Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5744
Peer-review started: March 21, 2021
First decision: April 29, 2021
Revised: May 12, 2021
Accepted: May 27, 2021
Article in press: May 27, 2021
Published online: July 16, 2021
Processing time: 107 Days and 11.5 Hours
Several cutaneous manifestations such as urticarial rash, erythematous patches and chilblain-like lesions have been described in young adults with coronavirus disease 2019 (COVID-19) and are present in up to 20% patients, but few reports exist describing histopathological and immunophenotypic characteristics of dermatological lesions in older patients. Our aim was to characterize skin lesions in elderly patients during late stages of COVID-19 from clinical, histological and immunophenotypic perspectives.
Three patients, admitted for COVID-19, and who developed cutaneous manifestations underwent skin biopsies. Immunophenotypic analysis for CD20, CD3, CD4 and CD8 was performed on skin biopsies to assess immune cell infiltrates. CD1a was used as a marker of Langerhans cells, and CD31 as a marker of endothelial cells. In the three study patients, cutaneous manifestations were evident in the late-stage of COVID-19 (mean time from the first positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) swab to rash onset was 35 d). Skin biopsies showed a similar pattern of T lymphocyte infiltration in all patients. Indeed, a chronic dermatitis with perivascular lymphocytic infiltrate was observed with predominance of CD3+ T-cell (CD3+).
Our study confirms previous reports. Histological and immunophenotypic patterns in our patients confirm results described in the two previous reported experiences. This pattern is similar to what is found in some lympho-proliferative disorders. Therefore, since these findings are non-specific, SARS-CoV-2 infection should be suspected.
Core Tip: We report results of histological and immunophenotypic analysis of skin rashes in three elderly patients with coronavirus disease 2019 (COVID-19). We found a pattern of T lymphocyte infiltration similar to that present in some lympho-proliferative disorders. In order to clarify the pathogenesis and the clinical significance if skin lesions in patients with COVID-19, further studies on histology and immunophenotyping are necessary.
- Citation: Mazzitelli M, Dastoli S, Mignogna C, Bennardo L, Lio E, Pelle MC, Trecarichi EM, Pereira BI, Nisticò SP, Torti C. Histopathology and immunophenotyping of late onset cutaneous manifestations of COVID-19 in elderly patients: Three case reports. World J Clin Cases 2021; 9(20): 5744-5751
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5744.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5744
Several cutaneous manifestations have been described in the context of coronavirus disease 2019 (COVID-19)[1-5]. An Italian study of 88 hospitalized patients with COVID-19 first reported the occurrence of skin lesions in up to 20% patients with COVID-19 in the form of erythematous rash, widespread urticaria and chickenpox-like vesicles[3]. A larger study in Spain described five major clinical patterns of cutaneous manifestations of COVID-19 with the following prevalence: Erythema with vesicles or pustules (pseudo-chilblain) (19%), other vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%) and livedo or necrosis (6%)[6]. Since then, several other studies reported skin lesions in the context of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with prevalence ranging from 0.19% to 20.45%, most of them present at diagnosis of COVID-19[7]. The pathogenesis of such skin manifestations in the context of SARS-CoV-2 infection remains unclear. As described for other diseases, urticarial rashes are related with alteration of local T-cell homeostasis[8,9]. However, only a few studies characterized the histopathology of skin lesions associated with COVID-19[10-13]. Skin lesions were characterized by a superficial perivascular lymphocytic infiltrate. To our knowledge, this is one of the first case series describing both the histopathology and immunophenotyping of COVID-19-related skin lesions with late onset.
Our objective was to describe the histology and immunophenotyping of late-onset cutaneous manifestations in patients diagnosed with COVID-19.
We report herein three cases of late onset COVID-19-related skin lesions, who were admitted to Infectious and Tropical Disease Unit for COVID-19.
Three elderly patients (Cases 1, 2, and 3) were diagnosed with COVID-19 on March 25, 2020, and all received hydroxychloroquine and azithromycin (HCQ/AZI)[14]. Skin rashes occurred late in all three patients. Skin biopsies were performed for all patients on May 5, 2020 after patient consent and per clinical indication of a Consultant Dermatologist. Patients gave written consent for publication. Data was retrospectively collected after approval from our Ethics Committee. Immunohistochemical staining of serial sections of formalin-fixed and paraffin embedded skin was performed using a standard automatized protocol with the Autostainer DAKO 48. Antibodies for CD20, CD3, CD4 and CD8 were used to assess immune cell infiltrates. CD1a was used as a marker of Langerhans cells, and CD31 as a marker of endothelial cells. For each patient, follow-up real time polymerase chain reaction (PCR) for SARS-CoV-2 were performed on nasopharyngeal swabs in order to evaluate viral shedding from respiratory samples. A positive PCR result was defined by the detection of one out of three target genes of SARS-CoV-2 and considering number of cycle threshold (CT).
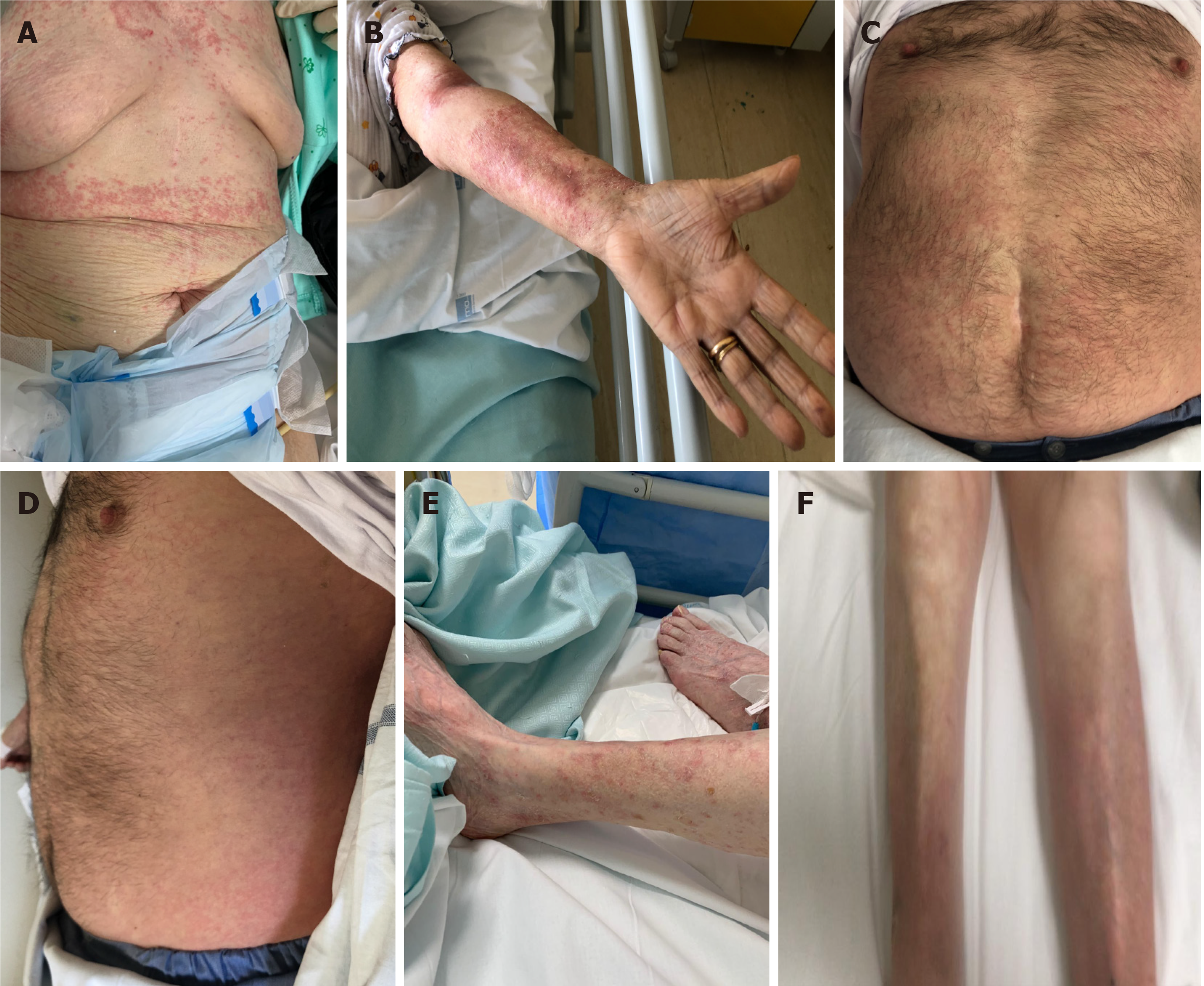
Case 1: Patient 1 was admitted on March 31, 2020 and prescribed HCQ/AZI, methylprednisolone, and enoxaparin at prophylactic dosing (all stopped after 10 d, but enoxaparin, which was continued for 14 d for the abed status). Thirty-eight days after the first positive swab, an erythematous and itchy skin rash appeared in the sub-mammary region (Figure 1A and B), rapidly extending to the trunk and the upper limbs, and spontaneously healing on May 11, 2020.
Case 2: Patient 2 was admitted on April 2, with severe COVID-19 pneumonia and was prescribed oxygen support, HCQ/AZI, piperacillin/tazobactam, methylprednisolone, and enoxaparin (all stopped within 10 d except enoxaparin, which was stopped after 20 d). Twenty-eight days after the first positive swab for SARS-CoV-2, an itchy and urticarial rash appeared on the chest and arms (Figure 1C and D), with mild increase in temperature (37.5 °C) and eosinophil count (from 50/μL to 550/μL). The rash extended to the face, chest, abdomen, wrists, and thighs, and spontaneously healed after 10 d.
Case 3: Patient 3 was admitted on April 2 with severe COVID-19 pneumonia, and prescribed oxygen support, HCQ/AZI, methylprednisolone, and enoxaparin. All drugs, except enoxaparin were stopped within 10 d. Thirty-eight days after the first positive swab for SARS-CoV-2, patient developed an itchy and erythematous rash on both the lower limbs (Figure 1E and F), worsening in the two following days, involving the trunk and the upper limbs, with spontaneous healing on May 16, 2020.
Case 1: An 89-year-old woman (patient 1) with a clinical history of hypertension, osteoporosis, chronic cerebral vasculopathy and on treatment with pantoprazole and allopurinol.
Case 2: A 65-year-old man (patient 2) with cognitive impairment and chronic psychosis on treatment with olanzapine.
Case 3: An 83-year-old female (patient 3) with hypertension, diabetes, osteoporosis, depression and cognitive impairment. She was on regular treatment with lansoprazole, tapentadol, ramipril and sertraline.
No further details are available aside from those mentioned in the history of past illness section above.
At admission, skin lesions were not detected in all three patients.
Nothing remarkable was found in blood tests for all three patients; however, mild leukopenia, increased C-reactive protein, ferritin and interleukin 6 levels were detected.
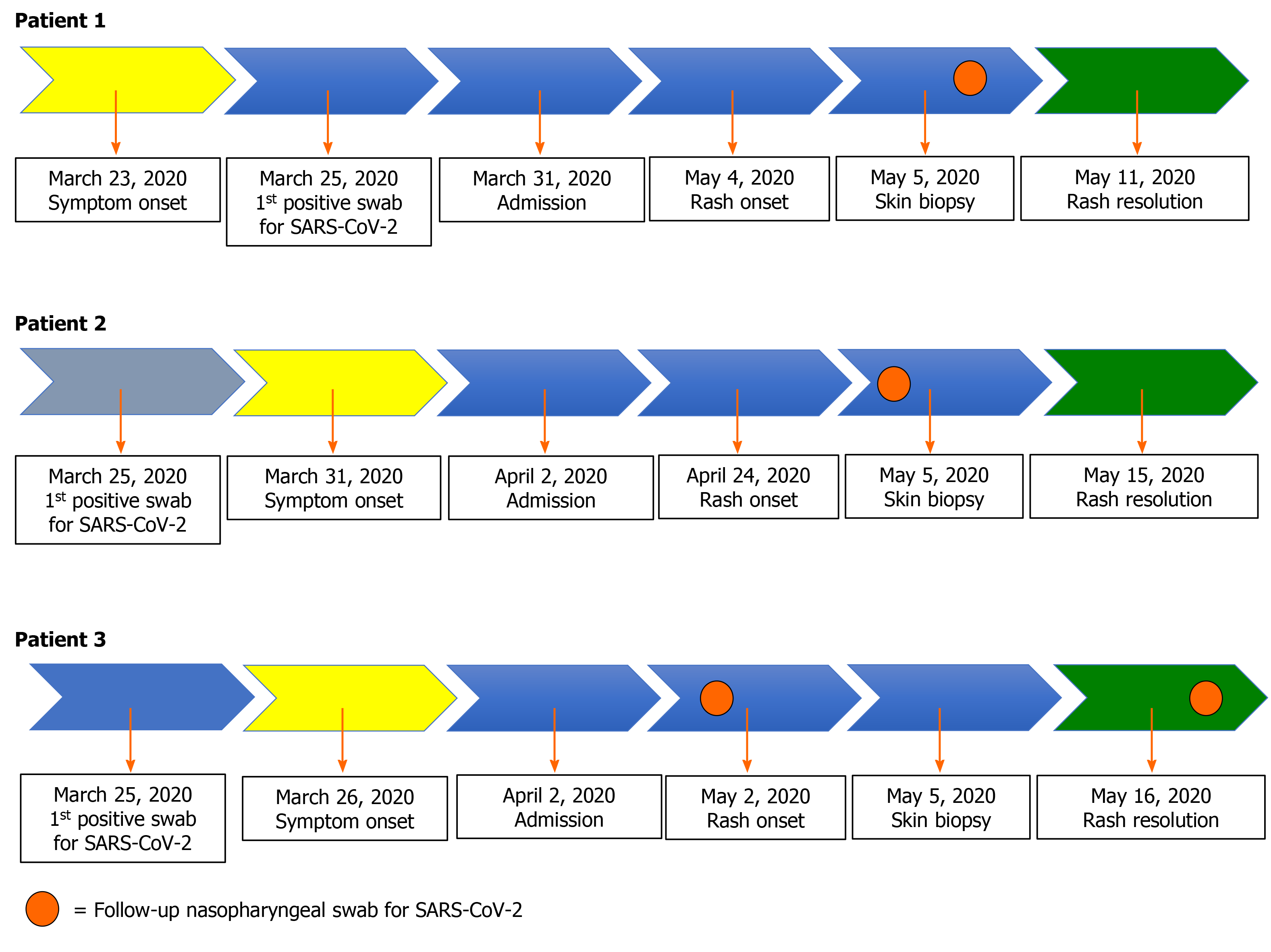
Figure 2 depicts the patient clinical course and results of PCR for SARS-CoV-2. For patient 1 the nasopharyngeal swab performed on May 5, 2020 came back as positive only for N gene (33 CT). For patient 2, the nasopharyngeal swab performed 2 d before the rash onset (April 22, 2020) came back as positive only for N gene (36 CT). For patient 3, PCR for SARS-CoV-2 on nasopharyngeal swab performed a week before the rash onset came back as negative, while the one performed a week later (May 16, 2020, the same day of rash healing) was positive only for the N gene (37 CT).
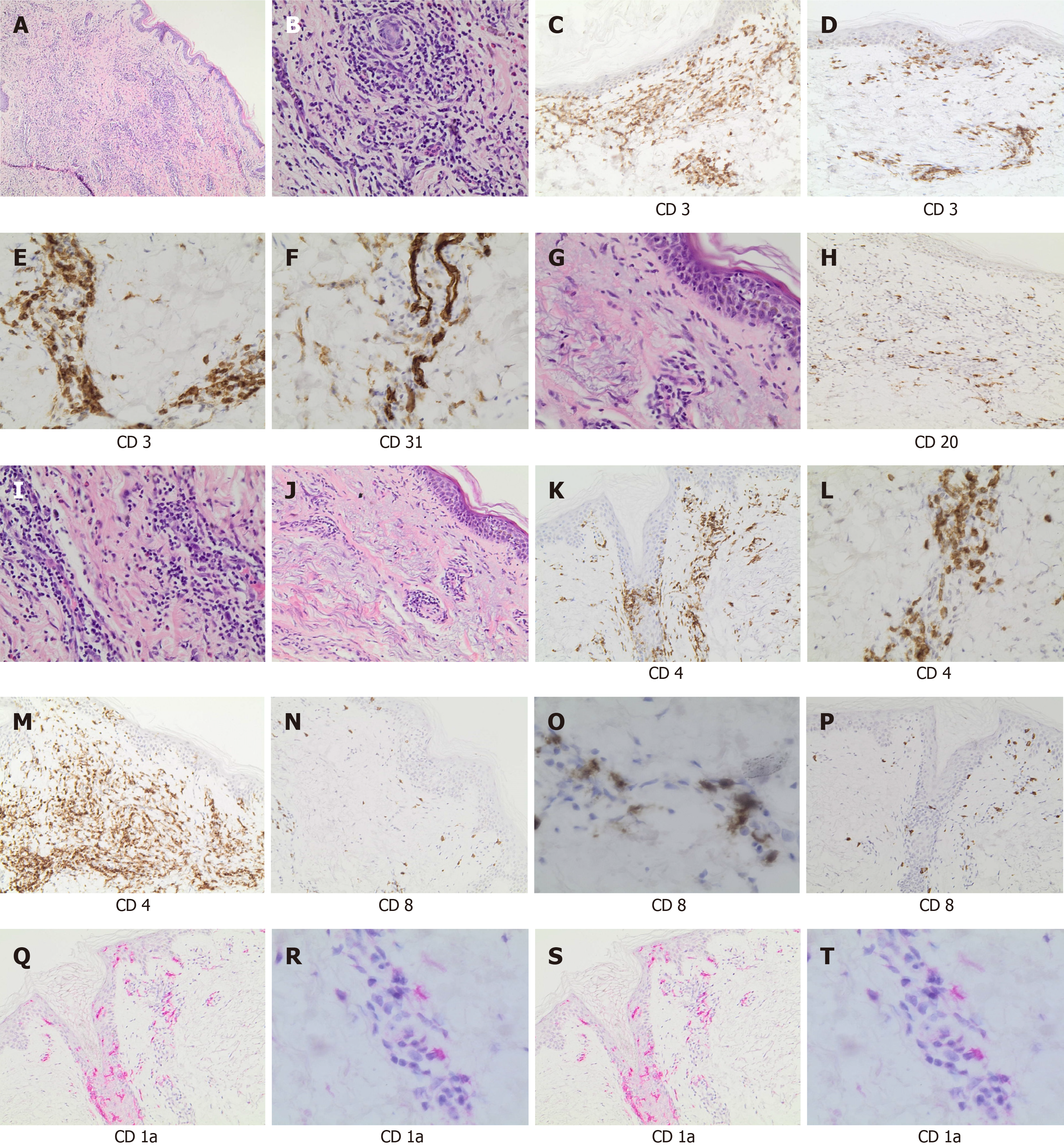
A similar histological pattern was found in all patients with a chronic dermatitis with perivascular lymphocytic infiltrate. The immunohistochemical analysis (Figure 3) showed dermatitis with a perivascular lymphocytic infiltrate (Figure 3A and B) in all three cases. There was a predominance of T-cell populations (CD3+) (Figure 3C), while B-cell infiltrates were scarce (Figure 3D), with a prevalence of CD4+ cells (T-helper lymphocytes) and few CD8+ cells (Figure 3E and F). Epidermotropism was visible in all cases (Figure 3G), with CD4+ cells predominance. CD31 staining showed small vessel damage with fibrinoid necrosis of the walls (N) whereas CD1a staining highlighted the presence of Langerhans cells in epidermidis and in perivascular infiltrates (Figure 3S and T).
No specific treatment was prescribed for skin rashes.
Skin rashes spontaneously healed in all three patients.
Our case series describes the histology and immunophenotype of late onset skin rashes in three elderly patients diagnosed with COVID-19. In spite of previously reported data, we observed skin rashes occurring in older patients well after the resolution of lung disease[2]. Our experience is similar to the one reported by Reymundo et al[12] who described clinical and histological characterization of late appearance maculopapular eruptions in 4 patients with COVID-19, but he did not temporally correlate cutaneous manifestations with SARS-CoV-2 shedding.
In two cases in the present study, there was a temporal correlation with viral shedding, demonstrated by the detection of SARS-CoV-2 genome at nasopharyngeal swab after many CT and with the expression of tardive target gene (N). For patient 3, RT PCR for SARS-CoV-2 was found positive 1 wk later (May 16, 2020) on the same day of rash healing[15]. So, it is possible that the appearance of late-onset cutaneous urticarial/erythema multiforme-like rashes are not directly related to the presence of the virus, but it may be due to a delayed immune response to COVID-19, as already hypothesized[12]. We observed a perivascular lymphocytic dermatitis with a cell infiltrate typical of other cutaneous diseases, such as cutaneous lymphomas, pseudoly
Unfortunately, this study is somewhat limited by the small number of patients. Moreover, we did not perform PCR for SARS-CoV-2 on skin samples.
Increased up-taking of skin biopsies with immunohistochemical analysis could improve our understanding of the clinical presentations and pathophysiology of cutaneous manifestations of COVID-19. Whether rashes associated with COVID-19 can simulate those occurring in lympho-proliferative disorders remains to be clarified.
We first want to thank our patients who accepted to undergo a skin biopsy, and our nurses. We also thank the Infectious Diseases and Tropical Medicine (IDTM) of the University “Magna Graecia” (UMG) COVID-19 Group, which is composed, besides the main authors, by the following: Arrighi E, Barreca GS, Biamonte F, Bertucci B, Bruni A, Busceti MT, Cancelliere A, Costanzo FS, Davoli C, De Francesco A, Fusco P, Gallo L, Garofalo E, Giancotti A, Giudice A, Greco G, La Gamba V, Laganà D, Lamberti A, Liberto MC, Lionello R, Longhini F, Marascio N, Matera G, Petullà M, Perri G, Procopio G, Quirino A, Ricchio M, Scaglione V and Tassone B.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang MK S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH
| 1. | Guarneri C, Rullo EV, Pavone P, Berretta M, Ceccarelli M, Natale A, Nunnari G. Silent COVID-19: what your skin can reveal. Lancet Infect Dis. 2021;21:24-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Guarneri C, Venanzi Rullo E, Gallizzi R, Ceccarelli M, Cannavò SP, Nunnari G. Diversity of clinical appearance of cutaneous manifestations in the course of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e449-e450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 786] [Article Influence: 157.2] [Reference Citation Analysis (1)] |
| 4. | Jamiolkowski D, Mühleisen B, Müller S, Navarini AA, Tzankov A, Roider E. SARS-CoV-2 PCR testing of skin for COVID-19 diagnostics: a case report. Lancet. 2020;396:598-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Marzano AV, Cassano N, Genovese G, Moltrasio C, Vena GA. Cutaneous manifestations in patients with COVID-19: a preliminary review of an emerging issue. Br J Dermatol. 2020;183:431-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 6. | Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A, Navarro Fernández I, Ruiz-Villaverde R, Falkenhain-López D, Llamas Velasco M, García-Gavín J, Baniandrés O, González-Cruz C, Morillas-Lahuerta V, Cubiró X, Figueras Nart I, Selda-Enriquez G, Romaní J, Fustà-Novell X, Melian-Olivera A, Roncero Riesco M, Burgos-Blasco P, Sola Ortigosa J, Feito Rodriguez M, García-Doval I. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 783] [Cited by in RCA: 877] [Article Influence: 175.4] [Reference Citation Analysis (1)] |
| 7. | Jia JL, Kamceva M, Rao SA, Linos E. Cutaneous manifestations of COVID-19: A preliminary review. J Am Acad Dermatol. 2020;83:687-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 8. | Caproni M, Giomi B, Volpi W, Melani L, Schincaglia E, Macchia D, Manfredi M, D'Agata A, Fabbri P. Chronic idiopathic urticaria: infiltrating cells and related cytokines in autologous serum-induced wheals. Clin Immunol. 2005;114:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Jain S. Pathogenesis of chronic urticaria: an overview. Dermatol Res Pract. 2014;2014:674709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, Pindado-Ortega C, Prieto-Barrios M, Jimenez-Cauhe J. Comment on: Cutaneous manifestations in COVID-19: a first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol. 2020;34:e252-e254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S, Ziv M, Leshem E, Dodiuk-Gad RP. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 12. | Reymundo A, Fernáldez-Bernáldez A, Reolid A, Butrón B, Fernández-Rico P, Muñoz-Hernández P, De Argila D, Wiesner T, Llamas-Velasco M. Clinical and histological characterization of late appearance maculopapular eruptions in association with the coronavirus disease 2019. A case series of seven patients. J Eur Acad Dermatol Venereol. 2020;34:e755-e757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Kaya G, Kaya A, Saurat JH. Clinical and Histopathological Features and Potential Pathological Mechanisms of Skin Lesions in COVID-19: Review of the Literature. Dermatopathology (Basel). 2020;7:3-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 14. | Mazzitelli M, Davoli C, Scaglione V, Fusco P, La Gamba V, Matera G, Trecarichi EM, Torti C. Apparent inefficacy of hydroxychloroquine combined with azithromycin on SARS-CoV-2 clearance in an incident cohort of geriatric patients with COVID-19. Travel Med Infect Dis. 2020;37:101826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Cento V, Colagrossi L, Nava A, Lamberti A, Senatore S, Travi G, Rossotti R, Vecchi M, Casati O, Matarazzo E, Bielli A, Casalicchio G, Antonello M, Renica S, Costabile V, Scaglione F, Fumagalli R, Ughi N, Epis OM, Puoti M, Vismara C, Faccini M, Fanti D, Alteri C, Perno CF. Persistent positivity and fluctuations of SARS-CoV-2 RNA in clinically-recovered COVID-19 patients. J Infect. 2020;81:e90-e92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Massone C, Cerroni L. Phenotypic variability in primary cutaneous anaplastic large T-cell lymphoma: a study on 35 patients. Am J Dermatopathol. 2014;36:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Hernández-Salazar A, García-Vera JA, Charli-Joseph Y, Ortiz-Pedroza G, Méndez-Flores S, Orozco-Topete R, Morales-Leyte AL, Domínguez-Cherit J, Lome-Maldonado C. Oral and Cutaneous Lymphomas other than Mycosis Fungoides and Sézary Syndrome in a Mexican Cohort: Recategorization and Evaluation of International Geographical Disparities. Indian J Dermatol. 2017;62:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 461] [Reference Citation Analysis (0)] |
| 19. | Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1635] [Cited by in RCA: 1590] [Article Influence: 318.0] [Reference Citation Analysis (1)] |