Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5709
Peer-review started: March 8, 2021
First decision: March 27, 2021
Revised: March 28, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: July 16, 2021
Processing time: 121 Days and 2.3 Hours
Chondroblastoma (CB) is an intermediate tumor of cartilage origin. CB involving the sacrum is a very rare pathology.
A 17-year-old male with sacral CB was diagnosed as CB during the first surgery, and 18 mo later, the tumor recurred and a second surgery was performed with the same pathology result of CB.
We recommend complete removal of the tumor in a timely manner, provided that surgical conditions are met. At the same time, other diseases should be carefully differentiated in terms of imaging or pathological features so as to avoid erroneous diagnostic conclusions.
Core Tip: Sacral chondroblastoma is a very rare pathology. Up to now, there was only one case involving the sacrum reported in the literature. In order to report this rare case of both location and pathology, we present a case with sacral chondroblastoma. According to the lessons learned from this rare case, we recommend complete removal of the tumor in a timely manner, provided that surgical conditions are met. At the same time, other diseases should be carefully differentiated in terms of imaging or pathological features so as to avoid erroneous diagnostic conclusions.
- Citation: Zheng BW, Niu HQ, Wang XB, Li J. Sacral chondroblastoma — a rare location, a rare pathology: A case report and review of literature. World J Clin Cases 2021; 9(20): 5709-5716
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5709.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5709
Chondroblastoma (CB) is a rare bone tumor of cartilage origin. Among all primary bone tumors, its incidence is 1%-1.4%[1]. The 80% of CBs occur in the epiphysis of the long bones, but CB that occurs in the spine is very rare, accounting for only 1.4% of all CB patients[2]. At present, the treatment of CB mainly relies on complete resection of the tumor, and traditional chemotherapy is often ineffective for CB patients, while radiotherapy can even cause malignant transformation of the disease[3]. Therefore, given our poor understanding of CB, the treatment and prognosis of patients with CB are not optimistic, which seriously affects their long-term quality of life and survival.
To date, only 48 cases of spinal CB have been retrieved from the English literature, and sacral CB is even rarer compared to cervical CB, which occurs less frequently than lumbar and thoracic CB[4]. Up to now, there has been only one case of sacral CB patients reported in the literature[5]. Therefore, in order to report this rare case of both location and pathology, we will present and discuss a 17-year-old male with sacral CB.
A 17-year-old male patient, who was admitted to our hospital in September 2018, initially underwent magnetic resonance images (MRI) at a local hospital due to discomfort and pain in the sacrum after strenuous exercise, which concluded sacral occupancy. In addition, the patient did not have symptoms of poor bowel control or weight loss.
The patient has no history of past illness.
The patient has no special personal and family history.
On admission, the patient's temperature and blood pressure were normal. Physical examination showed tenderness in the sacrococcygeal vertebrae, but sensory and motor functions of the extremities were normal. All were normal except for bilateral kyphoscopic signs (+). Head, neck and cardiac examination were normal. No rash or lymph node enlargement was noted. Abdominal examination was normal with no tenderness or hepatosplenomegaly and normal stools.
Laboratory tests: Erythrocyte sedimentation rate, C-reactive protein, procalcitonin and C12 were normal. Human immunodeficiency virus and hepatitis B virus tests were negative.
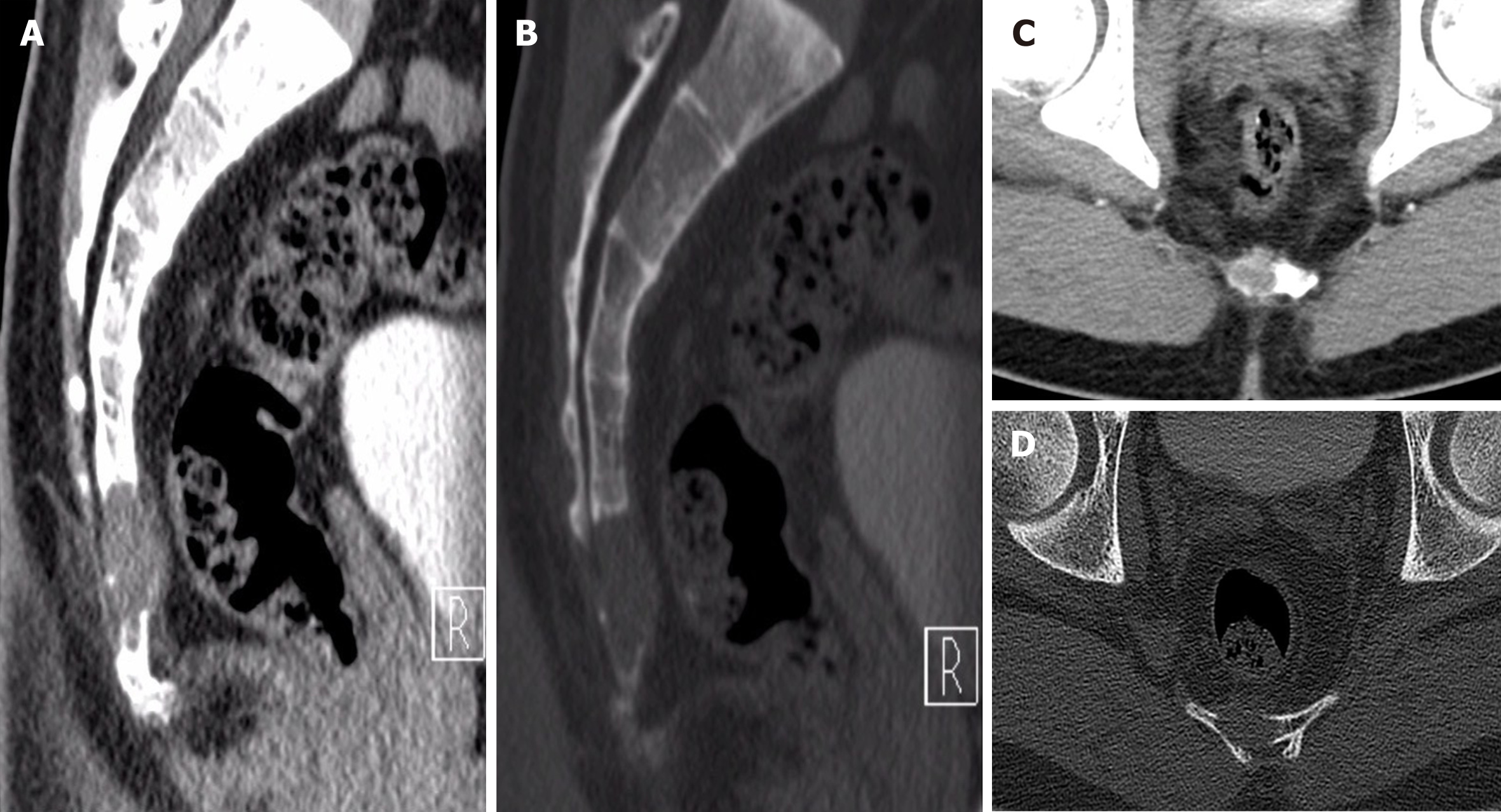
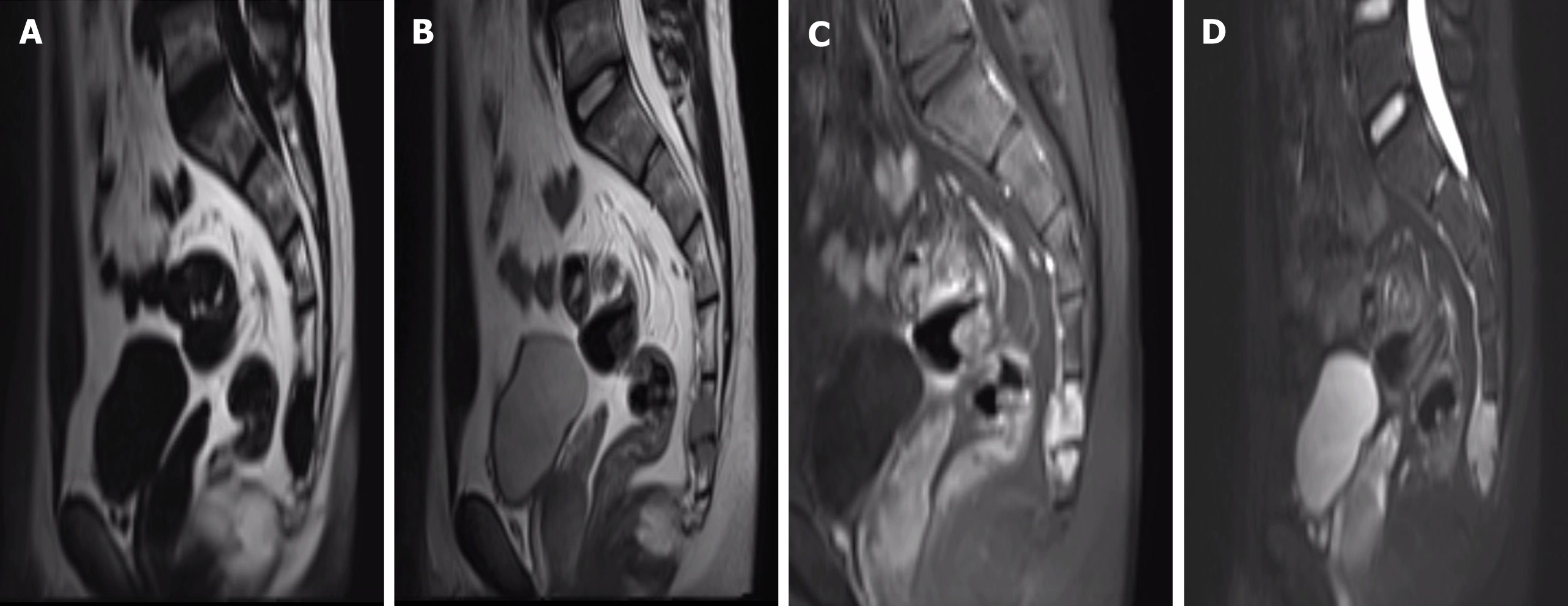
Computed tomography showed an osteolytic lesion with irregular margins and cortical breach (Figure 1); MRI showed that the sacrococcygeal an irregular nodular lesion, with low T1 and high T2 signal, with avid enhancement (Figure 2).
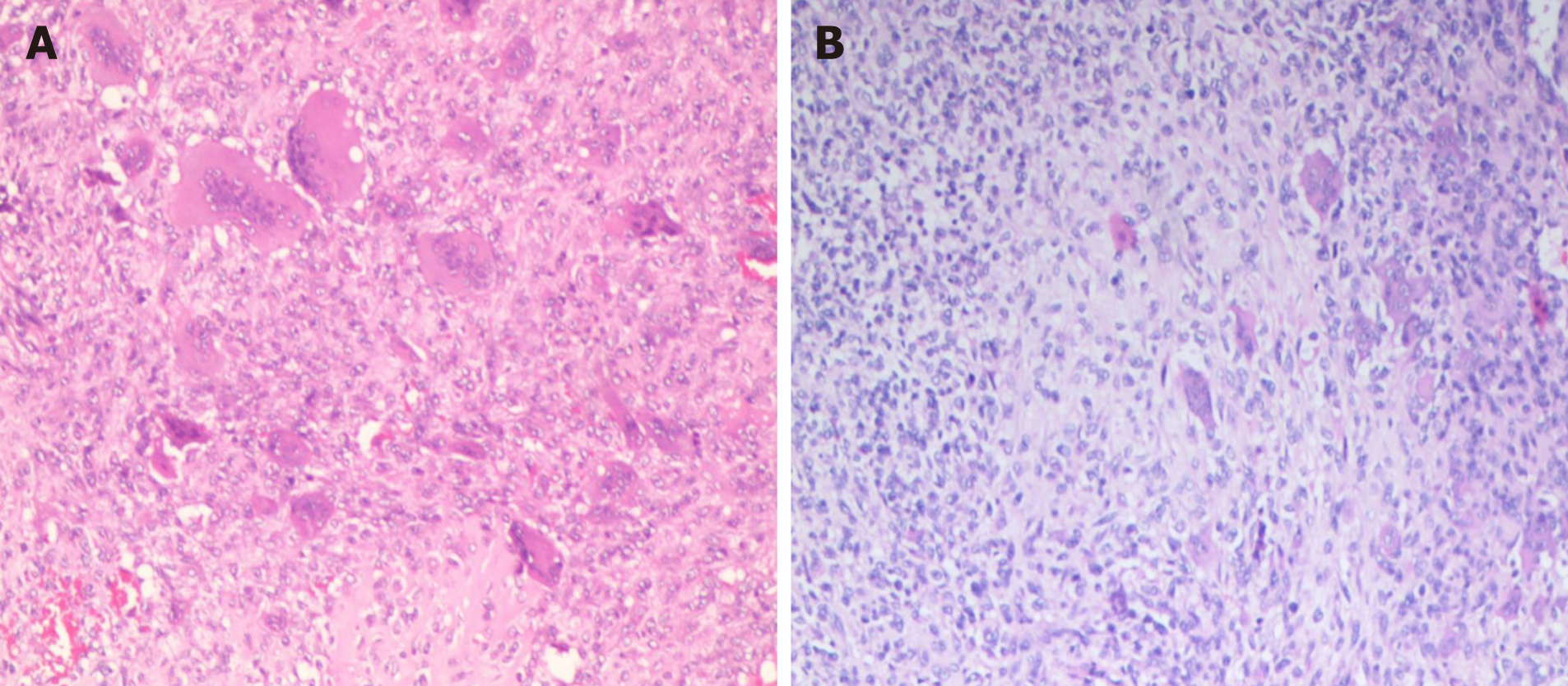
After a comprehensive evaluation, it was decided to perform surgery, and a tumor of about 3 cm × 2 cm in size was successfully removed from the right adnexa of the coccyx, which was poorly demarcated from the surrounding tissue and soft and fishy. Proliferating fibroblasts, multinucleated giant cells and proliferating cartilage tissue were seen in hematoxylin-eosin-stained pathological sections (Figure 3A). Immunohistochemistry showed S100 (+), P53 (5%+), CD34 (++), CD68 (+), Ki67 (40%+), smooth muscle actin (+), mouse double minute 2 homolog (+) and cytokeratin (-). The pathological findings were suggestive of CB.
CB.
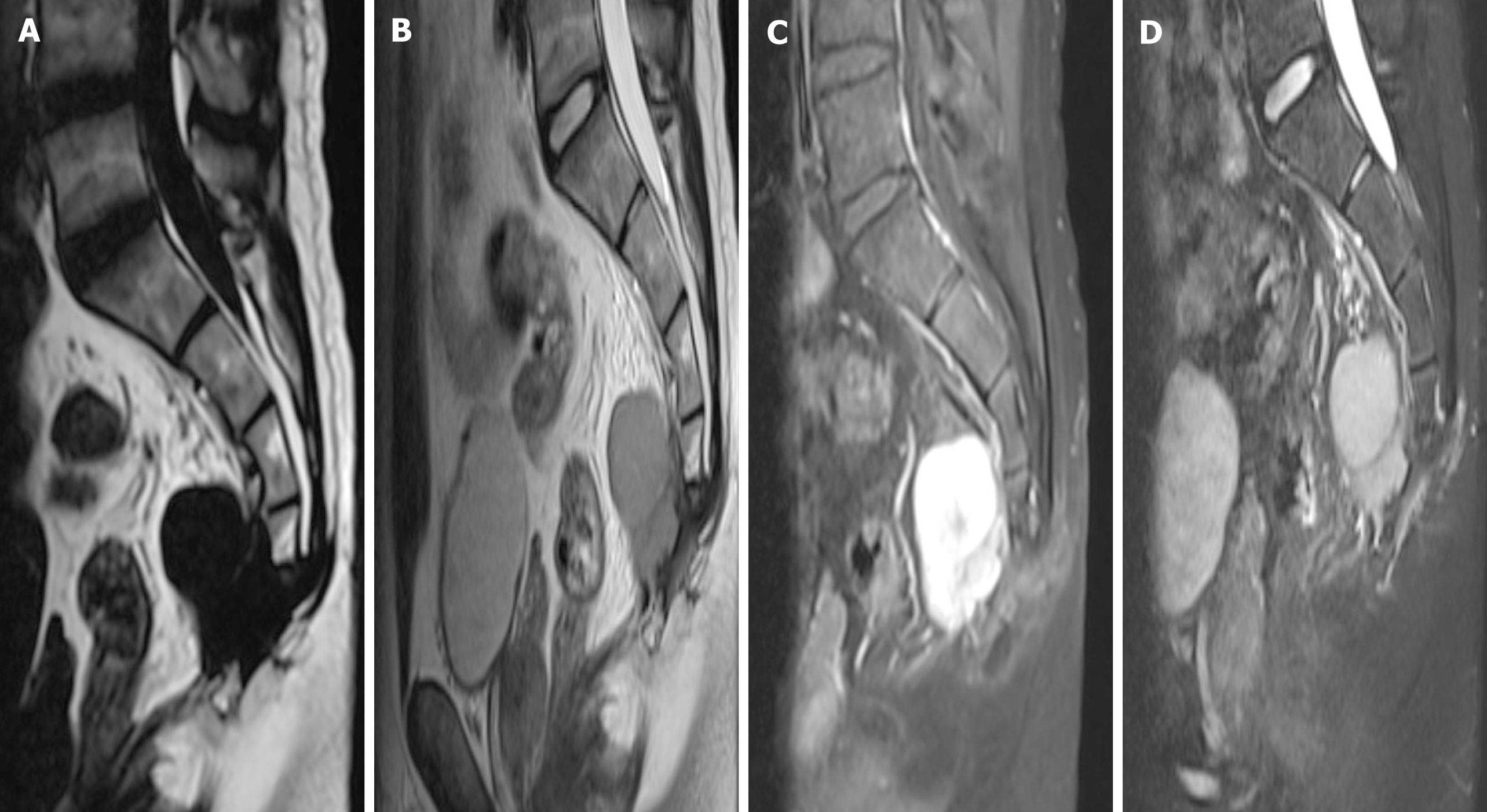
Postoperatively, the patient recovered and was discharged with regular follow-up. However, 18 mo later, the patient found a recurrence of the tumor at the original surgical site during a routine review and was admitted to our hospital again. The physical examination still did not reveal any meaningful positive signs. MRI showed an interval increase in the lesion with presacral extension, with low T1, high T2 signal, and avid enhancement (Figure 4).
The patient underwent surgical treatment again. During the operation, a mass about 7 cm × 6 cm × 5 cm in size on the ventral side of the caudal end of the four sacral vertebrae was removed. The tumor was fish-like, yellowish-brown, rich in blood, irregular in shape, without obvious envelope, with poorly defined border, adhesions to surrounding tissues and rectum, and scar tissue hyperplasia adhesions. Proliferating chondroblasts and cartilage-like matrix with scattered multinucleated giant cells were seen in hematoxylin-eosin-stained pathological sections (Figure 3B). Immunohistochemistry showed cytokeratin (-), vimentin (+), CD68 (+), P63 (+), SATB2 (+), S100 (+), smooth muscle actin (+), CD34 (++), CDK4 (-), mouse double minute 2 homolog (+), P53 (45%+) and Ki-67 (60%+). This pathology is consistent with CB, and the patient is still under close follow-up.
A second surgical treatment was performed and the patient is still under close follow-up.
According to the latest World Health Organization classification, CB is classified as an intermediate tumor that is locally aggressive and rarely metastasizes (< 2%), with a local recurrence rate of up to 38% and a 2% incidence of lung metastases[4]. Spinal CB has a higher recurrence rate and more aggressive tumor growth compared to long-bone CB[6,7], and Vialle et al[8] and Giri and Chavan[9] even stated that the local recurrence rate is 100% when CB involves the long bones. In view of the high invasiveness and high recurrence rate of this disease, it is necessary to understand fully this disease.
In demographics, the majority of CB patients are under the age of 50, mainly affecting people in their 20s and 30s, and the prognostic characteristics of CB patients at different sites are very different at different ages[10]. At the same time, there are significantly more male patients than female patients, with a male-to-female ratio of approximately 2:1[11,12].
Given the difficulty in distinguishing CB from many diseases and the misjudgment of this patient's first pathological findings, it is important to know the exact pathological features of CB. CB is often distinguished from giant cell tumors, aneurysmal bone cysts (ABC), eosinophilic granulomas, clear cell chondrosarcomas and osteoblastomas. The unique cytologic diagnostic features of CB are chondroblasts, multinucleated osteoclast-like giant cells and cartilage-like matrix[1]. Kilpatrick et al[13] concluded that in the absence of inflammatory cells, typical chondroblasts are sufficient for the diagnosis of CB, even in the absence of a chondrocyte-like stroma. The key to distinguishing CB from giant cell tumor is that the monocytes of giant cell tumor are spindle-shaped, cohesively arranged in clusters and lack nuclear groove and cartilage-like material in the background[14,15], while chondroblasts are round and often exist alone like a pebble. If the identification is difficult, immunohistochemistry can be used[14,16,17]. In addition, giant cell tumor and CB may be more difficult to differentiate if they are frequently associated with secondary ABC, but mutation-specific H3.3 immunohistochemistry (selectively complementary to next-generation sequencing-based DNA sequencing) and USP6 fluorescence in situ hybridization analysis can reliably distinguish cases with difficult morphological identification[18].
The two immunohistochemical results we reported for this patient revealed a high expression of Ki-67 index and CD34, and it has been reported that for Ki-67, as a proliferating cell-related antigen, a Ki-67 index greater than 10% is a high risk factor for bone giant cell tumor recurrence[19]. Therefore, we speculate that the recurrence in this sacral CB patient may be related to the high expression of Ki-67; in addition, CD34 can be used to measure the microvascular density within the tumor mask[20], and it is not difficult to understand that an adequate blood supply is conducive to the recurrent growth of the tumor. This is also suggestive of the highly aggressive nature of this sacral CB, and in addition, we observed that in the pathological findings after recurrence[21,22], P53, which is often present in high-grade bone tumors, had an extensive value-added compared to the first time. This finding is similar to a previous report where an extensive increase of P53 was detected in a patient with pelvic CB who relapsed and died[23].
Also, CB cannot be distinguished on imaging from many common diseases, such as ABC, tuberculous spondylitis, eosinophilic granulomas, osteoblastomas, giant cell tumor, cartilage mucinous, fibromas, chondrosarcomas, osteosarcomas and metastases[7,24,25]. In imaging examinations, CB tends to destroy one side of the vertebral bone, with extensive bone destruction and tissue infiltration[24,26]. The tumor's signal is heterogeneous on MRI T2-weighted and short-TI inversion recovery images, with regions of low signal intensity corresponding to solid components (calcification); non-osteoid tumor matrix and cartilage-like elements are associated with regions of high signal intensity[25,27]. There was significant enhancement of the tumor, and the thickness of the tumor border was usually less than 1 mm, which was also similar to the imaging features of the patient we reported. In addition, whole-body positron emission computed tomography examination can help determine the presence of distant metastases.
Currently, in the treatment of patients with CB of long bones, thorough tumor curettage with high-speed grinding drills, bone cement filling or radiofrequency ablation can lead to a good long-term prognosis and preserve as much normal function of the limb as possible[28,29]. In addition to chemical (phenol), electrocautery or cryosurgery can be used as adjuvant treatment for CB patients[30]. Whereas surgery is the only effective treatment for cranial CB, the probability of recurrence is greatly increased if residual lesions are present after surgery in patients with cranial CB[31]. Spinal CB has a higher recurrence rate compared to CB occurring in the long bones, and the growth of spinal CB is more aggressive[6,7]. Vialle et al[8] stated that the local recurrence rate is 24% to 100% when spinal CB involves the long bones, and for spine CB, a simple resection cannot achieve a good therapeutic effect[4]. In addition, most of the literature recommends complete resection of the vertebral body, complete nerve decompression and simultaneous reconstruction of spinal stability[2,7,10,32]. However, extensive resection may be difficult to perform in clinical practice due to the complex anatomy and infiltration of vital internal organs and neurovascular structures by tumor tissue, which can lead to an increased rate of patient recurrence. A single-center study of 13 spinal CBs found that patients with curettage resection had a 100% patient recurrence rate, whereas none of the patients with total en bloc spondylectomy resection recurred during follow-up[4]. Similarly, our patient experienced recurrence in less than 2 years after curettage resection. This also reflects the importance of performing complete resection of the spinal CB.
In this article, we report a case of a rare tumor occurring in a rare location, sacral CB. We recommend complete removal of the tumor in a timely manner, provided that surgical conditions are met. At the same time, other diseases should be carefully differentiated in terms of imaging or pathological features so as to avoid erroneous diagnostic conclusions. However, due to the rarity of this disease, a large sample of cases will be needed to explore further this disease in the future.
We thank Dr. Jiang Y and Dr. She XL from Department of Pathology, The Second Xiangya Hospital, Central South University for pathological analysis of the study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Almeida Prado RM, Muhammed A S-Editor: Gao CC L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Tathe SP, Parate SN, Jaiswal KN, Randale AA. Intraoperative crush smear cytology of vertebral chondroblastoma: A diagnostic challenge. Diagn Cytopathol. 2018;46:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Venkatasamy A, Chenard MP, Massard G, Steib JP, Bierry G. Chondroblastoma of the thoracic spine: a rare location. Case report with radiologic-pathologic correlation. Skeletal Radiol. 2017;46:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Angelini A, Hassani M, Mavrogenis AF, Trovarelli G, Romagnoli C, Berizzi A, Ruggieri P. Chondroblastoma in adult age. Eur J Orthop Surg Traumatol. 2017;27:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Jia Q, Liu C, Yang J, Ji Y, Wei H, Liu T, Yang X, Yang C, Xiao J. Clinical features, treatments and long-term follow-up outcomes of spinal chondroblastoma: report of 13 clinical cases in a single center. J Neurooncol. 2018;140:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Hoeffel JC, Brasse F, Schmitt M, Plenat F, Vignaud JM, Czorny A, Montaut J, Marchal AL. About one case of vertebral chondroblastoma. Pediatr Radiol. 1987;17:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Kim SA, Cho KJ, Park YK, Lee JS, Kwon HJ, Chung H, Kim MJ. Chondroblastoma of the Lumbar Spine - A Case Report and Review of the Literature. Korean J Pathol. 2011;45:532. |
| 7. | Ilaslan H, Sundaram M, Unni KK. Vertebral chondroblastoma. Skeletal Radiol. 2003;32:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Vialle R, Feydy A, Rillardon L, Tohme-Noun C, Anract P, Colombat M, De Pinieux G, Drapé JL, Guigui P. Chondroblastoma of the lumbar spine. Report of two cases and review of the literature. J Neurosurg Spine. 2005;2:596-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Giri PJ, Chavan VS. Chondroblastoma of thoracic vertebra in young adult causing paraparesis. Roman Neurosurg. 217;31:335-338. |
| 10. | Konishi E, Nakashima Y, Mano M, Tomita Y, Kubo T, Araki N, Morii E, Yoshikawa H, Haga H, Toguchida J, Ueda T, Osawa M, Hoshi M, Inoue T, Aono M, Yanagisawa A. Chondroblastoma of extra-craniofacial bones: Clinicopathological analyses of 103 cases. Pathol Int. 2017;67:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Suneja R, Grimer RJ, Belthur M, Jeys L, Carter SR, Tillman RM, Davies AM. Chondroblastoma of bone: long-term results and functional outcome after intralesional curettage. J Bone Joint Surg Br. 2005;87:974-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Lin PP, Thenappan A, Deavers MT, Lewis VO, Yasko AW. Treatment and prognosis of chondroblastoma. Clin Orthop Relat Res. 2005;438:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Kilpatrick SE, Pike EJ, Geisinger KR, Ward WG. Chondroblastoma of bone: use of fine-needle aspiration biopsy and potential diagnostic pitfalls. Diagn Cytopathol. 1997;16:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Fanning CV, Sneige NS, Carrasco CH, Ayala AG, Murray JA, Raymond AK. Fine needle aspiration cytology of chondroblastoma of bone. Cancer. 1990;65:1847-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Sneige N, Ayala AG, Carrasco CH, Murray J, Raymond AK. Giant cell tumor of bone. A cytologic study of 24 cases. Diagn Cytopathol. 1985;1:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Elsheikh T, Silverman JF, Wakely PE Jr, Holbrook CT, Joshi VV. Fine-needle aspiration cytology of Langerhans' cell histiocytosis (eosinophilic granuloma) of bone in children. Diagn Cytopathol. 1991;7:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Kobayashi TK, Ueda M, Nishino T, Bamba M, Echigo T, Oka H, Hino A, Fuse I, Fujimoto M, Katsumori T, Kaneko C. Langerhans cell histiocytosis of the skull on cytologic squash preparations. Diagn Cytopathol. 2007;35:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Rehkämper J, Steinestel K, Jeiler B, Elges S, Hekeler E, Huss S, Sperveslage J, Hardes J, Streitbürger A, Gosheger G, Wardelmann E, Baumhoer D, Trautmann M, Hartmann W. Diagnostic tools in the differential diagnosis of giant cell-rich lesions of bone at biopsy. Oncotarget. 2018;9:30106-30114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Weng JC, Li D, Wang L, Wu Z, Wang JM, Li GL, Jia W, Zhang LW, Zhang JT. Surgical management and long-term outcomes of intracranial giant cell tumors: a single-institution experience with a systematic review. J Neurosurg. 2018;131:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Roeke T, Sobral-Leite M, Dekker TJA, Wesseling J, Smit VTHBM, Tollenaar RAEM, Schmidt MK, Mesker WE. The prognostic value of the tumour-stroma ratio in primary operable invasive cancer of the breast: a validation study. Breast Cancer Res Treat. 2017;166:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Coughlan B, Feliz A, Ishida T, Czerniak B, Dorfman HD. p53 expression and DNA ploidy of cartilage lesions. Hum Pathol. 1995;26:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Wadayama B, Toguchida J, Yamaguchi T, Sasaki MS, Yamamuro T. p53 expression and its relationship to DNA alterations in bone and soft tissue sarcomas. Br J Cancer. 1993;68:1134-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Ostrowski ML, Johnson ME, Truong LD, Hicks MJ, Smith FE, Spjut HJ. Malignant chondroblastoma presenting as a recurrent pelvic tumor with DNA aneuploidy and p53 mutation as supportive evidence of malignancy. Skeletal Radiol. 1999;28:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Ehalt W, Ratzenhofer M. [On the case history of a benign chondroblastoma]. Z Orthop Ihre Grenzgeb. 1967;102:625-629. [PubMed] |
| 25. | Weatherall PT, Maale GE, Mendelsohn DB, Sherry CS, Erdman WE, Pascoe HR. Chondroblastoma: classic and confusing appearance at MR imaging. Radiology. 1994;190:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Osman W, Zaoui A, Abdelkrim SB, Hamida RB, Ayche MB. Chondroblastoma of the Lower Thoracic Spine. A Case Report and Review of the Literature. Am J Med Case Rep. 2014;2:78-81. |
| 27. | Kasthoori JJ, Wastie ML. Chondroblastoma of the spine presenting with tetraparesis. J Hong Kong Colle Radiolog. 2006;9:24. |
| 28. | Ebeid WA, Hasan BZ, Badr IT, Mesregah MK. Functional and Oncological Outcome After Treatment of Chondroblastoma With Intralesional Curettage. J Pediatr Orthop. 2019;39:e312-e317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Outani H, Kakunaga S, Hamada K, Takenaka S, Nakai S, Yasuda N, Imura Y, Naka N, Araki N, Ueda T, Yoshikawa H. Clinical outcomes of chondroblastoma treated using synthetic bone substitute: risk factors for developing radiographic joint degeneration. World J Surg Oncol. 2020;18:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Herget GW, Maier D, Südkamp NP, Uhl M, Füllgraf H, Dovi-Akue D. Anatomical Reconstruction of the Acromion Using an Autologous Iliac Crest Graft for Treatment of Recurrent Chondroblastoma: A Case Report. JBJS Case Connect. 2019;9:e0086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Muhammed A, Meshneb M, Saro H, Elnakib N, Elnakib E. Management of cranial chondroblastoma in adults; a pooled analysis. Am J Otolaryngol. 2020;41:102486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Sohn SH, Koh SA, Kim DG, Park SW, Lee KH, Kim MK, Choi JH, Hyun MS. A case of spine origin chondroblastoma metastasis to lung. Cancer Res Treat. 2009;41:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |