tsuda M, Hosaka H, Kawakami H, Oshima T, Omuro Y, Machida N, Esaki T, Yoshida K, Nishina T, Komatsu Y, Han SR, Shiratori S, Shitara K. Safety and efficacy of pembrolizumab in combination with S-1 plus oxaliplatin as a first-line treatment in patients with advanced gastric/gastroesophageal junction cancer: Cohort 1 data from the KEYNOTE-659 phase IIb study. Eur J Cancer. 2020;129:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Catenacci DVT, Kang YK, Park H, Uronis HE, Lee KW, Ng MCH, Enzinger PC, Park SH, Gold PJ, Lacy J, Hochster HS, Oh SC, Kim YH, Marrone KA, Kelly RJ, Juergens RA, Kim JG, Bendell JC, Alcindor T, Sym SJ, Song EK, Chee CE, Chao Y, Kim S, Lockhart AC, Knutson KL, Yen J, Franovic A, Nordstrom JL, Li D, Wigginton J, Davidson-Moncada JK, Rosales MK, Bang YJ; CP-MGAH22-5 Study Group. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21:1066-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 18. | Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, Cho H, Kang WK, Komatsu Y, Tsuda M, Yamaguchi K, Hara H, Fumita S, Azuma M, Chen LT, Kang YK. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. 2019;30:250-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 19. | Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171-6180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 525] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 20. | Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, Hernandez G, Mier J, He X, Hodi FS, Denker M, Leveque V, Cañamero M, Babitski G, Koeppen H, Ziai J, Sharma N, Gaire F, Chen DS, Waterkamp D, Hegde PS, McDermott DF. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 554] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 21. | Ott PA, Hodi FS, Buchbinder EI. Inhibition of Immune Checkpoints and Vascular Endothelial Growth Factor as Combination Therapy for Metastatic Melanoma: An Overview of Rationale, Preclinical Evidence, and Initial Clinical Data. Front Oncol. 2015;5:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 22. | Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150-4166. [PubMed] |
| 23. | Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4319] [Cited by in RCA: 3951] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 24. | Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15:310-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 465] [Article Influence: 66.4] [Reference Citation Analysis (1)] |
| 25. | Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 26. | Tada Y, Togashi Y, Kotani D, Kuwata T, Sato E, Kawazoe A, Doi T, Wada H, Nishikawa H, Shitara K. Targeting VEGFR2 with Ramucirumab strongly impacts effector/ activated regulatory T cells and CD8+ T cells in the tumor microenvironment. J Immunother Cancer. 2018;6:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 27. | Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y, Matsuki M, Matsuoka Y, Ghosh S, Kitano H, Nomoto K, Matsui J, Funahashi Y. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14:e0212513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 353] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 28. | Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 698] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 29. | Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013;62:203-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 30. | Ramakrishnan R, Huang C, Cho HI, Lloyd M, Johnson J, Ren X, Altiok S, Sullivan D, Weber J, Celis E, Gabrilovich DI. Autophagy induced by conventional chemotherapy mediates tumor cell sensitivity to immunotherapy. Cancer Res. 2012;72:5483-5493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Vacchelli E, Aranda F, Eggermont A, Galon J, Sautès-Fridman C, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch Onco Immunol. 2014;e27878. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 32. | Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 2175] [Article Influence: 310.7] [Reference Citation Analysis (0)] |
| 33. | Janjigian YY, Bendell JC, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Bono P, Jaeger D, Evans TRJ, De Braud FG, Chau I, Tschaika M, Harbison CT, Lin CS, Le DT. CheckMate-032: Phase I/II open-label study of safety and activity of nivolumab (nivo) alone or with ipilimumab (ipi) in advanced and metastatic (A/M) gastric cancer (GC). J Clin Oncol. 2016;34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Le DT, Bendell JC, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Bono P, Jaeger D, Evans TRJ, De Braud FG, Chau I, Christensen O, Harbison C, Lin CS, Janjigian YY. Safety and activity of nivolumab monotherapy in advanced and metastatic (A/M) gastric or gastroesophageal junction cancer (GC/GEC): Results from the CheckMate-032 study. J Clin Oncol. 2016;34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72:3967-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 36. | Yu S, Cai L, Lin F, Wu X, Zhang C, Liu X, Li W. Durable Response After Combination Of Concurrent Chemoradiotherapy And Anti-PD-1 Therapy In HER2-Negative Advanced Gastric Adenocarcinoma: A Case Report. Onco Targets Ther. 2019;12:7691-7698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C, Shen H, Weisenberger DJ, Schultz N, Shen RL, Weinhold N, Keiser DP, Bowlby R, Sipahimalani P, Cherniack AD, Getz G, Liu YC, Noble MS, Pedamallu C, Sougnez C, Taylor-Weiner A, Akbani R, Lee JS, Liu WB, Mills GB, Yang D, Zhang W, Pantazi A, Parfenov M, Gulley M, Piazuelo MB, Schneider BG, Kim J, Boussioutas A, Sheth M, Demchok JA, Rabkin CS, Willis JE, Ng S, Garman K, Beer DG, Pennathur A, Raphael BJ, Wu HT, Odze R, Kim HK, Bowen J, Leraas KM, Lichtenberg TM, Weaver L, McLellan M, Wiznerowicz M, Sakai R, Lawrence MS, Cibulskis K, Lichtenstein L, Fisher S, Gabriel SB, Lander ES, Ding L, Niu BF, Ally A, Balasundaram M, Birol I, Brooks D, Butterfield YSN, Carlsen R, Chu A, Chu J, Chuah E, Chun HJE, Clarke A, Dhalla N, Guin R, Holt RA, Jones SJM, Kasaian K, Lee D, Li HYA, Lim E, Ma Y, Marra MA, Mayo M, Moore RA, Mungall AJ, Mungall KL, Nip KM, Robertson AG, Schein JE, Tam A, Thiessen N, Beroukhim R, Carter SL, Cho J, DiCara D, Frazer S, Gehlenborg N, Heiman DI, Jung J, Lin P, Meyerson M, Ojesina AI, Pedamallu CS, Saksena G, Schumacher SE, Stojanov P, Tabak B, Voet D, Rosenberg M, Zack TI, Zhang HL, Zou LH, Protopopov A, Santoso N, Lee S, Zhang J, Mahadeshwar HS, Tang JB, Ren XJ, Seth S, Yang LX, Xu AW, Song XZ, Xi RB, Bristow CA, Hadjipanayis A, Seidman J, Chin L, Park PJ, Kucherlapati R, Ling SY, Rao A, Weinstein JN, Kim SB, Lu YL, Mills G, Bootwalla MS, Lai PH, Triche T, Van Den Berg DJ, Baylin SB, Herman JG, Murray BA, Askoy BA, Ciriello G, Dresdner G, Gao JJ, Gross B, Jacobsen A, Lee W, Ramirez R, Sander C, Senbabaoglu Y, Sinha R, Sumer SO, Sun YC, Iype L, Kramer RW, Kreisberg R, Rovira H, Tasman N, Haussler D, Stuart JM, Verhaak RGW, Leiserson MDM, Taylor BS, Black AD, Carney JA, Gastier-Foster JM, Helsel C, McAllister C, Ramirez NC, Tabler TR, Wise L, Zmuda E, Penny R, Crain D, Gardner J, Lau K, Curely E, Mallery D, Morris S, Paulauskis J, Shelton T, Shelton C, Sherman M, Benz C, Lee JH, Fedosenko K, Manikhas G, Voronina O, Belyaev D, Dolzhansky O, Rathmell WK, Brzezinski J, Ibbs M, Korski K, Kycler W, Lazniak R, Leporowska E, Mackiewicz A, Murawa D, Murawa P, Spychala A, Suchorska WM, Tatka H, Teresiak M, Abdel-Misih R, Bennett J, Brown J, Iacocca M, Rabeno B, Kwon SY, Kemkes A, Curley E, Alexopoulou I, Engel J, Bartlett J, Albert M, Park DY, Dhir R, Luketich J, Landreneau R, Janjigian YY, Kelsen DP, Cho E, Ladanyi M, Tang L, McCall SJ, Park YS, Cheong JH, Ajani J, Camargo MC, Alonso S, Ayala B, Jensen MA, Pihl T, Raman R, Walton J, Wan YH, Eley G, Shaw KRM, Tarnuzzer R, Wang ZN, Yang LM, Zenklusen JC, Davidsen T, Hutter CM, Sofia HJ, Burton R, Chudamani S, Liu J, Network CGAR. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4815] [Article Influence: 437.7] [Reference Citation Analysis (2)] |
| 38. | Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, Sessa F, Fleitas T, Freeman GJ, Rodig SJ, Rabkin CS, Bass AJ. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7:32925-32932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 250] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 39. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1452] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 40. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7201] [Article Influence: 720.1] [Reference Citation Analysis (0)] |
| 41. | Kono K, Nakajima S, Mimura K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer. 2020;23:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 42. | Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377:2500-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1693] [Cited by in RCA: 2401] [Article Influence: 300.1] [Reference Citation Analysis (0)] |
| 43. | Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, Piha-Paul SA, Yearley J, Seiwert TY, Ribas A, McClanahan TK. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930-2940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1686] [Cited by in RCA: 2687] [Article Influence: 335.9] [Reference Citation Analysis (0)] |
| 44. | Kim JW, Lee HS, Nam KH, Ahn S, Kim JW, Ahn SH, Park DJ, Kim HH, Lee KW. PIK3CA mutations are associated with increased tumor aggressiveness and Akt activation in gastric cancer. Oncotarget. 2017;8:90948-90958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Upadhaya S, Neftelino ST, Hodge JP, Oliva C, Campbell JR, Yu JX. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov. 2021;20:168-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 46. | Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (1)] |
| 47. | Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, Joshua AM, Hersey P, Dronca R, Joseph R, Hille D, Xue D, Li XN, Kang SP, Ebbinghaus S, Perrone A, Wolchok JD. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol. 2016;34:1510-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 581] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 48. | Bohnsack O, Hoos A, Ludajic K. Adaptation and modification of the immune related response criteria (IRRC): IrRECIST. J Clin Oncol. 2014;32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11750] [Article Influence: 783.3] [Reference Citation Analysis (0)] |
| 50. | Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33:3541-3543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 675] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 51. | Anagnostou V, Yarchoan M, Hansen AR, Wang H, Verde F, Sharon E, Collyar D, Chow LQM, Forde PM. Immuno-oncology Trial Endpoints: Capturing Clinically Meaningful Activity. Clin Cancer Res. 2017;23:4959-4969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 52. | Finn RS, Ryoo B-Y, Merle P, Kudo M, Bouattour M, Lim H-Y, Breder VV, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox JJ, Daniele B, Ebbinghaus S, Chen E, Siegel AB, Zhu AX, Cheng A-L. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2019;37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 53. | Hung HM, Wang SJ, O'Neill R. Statistical considerations for testing multiple endpoints in group sequential or adaptive clinical trials. J Biopharm Stat. 2007;17:1201-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Sah BK, Zhang B, Zhang H, Li J, Yuan F, Ma T, Shi M, Xu W, Zhu Z, Liu W, Yan C, Li C, Liu B, Yan M. Neoadjuvant FLOT versus SOX phase II randomized clinical trial for patients with locally advanced gastric cancer. Nat Commun. 2020;11:6093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 55. | Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, Ku G, Troncoso P, Logothetis CJ, Allison JP, Sharma P. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861-2871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 378] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 56. | Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, Sander C, Yin Y, Holtzman M, Johnson J, Rao UN, Kirkwood JM. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One. 2014;9:e87705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 57. | Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 694] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 58. | Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, Chakarov S, Rivera C, Hogstad B, Bosenberg M, Hashimoto D, Gnjatic S, Bhardwaj N, Palucka AK, Brown BD, Brody J, Ginhoux F, Merad M. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44:924-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 925] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 59. | Liu J, Blake SJ, Yong MC, Harjunpää H, Ngiow SF, Takeda K, Young A, O'Donnell JS, Allen S, Smyth MJ, Teng MW. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016;6:1382-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 639] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 60. | Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR, Broderick S, Battafarano RJ, Velez MJ, Rekhtman N, Olah Z, Naidoo J, Marrone KA, Verde F, Guo H, Zhang J, Caushi JX, Chan HY, Sidhom JW, Scharpf RB, White J, Gabrielson E, Wang H, Rosner GL, Rusch V, Wolchok JD, Merghoub T, Taube JM, Velculescu VE, Topalian SL, Brahmer JR, Pardoll DM. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;378:1976-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 1462] [Article Influence: 208.9] [Reference Citation Analysis (0)] |
| 61. | Jin H, Li P, Mao C, Zhu K, Chen H, Gao Y, Yu J. Pathological Complete Response After a Single Dose of Anti-PD-1 Therapy in Combination with Chemotherapy as a First-Line Setting in an Unresectable Locally Advanced Gastric Cancer with PD-L1 Positive and Microsatellite Instability. Onco Targets Ther. 2020;13:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |