Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5737
Peer-review started: March 16, 2021
First decision: April 24, 2021
Revised: May 6, 2021
Accepted: May 24, 2021
Article in press: May 24, 2021
Published online: July 16, 2021
Processing time: 113 Days and 3.7 Hours
Adrenocortical carcinoma (ACC) is a rare malignant epithelial tumor originating from adrenocortical cells that carries a very poor prognosis. Metastatic or inoperable diseases are often considered incurable, and treatment remains a challenge. Especially for advanced cases such as ACC complicated with renal venous cancer thrombus, there are few cumulative cases in the literature.
The patient in this case was a 39-year-old middle-aged male who was admitted to the hospital for more than half a month due to dizziness and chest tightness. Computed tomography (CT) findings after admission revealed a left retroperitoneal malignant space-occupying lesion, but the origin of the formation of the left renal vein cancer thrombus remained to be determined. It was speculated that it originated from the left adrenal gland, perhaps a retroperitoneal source, and left adrenal mass + left nephrectomy + left renal vein tumor thrombus removal + angioplasty were performed under general anesthesia. Postoperative pathology results indicated a diagnosis of ACC. Postoperative steroid therapy was administered. At 3 mo after surgery, abdominal CT reexamination revealed multiple enlarged retroperitoneal lymph nodes and multiple low-density shadows in the liver, and palliative radiotherapy and mitotane were administered, considering the possibility of metastasis. The patient is currently being followed up.
ACC is a highly malignant tumor. Even if the tumor is removed surgically, there is still the possibility of recurrence. Postoperative mitotane and adjuvant chemoradiotherapy have certain benefits for patients, but they cannot fully offset the poor prognosis of this disease.
Core Tip: Adrenocortical carcinoma (ACC) is a highly malignant tumor that is rarely seen in the clinic, especially with renal venous cancer thromboembolism, and the prognosis is very poor. In metastatic or locally advanced cases of ACC, there are a limited number of available treatments, and the curative effect is not significant. Priority is often given to surgical tumor reduction treatment, with mitotane as the basis of treatment. If the condition deteriorates, cytotoxic drugs should be added and radiotherapy taken into consideration.
- Citation: Zhou Z, Luo HM, Tang J, Xu WJ, Wang BH, Peng XH, Tan H, Liu L, Long XY, Hong YD, Wu XB, Wang JP, Wang BQ, Xie HH, Fang Y, Luo Y, Li R, Wang Y. Multidisciplinary team therapy for left giant adrenocortical carcinoma: A case report. World J Clin Cases 2021; 9(20): 5737-5743
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5737.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5737
Adrenocortical carcinoma (ACC) is a rare malignant epithelial tumor that originates from adrenocortical cells and is infrequently seen in the clinic. ACC can be divided into functional and nonfunctional types, with the latter accounting for approximately 40% of cases[1]. Its incidence in the population is 1-2 per million cases per year in North America and 4-12 cases per million cases per year worldwide[2]. The 5-year survival rate and median survival time reported by various medical centers vary to some extent[3-5], with 5-year survival ranging from 16%-47%[6]. The molecular mechanism of ACC is not completely clear at present and may be related to the inactivation of tumor suppressor genes (TP53, Men-1, p57Kip2, and H19), abnormal activation of proto-oncogenes (deletion of GAS, RAS, ACTH receptors), overexpression of growth factor IGF-2, and abnormal activation of the B-catenin gene[7]. In general[8], ACC patients have a poor prognosis and high recurrence rate, even if they actively undergo surgical resection. Ki-67 is a very important prognostic index for determining localized ACC immunohistochemically; if Ki-67 > 10%, the risk of recurrence is significantly increased. In this case, the patient underwent surgical resection of a large ACC from his left side. In a short period of time, the patient’s weight had significantly decreased, and his clinical symptoms had improved. However, postoperative computed tomography (CT) reexamination showed obvious metastatic lesions. After describing this case in detail and providing a review of the literature, we further discuss the treatment and prognosis of ACC patients.
The patient was a 39-year-old middle-aged male who was admitted to the hospital for more than half a month due to dizziness and chest tightness, and he had gained approximately 30 kg in the past 3 mo. CT examination conducted in the local hospital 5 d before admission suggested a left retroperitoneal space-occupying lesion.
The patient presented with numbness of the hands and feet, increased nocturia, nocturia approximately 3-4 times/night, no obvious abdominal pain, abdominal distension, no frequent urination, no urgency, and no hematuria or other symptoms.
The patient was generally healthy and had no special disease history.
His medical history and family history were unremarkable.
T: 36 ℃, P: 78 times/min, R: 19 times/min, BP: 164/120 mmHg. There was no localized bulge in the bilateral kidney area, no tenderness at the bilateral costal ridge angles, no percussion pain the kidneys, and no obvious tenderness or rebound pain in the entire abdomen.
The results of laboratory examination upon admission revealed the following: alanine aminotransferase: 115 U/L (reference < 40 U/L), uric acid: 526 μmol/L (reference< 420 μmol/L), brain natriuretic peptide: 14,157 pg/mL (reference < 400 pg/mL), aldosterone (sitting up): 616 pg/mL (reference 70-300 pg/mL), aldosterone (recumbent position) 610 pg/mL (reference 30-160 pg/mL), angiotensin II: 86 pg/mL (reference 25-60 pg/mL), K: 2.58 mmol/L (reference 3.5-5.5 mmol/L).
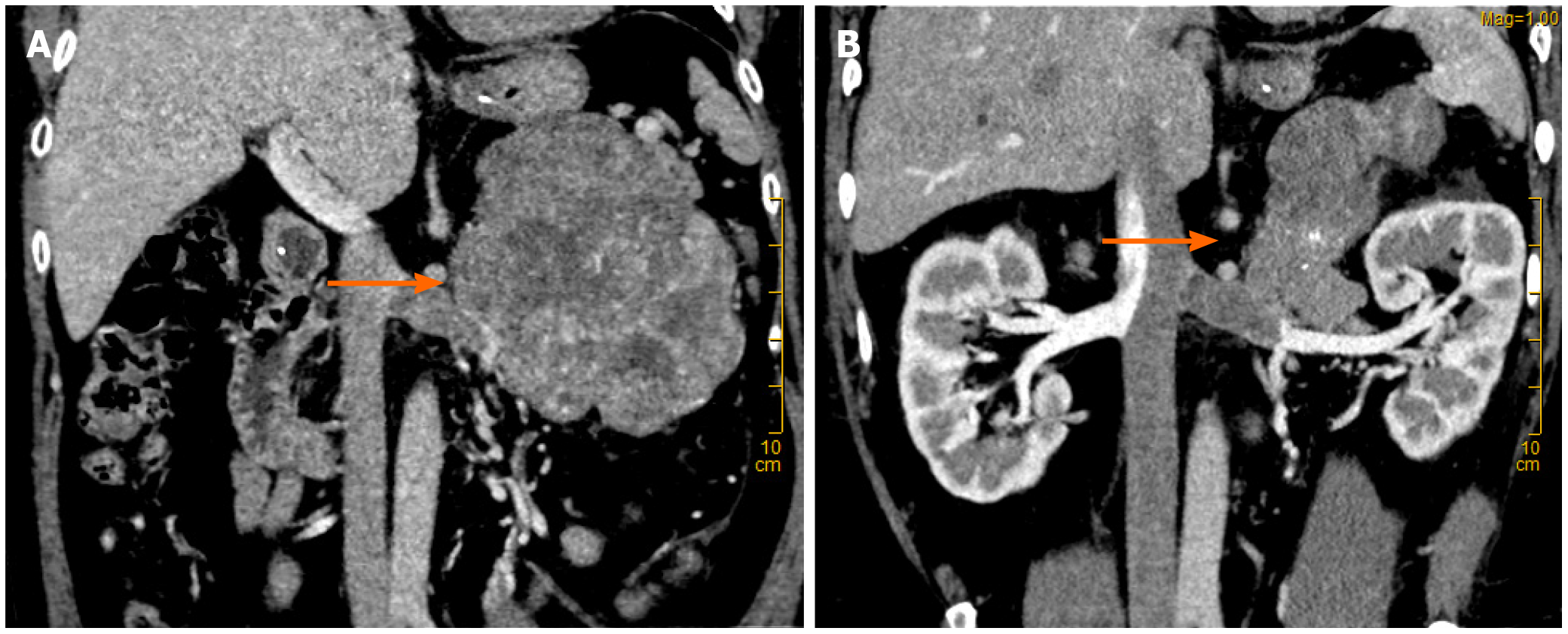
CT suggested malignant left retroperitoneal mass with left renal vein cancer thrombus, source to be determined: Left adrenal gland source? (Pheochromocytoma? Cortical cancer?) Retroperitoneal origin? (Leiomyolipoma? Sarcoma?) (Figure 1) and right adrenal atrophy.
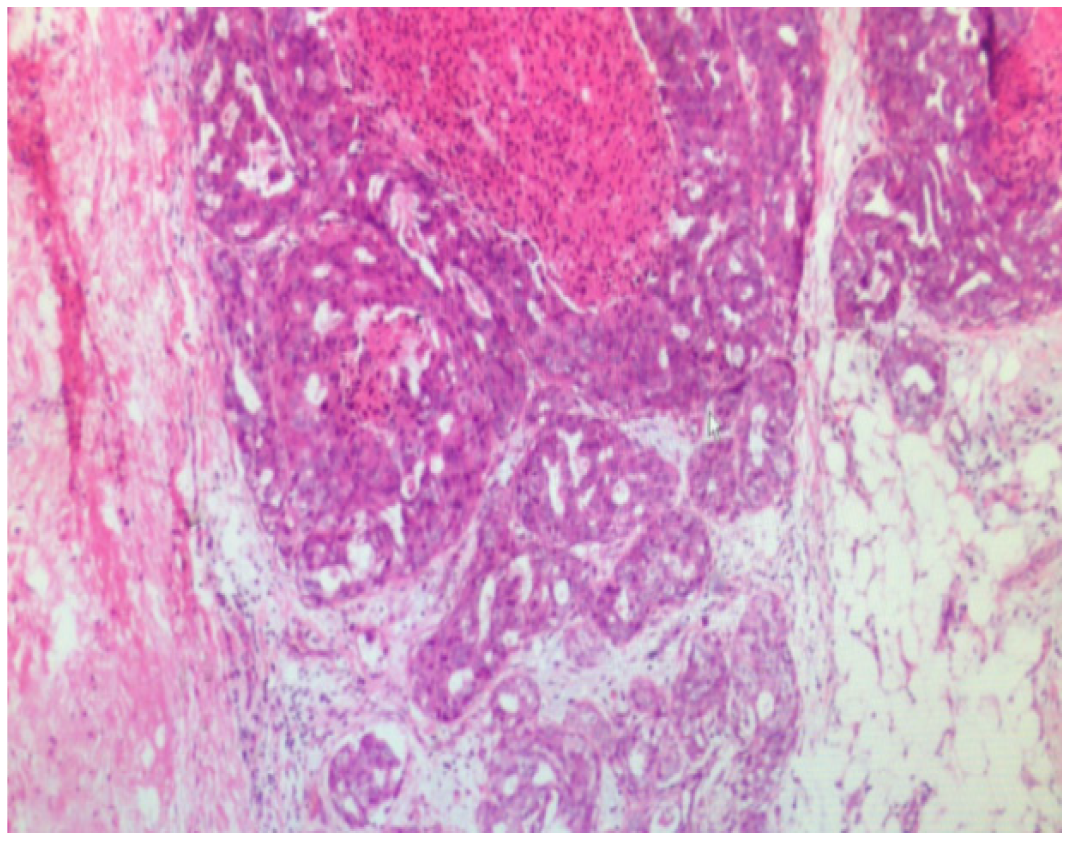
The final diagnosis of the presented case is ACC complicated with renal venous cancer thrombus. Postoperative pathological results are shown in the (Figure 2).
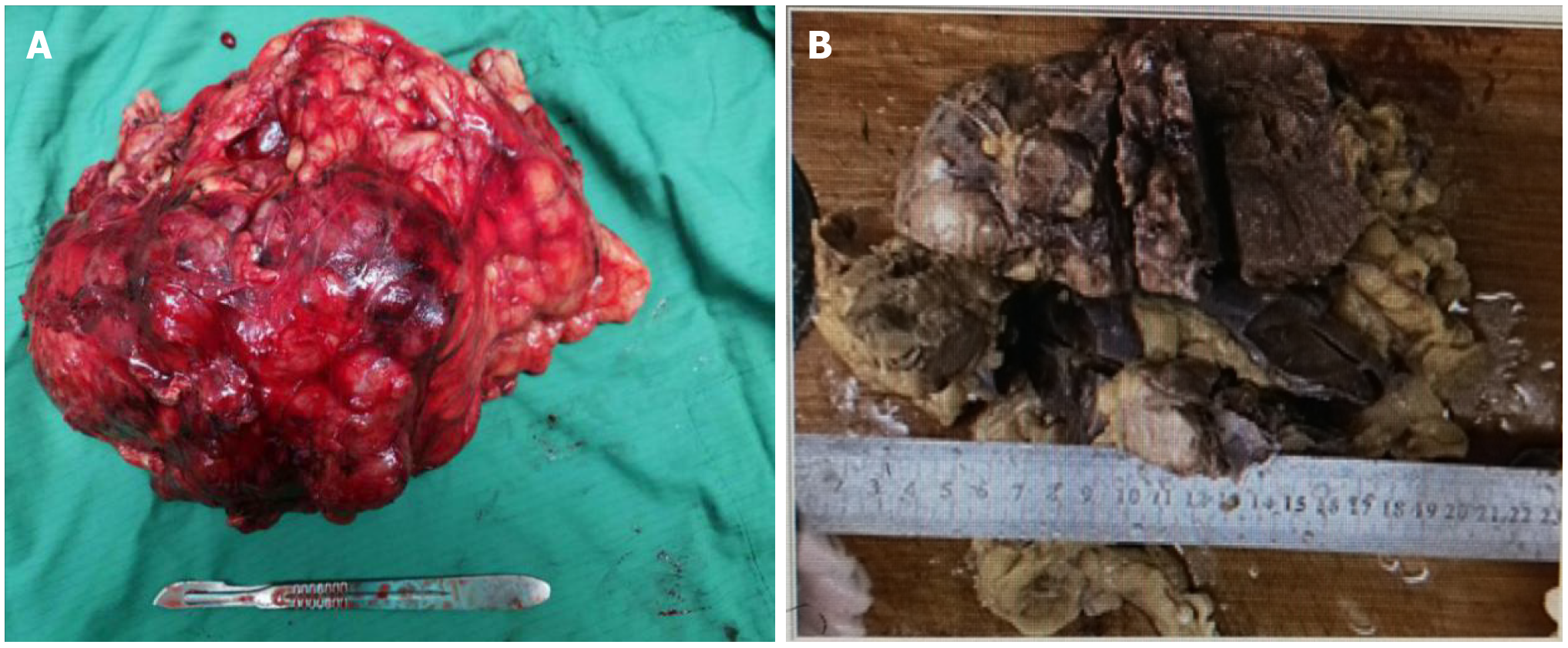
After admission, the patient was treated with symptomatic treatment to lower blood pressure, correct electrolyte imbalances, protect the liver and diuresis. At 2 d after admission, the patient experienced sudden chest pain and shortness of breath without obvious inducement and coughed up pink foam-like sputum. Considering acute left heart failure, the patient was transferred to the intensive care unit (ICU) for treatment to strengthen the heart, diuresis, protect the liver and stomach, lower blood pressure and correct electrolyte imbalances. After 1 wk of conservative treatment, multiple departments within the hospital were consulted, and surgical treatment was considered based on the opinions of various departments. It was determined that preoperative vasodilation for 2 wk (prazosin 2 mg q8h + spirolactone 80 mg q8h) would be administered. Under general anesthesia, the patient underwent laparoscopic resection of left renal retroperitoneal mass resection + left nephrectomy + left renal vein tumor thrombus removal + angioplasty. A large mass with a hard texture approximately 20 cm × 10 cm × 7 cm in size located in the adrenal region was observed intraoperatively (Figure 3), and the patient’s blood pressure increased significantly when the mass was squeezed. Due to the difficult of laparoscopic separation of the renal pedicle and the inner and lower parts of the mass, a transabdominal laparoscopic approach was used to cut the left peritoneum, and the mass was found to be closely adhered to the pancreas. After careful separation, the pancreatic capsule was sutured with silk thread. After the pedicle was exposed, obvious varicose renal veins were observed, and thrombectomy became difficult under laparoscopic. Therefore, conversion to open surgery was decided for thrombectomy, which was performed by a vascular surgeon. The liver, pancreas, stomach, colon and adrenal gland were not invaded, and no lymph node metastasis was observed. After surgery, he was transferred to the intensive care unit for symptomatic supportive treatment such as correcting heart failure, improving cardiac function, controlling blood pressure, and maintaining homeostasis. In addition, based on the related problems arising from treatment, consultation with a multidisciplinary team was performed half a month after the operation to provide supportive management such as anti-infection treatment, stomach and kidney support, hypotensive control, nutritional support, hemodialysis, and steroid therapy. During the first 3 d after surgery, the patient received hydrocortisone (100 mg q8h). The postoperative multidisciplinary team summary plan was as follows: intravenous hydrocortisone (100 mg q8h) with a gradual dose reduction (50 mg q8h). After 38 d, the prednisone dosage was changed to oral administration of 15 mg early and 5 mg late and hydrocortisone tablets at a dosage of 10 mg early and 5 mg late. After treatment, the general condition of the patient improved, his tolerance for activity increased, and the patient was discharged 48 d after surgery.
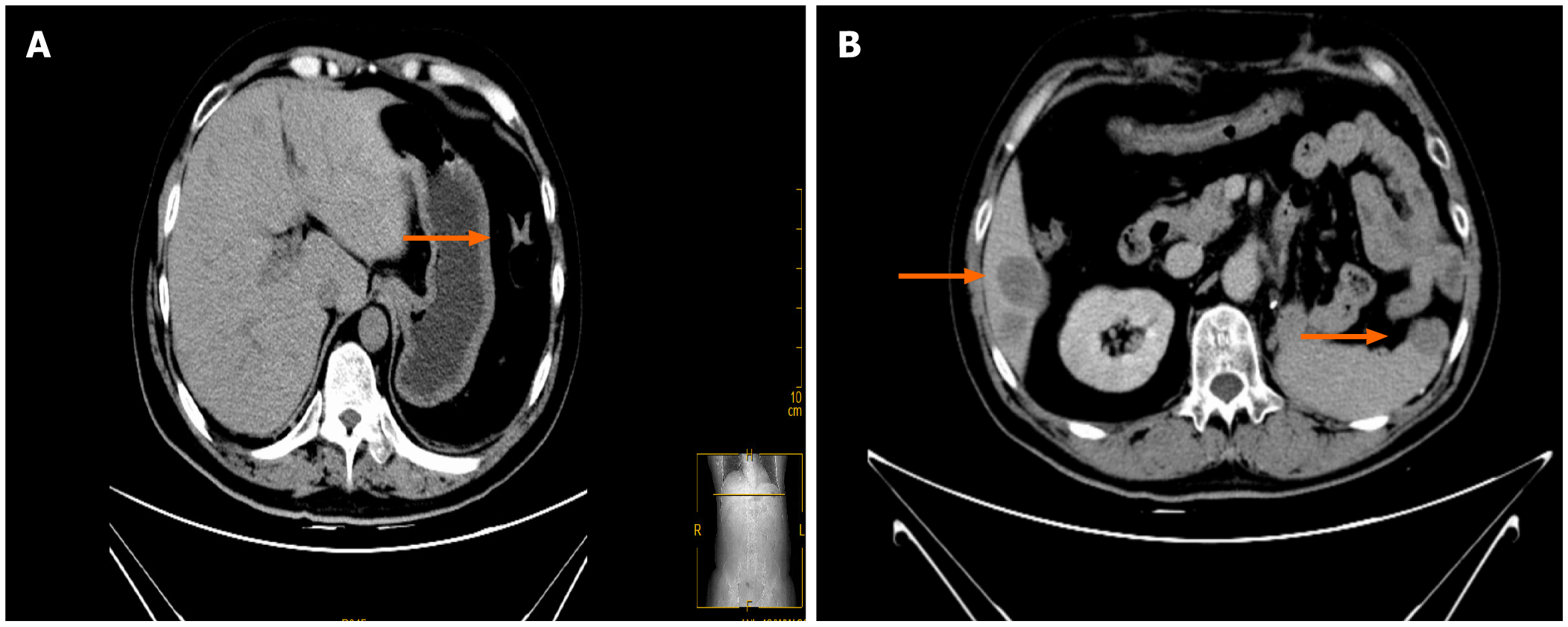
CT reexamination 3 mo after surgery indicated the following (Figure 4A): changes after left retroperitoneal mass resection + changes after left nephrectomy; multiple low-density shadows adjacent to enlarged retroperitoneal lymph nodes and the liver indicating a high possibility of metastasis; and right adrenal atrophy was the same as before. Palliative radiotherapy for metastatic left adrenal cortical carcinoma (PTV1: 30 Gy/10F, PTV2: 35 Gy/10F, PTV3: 35 Gy/10F 5 times/wk twice. Mitotane 500 mg q6h. CT reexamination 5 mo after surgery indicated (Figure 4B) local recurrence with invasion and metastasis of the spleen, along with multiple liver metastases and multiple retroperitoneal lymph node metastases that were more advanced than before. Metastases of the sacrum, chest, and lumbar vertebrae were also present. The patient was treated with amlotinib 12 mg qd × 12 d + mitotane 1 g qd + hydrokedasone 30 mg qd. The patient is currently being followed up.
ACC is a rare highly invasive endocrine tumor. The vast majority of ACC tumors are larger than 5 cm in diameter, but the size is not the only characteristic for the identification of benign and malignant tumors of the adrenal gland because some of the smaller adrenocortical tumors can shift[9]. Surgery is the main treatment for early metaphase ACC. Resection combined with postoperative adjuvant therapy provides the best possibility for long-term survival and a potential cure for local disease against a recurrent attack on tissues and organs. Interventional surgery, radiofrequency ablation or radiotherapy are options for oligometastases in parenchymal organs. In late ACC, operations cannot generally be performed, and patients may benefit from systemic treatment, including chemotherapy, radiotherapy, immunotherapy, and targeted therapy. Mitotane is now the most effective drug in the treatment of ACC and is applicable to cases that cannot be operated, have failed to shift or where surgery failed to completely resect the ACC. The pharmacological effects of mitotane can lead to adrenal cortical zona fasciculata and zona reticularis necrosis, and it is the only drug approved for use in the United States by the Food and Drug Administration for ACC clinical stages. It was listed as a rare disease drug by the European Medicines Agency in 2004[10]. To avoid drug-induced adrenal insufficiency, appropriate glucocorticoid supplementation can be considered[11]. A total of 177 ACC patients in Italy and Germany were treated with mitotane after radical resection[12], and the results showed that the duration of recurrence-free survival (RFS) in the mitotane group was significantly longer (42 mo) than that in the two control groups (10 mo and 25 mo), and the overall survival rate increased. Other foreign studies have shown that adjuvant therapy with mitotane can prolong the time of RFS (but cannot improve the overall survival rate), especially in combination with radiotherapy. Some patients diagnosed with advanced ACC and low tumor load benefit from monotherapy with mitotane. In patients with metastatic disease, mitotane monotherapy may not be an option. In such patients, the opportunity to combine mitotane with cytotoxic agents should be evaluated. Fassnacht et al[13] conducted a randomized study (including 304 patients) that confirmed that patients treated with mitotane combined with EDP (etoposide + cisplatin + epirubicin) had a significantly higher response and longer median progression-free survival than those treated with mitotane combined with streptocin. Mitotane in combination with EDP is also considered to be the preferred therapy in cases requiring a more aggressive treatment approach. Immunotherapy for ACC is still in the preliminary research stage. Studies have shown that adjuvant radiotherapy for ACC patients shows no significant improvement in overall survival and disease-free survival, but it can reduce the possibility of local tumor recurrence[14]. High tumor load, high tumor grade, high Ki-67 index and uncontrollable symptoms are the main factors affecting the prognosis of patients with advanced disease[15].
ACC is a rare disease that has a very poor prognosis, with a 5-year survival rate of less than 50%. Surgical treatment can be used for patients with early-/mid-stage disease confined to the adrenal gland. If the tumor is already in an advanced stage and has metastasized, tumor reduction surgery combined with radiotherapy and chemotherapy is the main treatment, and the overall prognosis of these patients is poor. We report the case of a patient with a large left adrenal mass and tumor thrombus in the left renal vein. The patient experienced recurrence 3 mo after surgery and is currently undergoing radiotherapy and chemotherapy and receiving regular follow-up.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shi B S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35:282-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 611] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 2. | Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:4551-4564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 276] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 3. | Libé R. Adrenocortical carcinoma (ACC): diagnosis, prognosis, and treatment. Front Cell Dev Biol. 2015;3:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Ayala-Ramirez M, Jasim S, Feng L, Ejaz S, Deniz F, Busaidy N, Waguespack SG, Naing A, Sircar K, Wood CG, Pagliaro L, Jimenez C, Vassilopoulou-Sellin R, Habra MA. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol. 2013;169:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Schimmack S, Strobel O. [Resection strategies for adrenocortical carcinoma]. Chirurg. 2019;90:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Nikoleishvili D, Koberidze G, Kutateladze M, Zumbadze G, Mariamidze A. Bilateral adrenocortical carcinoma: case report and review of literature. Georgian Med News. 2018;19-24. [PubMed] |
| 7. | Wasserman JD, Zambetti GP, Malkin D. Towards an understanding of the role of p53 in adrenocortical carcinogenesis. Mol Cell Endocrinol. 2012;351:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Mota JM, Sousa LG, Braghiroli MI, Siqueira LT, Neto JEB, Chapchap P, Hoff AAO, Hoff PM. Pembrolizumab for metastatic adrenocortical carcinoma with high mutational burden: Two case reports. Medicine (Baltimore). 2018;97:e13517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Crucitti F, Bellantone R, Ferrante A, Boscherini M, Crucitti P. The Italian Registry for Adrenal Cortical Carcinoma: analysis of a multiinstitutional series of 129 patients. The ACC Italian Registry Study Group. Surgery. 1996;119:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Maiter D, Bex M, Vroonen L, T'Sjoen G, Gil T, Banh C, Chadarevian R. Efficacy and safety of mitotane in the treatment of adrenocortical carcinoma: A retrospective study in 34 Belgian patients. Ann Endocrinol (Paris). 2016;77:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Reimondo G, Puglisi S, Zaggia B, Basile V, Saba L, Perotti P, De Francia S, Volante M, Zatelli MC, Cannavò S, Terzolo M. Effects of mitotane on the hypothalamic-pituitary-adrenal axis in patients with adrenocortical carcinoma. Eur J Endocrinol. 2017;177:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Erickson LA. Challenges in surgical pathology of adrenocortical tumours. Histopathology. 2018;72:82-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardière C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Müller HH, Skogseid B; FIRM-ACT Study Group. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 589] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 14. | Sabolch A, Feng M, Griffith K, Hammer G, Doherty G, Ben-Josef E. Adjuvant and definitive radiotherapy for adrenocortical carcinoma. Int J Radiat Oncol Biol Phys. 2011;80:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Libé R, Borget I, Ronchi CL, Zaggia B, Kroiss M, Kerkhofs T, Bertherat J, Volante M, Quinkler M, Chabre O, Bala M, Tabarin A, Beuschlein F, Vezzosi D, Deutschbein T, Borson-Chazot F, Hermsen I, Stell A, Fottner C, Leboulleux S, Hahner S, Mannelli M, Berruti A, Haak H, Terzolo M, Fassnacht M, Baudin E; ENSAT network. Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): an European Network for the Study of Adrenal Tumor (ENSAT) study. Ann Oncol. 2015;26:2119-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |