Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5689
Peer-review started: March 5, 2021
First decision: April 4, 2021
Revised: April 7, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: July 16, 2021
Processing time: 124 Days and 8.2 Hours
Healthcare workers (HCWs) are at an increased risk for exposure to infections. Serratia marcescens (S. marcescens) is a gram-negative, opportunistic and nosoco
A 33-year-old woman, a resident in anesthesiology, was admitted at 14 wk gestation for fever with chills. She had no medical history other than contact dermatitis of both hands that started from the beginning of the trainee. There was no obvious infection focus and no bacterial growth in blood cultures. She was discharged after 1 wk of empirical antibiotic treatment. At three weeks before the fever started, she had a blister on the site of contact dermatitis on both hands, she applied antibiotic ointment for three days and the blisters had healed. At 19 wk gestation, she had a high fever and was readmitted. Physical examination and image studies were nonspecific and the patient had no other symptoms. S. marcescens grew in blood cultures at 19 wk gestation. Treatment with intravenous antibiotics was started. However, she suffered a miscarriage at 224/7 wk gestation. Pathologically, the amniotic membrane showed chorioamnionitis with a focal infarct. Subsequently, a placenta tissue culture grew S. marcescens.
HCWs can be exposed to pathogens that can cause opportunistic infections such as S. marcescens. Pregnancy affects the immune system, making it susceptible to opportunistic infections. Therefore, pregnant HCWs may require more preventive measures, including hand hygiene and avoid risk factors (ex. wrapping the skin).
Core Tip: In this case, a 33-year-old female patient suffered a miscarriage due to chorioamnionitis caused by Serratia marcescens infection. Although she performed aseptic procedures and hand hygiene during medical practice, it is suspected that the cause of the infection was the weakening of the skin line due to contact dermatitis. In addition, it is thought that changes in the immune system caused by pregnancy may also have an effect. Therefore, it is recommended that pregnant healthcare workers perform more meticulous hand hygiene and avoid infection risk factors (ex. wrapping the skin).
- Citation: Park SY, Kim MJ, Park S, Kim NI, Oh HH, Kim J. Chorioamnionitis caused by Serratia marcescens in a healthcare worker: A case report. World J Clin Cases 2021; 9(20): 5689-5694
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5689.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5689
Serratia marcescens (S. marcescens) is a Gram-negative anaerobic bacillus in the Enterobacteriaceae family. It is not a normal member of the human bacterial flora, although it can colonize the gastrointestinal, urinary, and respiratory tracts. The incidence of nosocomial infection of S. marcescens is 1%-2%, but it shows high mortality rate of 37%[1,2]. There have been a few case reports of Serratia bacteremia associated with pregnant women, four of which resulted in miscarriage[3-8]. Hand-to-hand transmissions by HCWs is the major mode of spread[9].
Healthcare workers (HCWs) are at increased risk for exposure to infections, including blood-borne, air-borne, and contact-transmitted infection. Blood-borne infections include human immunodeficiency virus and hepatitis B and C viruses[10]. Air-borne transmitted infectious diseases include tuberculosis and viruses contained in operating room smoke[11]. There are some reports of methicillin-resistant Staphylococcus aureus (MRSA) carriage and infection in HCWs, which is spread by contact[12].
Pregnant women tend to be more severely affected by infections due to the immunological changes caused by altered hormone levels[13]. Therefore, pregnant HCWs may need more attention and care for infection prevention. However, hospital-acquired bacterial infection in pregnant HCWs has been studied less often. Here, we report a pregnant HCW with S. marcescens bacteremia that resulted in a spontaneous abortion.
A 33-year-old woman was admitted at 19 wk gestation for a fever with chills.
She was hospitalized at 14 wk of pregnancy with fever and dry cough. At that time, after receiving empirical antibiotic treatment (intravenous ceftriaxone 2 g and azithromycin 500 mg orally) for 1 wk, it improved. After discharge, she had an intermittent mild fever (37.5-38.0 °C) for 3 wk.
She had contact dermatitis of both hands that started from the beginning of trainee. She applied steroid ointment intermittently to control the symptoms of contact dermatitis. At three weeks before the 1st fever event (gestation 11 wk) had a blister on the site of contact dermatitis on both hands, she applied antibiotic ointment for three days and the blisters had healed.
She was an anesthesiology resident and worked in intensive care units at gestational periods. She rounded the patients and, if necessary, performed endotracheal intubation or central venous catheterization. Family history was unremarkable.
The physical examination was unremarkable and the patient did not complain abdominal pain or urinary symptoms. A vaginal examination showed no leaking amniotic fluid.
Laboratory tests indicated mildly increased white blood cell count, high C-reactive protein (CRP) (15.8 mg/dL; normal 0-0.3 mg/dL), and mildly elevated procalcitonin level (0.503 ng/mL; normal 0-0.5 ng/mL). Her thyroid, liver, and renal functions, coagulation profile, electrolytes, and lactate levels were normal. Polymerase chain reaction results of a vaginal discharge for sexually transmitted diseases were normal. Laboratory studies to rule out rheumatological diseases were all normal. After 2 d, the blood cultures grew S. marcescens.
Abdominal ultrasonography was nonspecific other than a 0.5 cm gallbladder polyp. The fetus and placenta appeared normal in obstetric ultrasonography. An additional abdominal and pelvic magnetic resonance imaging scan was also performed to find the cause of the fever, but it was also normal.
Fever was caused by S. marcescens cultured in the blood, and the site of infection was the chorionic tissue (later) found after abortion.
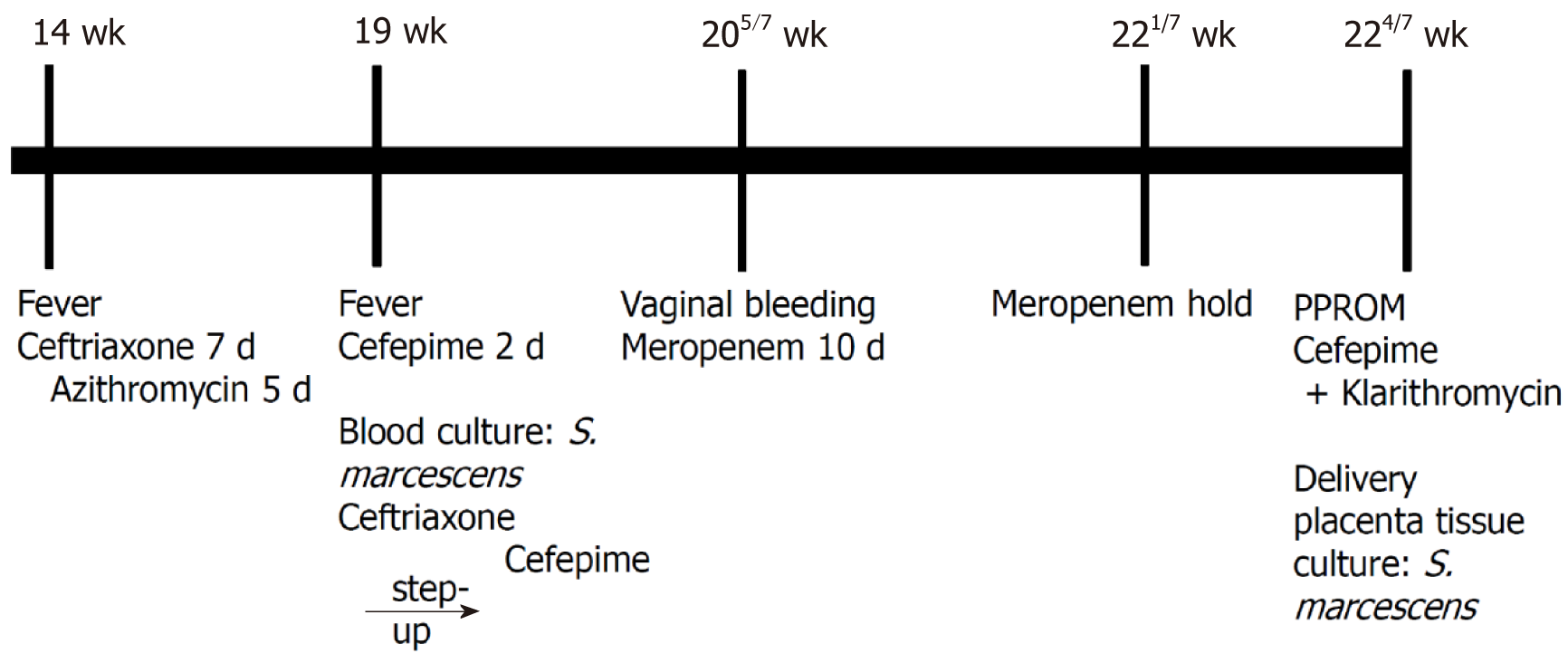
Empirical cefepime 2 g intravenous (IV) was given every 8 h. After 2 d, the blood cultures grew S. marcescens. The antibiotic was changed to ceftriaxone 2 g IV daily as the bacteria were susceptible to ceftriaxone. On the 4th day of admission, she had a fever again, so the antibiotic was stepped up to cefepime. Figure 1 shows the timeline of the major clinical events.
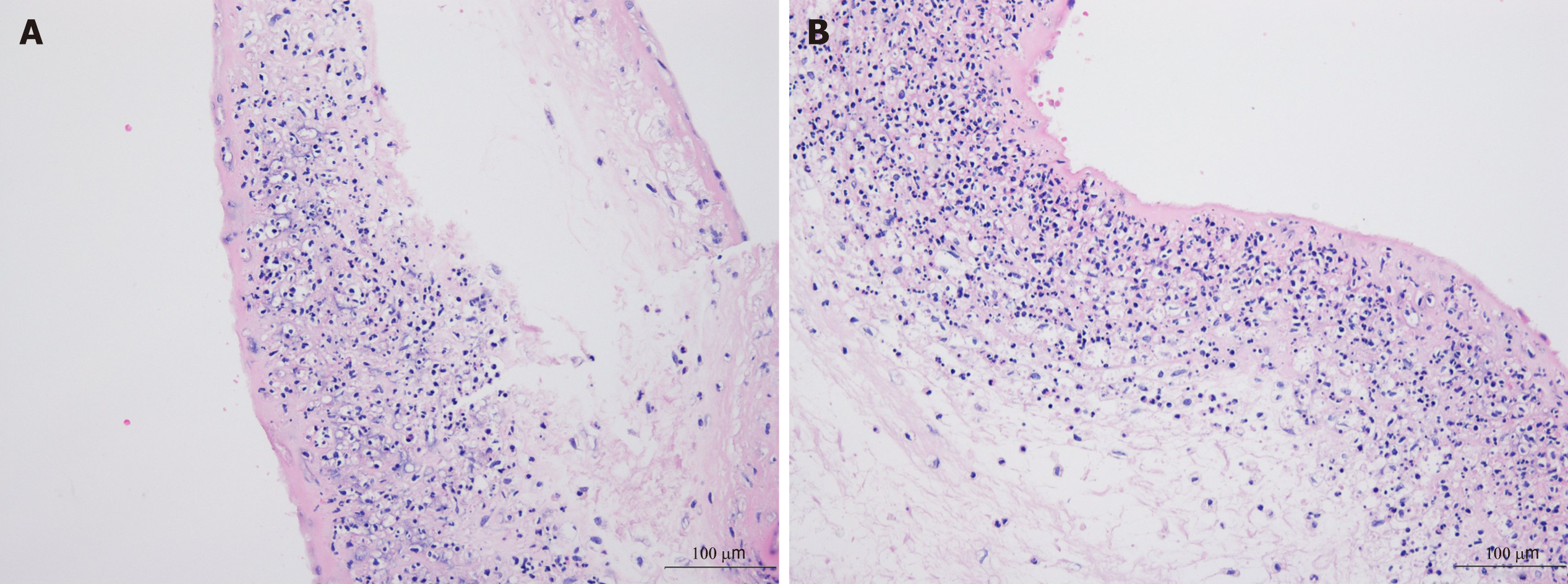
Twenty-three days after admission, at 224/7 wk gestation, she had a watery discharge and preterm premature rupture of the membrane (PPROM) was diagnosed as the vaginal discharge showed pH 8, pooling (+), Fern (+), and AmniSure (+). Cefepime 2 g IV every 8 h and clarithromycin 500 mg PO were started after taking blood cultures. Labor was induced and she delivered a 360 g dead male fetus. After 7 d of IV cefepime and 5 days of PO clarithromycin, the patient was afebrile and the leukocytosis and CRP level had normalized. She was discharged in good condition. The tissue culture from the placenta subsequently grew S. marcescens. Pathologically, the amniotic membrane showed chorioamnionitis with a focal infarct (Figure 2).
This present case reports healthy pregnant HCW developed an atypical reproductive system infection by S. marcescens, a Gram-negative bacillus that survives in environments such as drinking water pipes, hospital disinfectants, and medical instruments[14]. It can also colonize the human gastrointestinal tract and skin for extended periods and is important, frequently found nosocomial pathogen. Many studies have traced S. marcescens outbreaks in hospitals to medical instruments[9,15-17]. It is exogenously acquired, mostly via the hands of healthcare providers[9].
A few cases of S. marcescens infection during pregnancy have been reported[3,5-8]. In four cases, the suspected routes of infection were chorionic-villus sampling[6], urinary tract infection due to a double ureter[6], the placement of a peripherally inserted central catheter[5], and prolonged PPROM[3]. In another three cases, no route of infection was found. Our patient had no S. marcescens infection route other than skin route. Of the previous cases, S. marcescens infection during pregnancy has a poor prognosis, three had spontaneous abortions, one delivered a dead fetus, and three had live babies. Of the three cases that had live babies, one showed signs of chorioamnionitis[3], and two had placental abscesses on ultrasonography and continued the antibiotic treatment[4,5]. In our patient, however, no infection focus was found until after the spontaneous abortion, so deciding the duration of antibiotic treatment was difficult.
There have been many reviews of adverse pregnancy outcomes among HCWs, such as congenital anomalies, fetal death, and fertility disorders[18-20]. Two reviews considered the relationships between adverse pregnancy outcomes and specific exposures, such as anesthetic gases, antineoplastic agents, sterilizing agents, and radiation. But neither assessed hospital-acquired bacterial infection in a pregnant HCW. March et al[21] reported that 66.1% of HCWs in an Italian long-term care facility were colonized by multidrug-resistant bacteria. Liu et al[22] reported that intensive care unit HCWs have a higher proportion of multidrug-resistant Gram-negative bacteria colonization than non-medical workers (41.87% vs 28.57%, respectively). Peters et al[23] reviewed the occupational infection risk with multidrug-resistant organisms, such as MRSA, vancomycin-resistant Enterococcus, and Gram-negative bacteria, in HCWs, but the comparison of results was limited due to data heterogeneity.
The occurrence of this rare case is suspected to be due to the patient's underlying disease, career and work environment. Skin is essential to maintaining a normal immune state from pathogens. If this barrier breaks down, the normal flora can become pathogens[24]. The patient was in a state of collapsed skin defenses due to contact dermatitis. She applied steroid ointment intermittently to control the symptoms of contact dermatitis. Pregnant women may be more severely affected by infection than non-pregnant women due to major changes in their immune systems[25.] In addition, since the patient is an HCW, it is a situation in which a lot of exposure to S. marcescens. is possible, and it is an environment in which medicines can be easily prescribed. Although there is no evidence that the skin lesions that occurred before fever was caused by S. marcescens, there is a case report that appeared in the form of blisters during Serratia infection[26].
This case is important because even a healthy HCW may be vulnerable to infection due to the immune system changes that occur during pregnancy. HCWs should be aware and cautioned that there is a risk of opportunistic infections at work. Also, HCWs who are pregnant may require more preventive precautions, including hand hygiene, and avoid risk factors (ex. wrapping the skin).
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anzola L S-Editor: Wang JL L-Editor: A P-Editor: Zhang YL
| 1. | Schaberg DR, Highsmith AK, Wachsmuth IK. Resistance plasmid transfer by Serratia marcescens in urine. Antimicrob Agents Chemother. 1977;11:449-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Engel HJ, Collignon PJ, Whiting PT, Kennedy KJ. Serratia sp. bacteremia in Canberra, Australia: a population-based study over 10 years. Eur J Clin Microbiol Infect Dis. 2009;28:821-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Erenberg M, Yagel Y, Press F, Weintraub AY. Chorioamnionitis caused by Serratia marcescens in a healthy pregnant woman with preterm premature rupture of membranes: A rare case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2017;211:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Mak ASL, Tang THC, Lam KW, Kwok ALM, Cheuk W, Wu TC, Leung KY. Prenatal sonography of placental abscess and prolonged antibiotic treatment for Serratia marcescens bacteremia. Clin Case Rep. 2018;6:537-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Meirowitz NB, Fleischer A, Powers M, Hippolyte F. Diagnosis of placental abscess in association with recurrent maternal bacteremia in a twin pregnancy. Obstet Gynecol. 2006;107:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Prosser BJ, Horton J. A rare case of serratia sepsis and spontaneous abortion. N Engl J Med. 2003;348:668-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Shimizu S, Kojima H, Yoshida C, Suzukawa K, Mukai HY, Hasegawa Y, Hitomi S, Nagasawa T. Chorioamnionitis caused by Serratia marcescens in a non-immunocompromised host. J Clin Pathol. 2003;56:871-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Vale-Fernandes E, Moucho M, Brandão O, Montenegro N. Late miscarriage caused by Serratia marcescens: a rare but dire disease in pregnancy. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | de Vries JJ, Baas WH, van der Ploeg K, Heesink A, Degener JE, Arends JP. Outbreak of Serratia marcescens colonization and infection traced to a healthcare worker with long-term carriage on the hands. Infect Control Hosp Epidemiol. 2006;27:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Beltrami EM, Williams IT, Shapiro CN, Chamberland ME. Risk and management of blood-borne infections in health care workers. Clin Microbiol Rev. 2000;13:385-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Albrich WC, Harbarth S. Health-care workers: source, vector, or victim of MRSA? Lancet Infect Dis. 2008;8:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 305] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 13. | Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;371:1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 384] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Arslan U, Erayman I, Kirdar S, Yuksekkaya S, Cimen O, Tuncer I, Bozdogan B. Serratia marcescens sepsis outbreak in a neonatal intensive care unit. Pediatr Int. 2010;52:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Cullen MM, Trail A, Robinson M, Keaney M, Chadwick PR. Serratia marcescens outbreak in a neonatal intensive care unit prompting review of decontamination of laryngoscopes. J Hosp Infect. 2005;59:68-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Sokalski SJ, Jewell MA, Asmus-Shillington AC, Mulcahy J, Segreti J. An outbreak of Serratia marcescens in 14 adult cardiac surgical patients associated with 12-lead electrocardiogram bulbs. Arch Intern Med. 1992;152:841-844. [PubMed] |
| 18. | Park C, Kang MY, Kim D, Park J, Eom H, Kim EA. Adverse pregnancy outcomes in healthcare workers: a Korean nationwide population-based study. Int Arch Occup Environ Health. 2017;90:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Quansah R, Jaakkola JJ. Occupational exposures and adverse pregnancy outcomes among nurses: a systematic review and meta-analysis. J Womens Health (Larchmt). 2010;19:1851-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Warembourg C, Cordier S, Garlantézec R. An update systematic review of fetal death, congenital anomalies, and fertility disorders among health care workers. Am J Ind Med. 2017;60:578-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | March A, Aschbacher R, Sleghel F, Soelva G, Kaczor M, Migliavacca R, Piazza A, Mattioni Marchetti V, Pagani L, Scalzo K, Pasquetto V, Pagani E. Colonization of residents and staff of an Italian long-term care facility and an adjacent acute care hospital geriatric unit by multidrug-resistant bacteria. New Microbiol. 2017;40:258-263. [PubMed] |
| 22. | Liu H, Fei CN, Liu J, Ji XY, Song J. [Investigation on the status of multidrug-resistant gram-negative bacteria induced by occupational exposure among medical staff in ICU]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2018;36:734-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Peters C, Dulon M, Nienhaus A, Schablon A. Occupational Infection Risk with Multidrug-Resistant Organisms in Health Personnel-A Systematic Review. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Abdallah F, Mijouin L, Pichon C. Skin Immune Landscape: Inside and Outside the Organism. Mediators Inflamm. 2017;2017:5095293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Chai LY, Rauff M, Ong JS, Kee AC, Teo FS. Serratia septicaemia in pregnancy: further evidence of altered immune response to severe bacterial infection in pregnancy. J Infect. 2011;63:480-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Bahadir A, Erduran E. Serratia marcessens infection presenting with papillovesicular rash similar to varicella zoster infection: a case report. North Clin Istanb. 2015;2:55-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |