Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5675
Peer-review started: March 3, 2021
First decision: April 14, 2021
Revised: April 25, 2021
Accepted: May 8, 2021
Article in press: May 8, 2021
Published online: July 16, 2021
Processing time: 125 Days and 19.5 Hours
Oncocytic adrenocortical tumor (OACT) is rare, with few cases reported in the literature. No more than 20 cases in children have been reported. The clinical characteristics, diagnosis, treatment and prognosis of children with OACT are summarized based on a literature review, in order to improve the understanding of OACT in children.
We report a case of a 17-mo-old patient who was admitted to our hospital due to symptoms of odynuria and fever, which are clinical features consistent with a functional adrenocortical tumor. The patient was diagnosed with OACT of uncertain malignant potential. Computed tomography indicated a soft tissue giant tumor in the right adrenal region, approximately 4.3 cm × 5.5 cm in size. Multiple nodular and speckled calcifications were observed in the lesion. The patient received robot-assisted laparoscopic right adrenal tumor resection. Postoperative pathological results were consistent with OACT, and immunohistochemical results showed cytokeratin+/-, chromogranin A+, synaptophysin-, neuron-specific enolase-, S100-, Ki67 about 10%, CD34- and D2-40-. After surgery, urinary tract ultrasonography was reviewed monthly, catecholamine hormone and sex hormone levels were examined every 2 mo and computed tomography was performed every 6 mo. To date, no tumor metastasis or recurrence has been identified in this patient. The levels of sex hormones and catecholamine hormones decreased to normal 1 mo after surgery.
OACT is rare in the pediatric population, with few cases reported in the literature. Although most pediatric OACTs are benign, malignant cases have been reported. Surgical resection is the preferred option in most patients.
Core Tip: Oncocytic adrenocortical tumor (OACT) is a very rare tumor, with few cases reported in the literature. Surgical resection is the preferred treatment option in most cases. We report the first case of robot-assisted laparoscopic resection of an OACT in a pediatric patient. The findings from this case report and the literature review show that robot-assisted laparoscopic resection of pediatric OACT with a complete tumor capsule, clear boundary between the tumor and surrounding tissues and no regional lymph node invasion is safe. Radiation and chemotherapy do not seem to be necessary in pediatric OACT without metastasis.
- Citation: Chen XC, Tang YM, Mao Y, Qin DR. Oncocytic adrenocortical tumor with uncertain malignant potential in pediatric population: A case report and review of literature. World J Clin Cases 2021; 9(20): 5675-5682
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5675.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5675
Oncocytic adrenocortical tumor (OACT) is a very rare tumor[1]. At present, less than 200 cases have been reported[2]. Fewer than 20 pediatric patients have been reported worldwide[3-16]. OACT can be functional or nonfunctional, and most are nonfunctional[17]. Herein, we report a functional OACT with uncertain malignant potential in a 17-mo-old patient.
A 17-mo-old male patient was admitted to our hospital due to symptoms of odynuria and fever.
One day previously, the child showed no obvious cause of his urination pain, which was accompanied by fever, with a maximum temperature of 38.2 ℃.
The patient had no previous medical history.
The patient had no specific personal and family history.
Forehead and the outer thighs showed scattered acne, and development of the penis and scrotum was more mature than his peers. Blood pressure was normal without significant fluctuations. No central obesity or other Cushing's syndrome signs were observed.
Serum tumor marker examination revealed alpha-fetoprotein 5.40 ng/mL (< 8.78 ng/mL), cancer antigen-125 22.50 U/mL (< 35.0 U/mL), carbohydrate antigen 19-9 16.9 52 U/mL (< 34.0 U/mL), carcinoembryonic antigen 1.59 ng/mL (< 6.2 ng/mL) and neuron-specific enolase 43.26 ng/mL (< 20 ng/mL). Sex hormone levels were as follows: Dehydroepiandrosterone sulfate > 40.71 mol/L (3.7-16.1 mol/L), estradiol 87.6 pmol/L (40.4-161.5 pmol/L) and testosterone 12.97 nmol/L (4.94-32.01 nmol/L). Catecholamine hormone levels were as follows: Methoxy noradrenaline 1.22 nmol/L (< 0.90 nmol/L), 3-methoxytyramine 2.12 pg/mL (0-18.4 pg/mL), dopamine 0.01 nmol/L (< 0.02 nmol/L), adrenaline 0.04 nmol/L (0-0.77 nmol/L) methoxy adrenaline 0.03 nmol/L (< 0.05 nmol/L) and noradrenaline 3.15 nmol/L (0.41-10.06 nmol/L). The biochemical evaluation showed normal serum electrolyte levels.
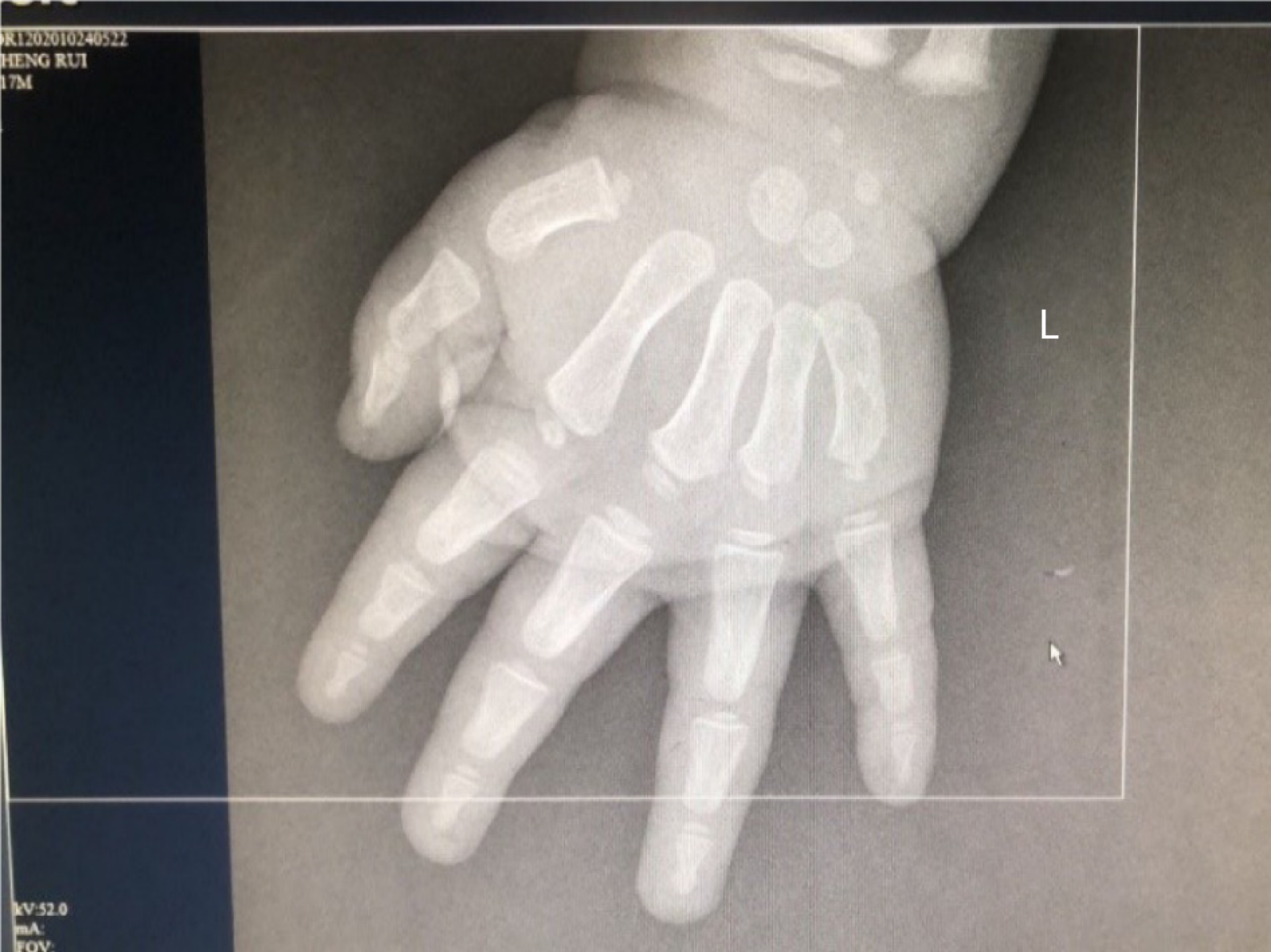
Computed tomography showed a soft tissue tumor in the right adrenal region approximately 4.3 cm × 5.5 cm in size. There were multiple nodular and speckled calcifications in the lesion, which were considered to be tumor occupying changes. Photographs showed that the patient’s bone age was ahead of schedule (Figure 1).
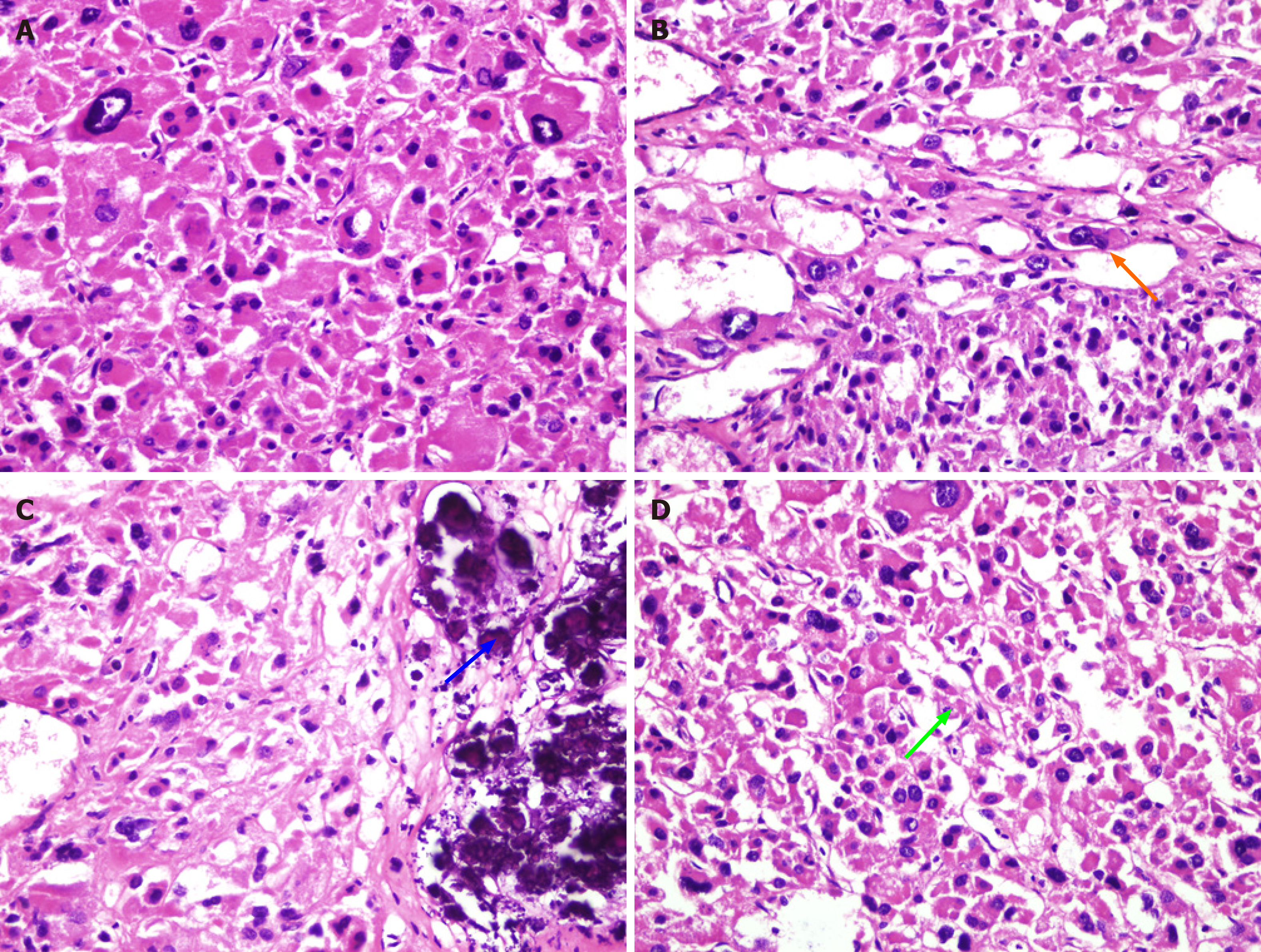
Pathology and immunohistochemistry confirmed an adrenocortical tumor of oncocytic subtype, with a maximum diameter of about 5.5 cm. Postoperative tissue is shown in Figure 2, and a microscopic image is shown in Figure 3. Eosinophils were predominant; clear cells were < 25% with visible atypical wide cells. No definite necrosis, capsular invasion or vein invasion were found. Visible focal sinus infiltration was identified, nuclear fission image count was 1/10 hibernation-promoting factor (HPF) and reticular fiber staining was noted showing partial damage. Immunohistochemical results were as follows: Cytokeratin+/-, chromogranin A+, synaptophysin-, neuron-specific enolase-, S100-, Ki67 about 10%, CD34- and D2-40-. According to the World Health Organization 2016/4thE Lin-Weiss-Bisceglia standard, the tumor was considered an OACT with uncertain malignant potential.
Malignant features in oncocytic adrenocortical tumor are determined using the World Health Organization 2016/4thE Lin–Weiss–Bisceglia criteria, the patient was finally diagnosed as an OACT with uncertain malignant potential.
The maximum diameter of the tumor was 5.5 cm, and it was confirmed to be an OACT. The patient underwent robot-assisted laparoscopic right adrenal tumor resection.
During the follow-up, urinary tract ultrasonography was reviewed monthly, catecholamine hormone and sex hormone levels were examined every 2 mo and CT was performed every 6 mo. To date, no tumor metastasis or recurrence has been identified. The sex hormone and catecholamine hormone levels reduced to normal 1 mo after surgery.
At present, the etiology of OACT is still unclear. Kakimoto et al[18] were the first to report an OACT in 1986. Oncocytic tumors are neoplasms composed predominantly of cells with abundant granular, eosinophilic cytoplasm termed “oncocytes”. These cytoplasmic changes are attributed to the abundant mitochondria[19]. Gasparre et al[20] believed that the increase in mitochondria in this disease is a compensatory reaction to cellular dysfunction, which may be due to the excessive proliferation of mitochondrial DNA without corresponding transcription of RNA, resulting in the excessive increase of mitochondria in cells[20].
Limited reports on OACT epidemiology were found in the literature review. Available statistics are from an epidemiological study by Kanitra et al[21] that included 140 OACT patients, 66% were found in female patients, 64% were found in the left adrenal gland and 66% were nonfunctional. The mean age at diagnosis was 44 years (2.5-77 years), and the mean tumor size was 80 mm (16-285 mm). A total of 35% were benign, 41% were of uncertain malignant potential and 24% were malignant. Compared with female patients, male patients were more likely to develop malignancy (36% vs 18%, P = 0.035). The 5-year overall survival rate of benign OACT was 100%, was 88% in those with uncertain malignant potential and 47% in those with malignant potential[21]. However, there are limitations in the statistics, which did not include children under 2.5 years. There are no epidemiological data for large populations of children, as only a few cases have been reported.
An OACT can be functional or nonfunctional, most of which are nonfunctional and only a few are functional[17], resulting in pseudopuberty, gynecomastia, virilization, feminization and Cushing's syndrome[22,23]. Advanced bone age has also been reported in children[13]. Details of children with OACT are shown in Table 1.
| Ref. | Age in yr | G | Clinical features | Size | Treatment | Follow-up | Prognosis |
| Gumy-Pause et al[3], 2008 | 12 | F | Fatigue, headache, acne vulgaris, and abdominal pain | 5.0 cm × 4.3 cm × 2.2 cm | Open adrenalectomy | Normal hormone levels 18 mo after diagnosis | No recurrence |
| Lim et al[4], 2010 | 14 | F | Deepening of the voice and excessive hair | 17.5 cm × 15 cm × 14 cm | Open adrenalectomy | Normal hormone levels 2 wk after operative resection | No recurrence |
| Tahar et al[5], 2008 | 6 | F | Precocious puberty | 3.0 cm × 2.0 cm × 1.5 cm | Open adrenalectomy | 12 mo after operative resection, he manifestations of pseudoprecocious puberty were effectively reduced | No recurrence |
| Subbiah et al[6], 2013 | 3 1/2 | F | Premature pubarche, clitoromegaly | 2.5 cm × 2.0 cm | Open adrenalectomy | Normal hormone levels 1 mo after operative resection | No recurrence |
| Kawahara et al[7], 2014 | 11 | F | Fever, weight loss,increased inflammatory markers | 4.5 cm × 4.5 cm × 2.5 cm | Open adrenalectomy | The inflammatory markers and IL-6 levels normalized within 2 wk after tumor resection | No recurrence |
| Yoon et al[8], 2014 | 10 | F | Precocious puberty | 6.0 cm × 4.0 cm | Open adrenalectomy | 1 yr after surgery without new lesions | No recurrence |
| Akin et al[9], 2014 | 11 | M | Metabolic, alkalosis, polyuria, polydipsia, hypokalemia | 4.5 cm × 3.5 cm × 2.5 cm | Laparoscopic surgery | After the operation, the patient's polyuria and hypokalemia resolved, and his aldosterone level returned to normal | No recurrence |
| Ranganathan et al[10], 2005 | 5 | M | Precocious puberty, acne | 4.2 cm × 3.9 cm × 2.6 cm | Laparoscopic surgery | 3 mo later, the patient had lost 3.2 kg and had grown 3.5 cm. Clinically, his symptoms resolved with no progression of pubic hair, axillary hair, or acne | No recurrence |
| Mardi et al[11], 2016 | 14 | F | Hirsuitism | 18 cm × 8.0 cm × 7.0 cm | Open adrenalectomy | The hirsuitism resolved gradually following surgery | No recurrence |
| Chen et al[12], 2018 | 15 | M | Lower back pain | 9.0 cm × 6.3 cm | Laparoscopic surgery | Lower back pain relief | No recurrence |
| Yordanova et al[13], 2015 | 9 | F | Virilization | 2.2 cm × 2.2 cm | Laparoscopic surgery | 11 mo after the surgery, the girl’s appearance was less masculine, with significantly reduced body hairs but still no changes in the voice | No recurrence |
| Pereira et al[14], 2014 | 5.8 | F | Weight gain, precocious puberty | 3.2 cm × 4.5 cm | Open adrenalectomy | The patient is in complete remission after 64 mo of follow-up | No recurrence |
| Kolev et al[15], 2013 | 9 | F | Deepening of thevoice and excessive hair | 3.0 cm × 2.8 cm × 3.5 cm | Laparoscopic surgery | Normal hormone levels 2 wk after operative resection | No recurrence |
| Agarwal et al[16], 2011 | 2.5 | F | Virilization | - | Open adrenalectomy, biopsy | Poor prognosis | No resection, infiltration into adjacent organs |
| Current case | 17 mo | M | Odynuria, fever | 5.5 cm × 5.0 cm × 3.0 cm | Robot assisted laparoscopic operation | Normal hormone levels 1 mo after operative resection | No recurrence |
Clinical manifestations, auxiliary examination and tumor size are limited in the diagnosis of OACT, and the Weiss criteria are not completely reliable for the diagnosis of OACT[24]. Bisceglia et al[17] improved the Weiss criteria according to the pathological characteristics of OACT. At present, the Lin Weiss Bisceglia (LWB) criteria are commonly used in the pathological diagnosis of OACT: OACTs that meet any of the following major criteria are classified as malignant: Mitotic rate > 5 per 50 HPF, atypical mitoses and venous invasion. OACTs that meet any of the following secondary criteria are classified as having uncertain malignant potential; larger size (> 10 cm and/or weight > 200 g), necrosis, capsular invasion or sinusoidal infiltration. In the absence of major or secondary criteria, OACTs are considered benign[21]. In the present case, the tumor was nodular, 5.5 cm × 5.0 cm × 3.0 cm in size with a complete capsule, the section was gray-yellow and soft and calcification was visible, with no obvious bleeding and necrosis. No definite capsular invasion, vascular invasion or focal sinus infiltration were observed, and the mitotic count was one per 10 HPF. Histologically, one item met the secondary criteria; thus, it was considered an OACT with uncertain malignant potential.
Pathological examination is helpful in the diagnosis of pediatric OACT. A typical OACT is dark brown. In the present case, under a light microscope, the tumor cells were the same size, with low-fat droplets of granular and oncocytic cytoplasm. The nucleus was in the center, arranged into a beam-like and hollow tubular structure, occasionally forming microcapsules surrounded by thin fibrous septa, and a small amount of lymphocytes infiltrated the mesenchyme. Under an electron microscope, there were a number of mitochondria with lamellar and tubulovesicular cristae and small electron dense inclusion bodies in the cytoplasm. OACTs rarely show mitosis and necrosis[25]. At present, the LWB criteria are commonly used in the pathological diagnosis of children with OACT. The pathological findings in this case were consistent with the diagnosis of OACT. Following immunohistochemical analysis, eosinophilic cells were markedly and diffusely positive for vimentin, steroidogenic factor 1, alpha-inhibin, Melan A, synaptophysin, neuron-specific enolase and CD56[26]. Equilibrative nucleoside transporter 1 and synaptophysin are the two most specific markers in OACT[27].
Surgical resection is the main treatment for OACT, and long-term follow-up is required. Traditional open surgery is established. However, with the development and popularization of laparoscopic technology, this technology is more commonly adopted for the resection of adrenal cortical tumors. At present, robot-assisted laparoscopic resection of adrenal cortical tumors has not been reported. In 2014, Akin et al[9] reported the first laparoscopic resection of an OACT in children[9]. However, whether laparoscopic adrenalectomy should be used for potential malignancies with tumor diameter > 6 cm is controversial. Up to now the consensus has been to restrict the endoscopic approach to adrenal tumors measuring < 5 to 6 cm in diameter[28]. We believe that robot-assisted laparoscopic resection of OACT with a complete tumor capsule, clear boundary between the tumor and surrounding tissue and no regional lymph node invasion is safe in pediatric patients. Radiotherapy and chemotherapy have been reported for metastatic OACT[29]. In children with OACT without metastasis, there may be no need for radiotherapy or chemotherapy. OACTs are mostly benign tumors, but there are also reports of malignancy. Contrary to previous literature, John et al[30] found that the majority of adrenocortical oncocytic neoplasms (65%) were either malignant or had malignant potential. Hormone function cannot distinguish benign from malignant or prognosis. However, the majority of OACTs that are synaptophysin positive (50%, P < 0.001) and vimentin negative (62%, P = 0.009) were benign[21]. It has also been reported that Ki67 can reflect the degree of malignancy and the prognosis of OACT[31,32]. To date, no tumor metastasis or recurrence has been identified in the present case.
OACTs in children are rare. There are a few reports on pediatric OACTs, and the etiology is still unclear. Most OACTs are nonfunctional benign tumors, but there are also reports of malignancy. The LWB criteria are commonly used for the diagnosis of pediatric OACTs, and the diagnosis is mainly based on histological and pathological examination. Equilibrative nucleoside transporter 1 and synaptophysin are two of the most specific markers in eosinophilic tumors. Radical resection is the key to early treatment. We believe that robot-assisted laparoscopic resection of pediatric OACT with a complete tumor capsule, clear boundary between the tumor and surrounding tissues and no regional lymph node invasion is safe. Children with OACT do not seem to require radiotherapy and chemotherapy. Ki67 can reflect the degree of malignancy and the prognosis of OACT.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Keikha M S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Ohtake H, Kawamura H, Matsuzaki M, Yokoyama E, Kitajima M, Onizuka S, Yamakawa M. Oncocytic adrenocortical carcinoma. Ann Diagn Pathol. 2010;14:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Yan K, Xu X, Liu X, Wang X, Hua S, Wang C. Corrigendum: The Associations Between Maternal Factors During Pregnancy and the Risk of Childhood Acute Lymphoblastic Leukemia: A Meta-Analysis. Pediatr Blood Cancer. 2016;63:953-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Gumy-Pause F, Bongiovanni M, Wildhaber B, Jenkins JJ, Chardot C, Ozsahin H. Adrenocortical oncocytoma in a child. Pediatr Blood Cancer. 2008;50:718-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Lim YJ, Lee SM, Shin JH, Koh HC, Lee YH. Virilizing adrenocortical oncocytoma in a child: a case report. J Korean Med Sci. 2010;25:1077-1079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Tahar GT, Nejib KN, Sadok SS, Rachid LM. Adrenocortical oncocytoma: a case report and review of literature. J Pediatr Surg. 2008;43:E1-E3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Subbiah S, Nahar U, Samujh R, Bhansali A. Heterosexual precocity: rare manifestation of virilizing adrenocortical oncocytoma. Ann Saudi Med. 2013;33:294-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kawahara Y, Morimoto A, Onoue A, Kashii Y, Fukushima N, Gunji Y. Persistent fever and weight loss due to an interleukin-6-producing adrenocortical oncocytoma in a girl--review of the literature. Eur J Pediatr. 2014;173:1107-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Yoon JH, Cha SS, Yoon SK. Computed tomography and magnetic resonance images of adrenocortical oncocytoma cases. J Korean Med Sci. 2014;29:445-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Akin M, Erginel B, Tanik C, Akinci N, Yildiz A, Beker B, Karadag CA, Sever N, Turk S, Dokucu AI. The first laparo- scopic resection of an aldosterone-secreting adrenocortical oncocytoma in a child. J Pediatr Surg Case Rep. 2014;2:424-427. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Ranganathan S, Lynshue K, Hunt JL, Kane T, Jaffe R. Unusual adrenal cortical tumor of unknown biologic potential: a nodule in a nodule in a nodule. Pediatr Dev Pathol. 2005;8:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Mardi K. Virilizing adrenocortical oncocytic neoplasm with uncertain malignant potential in a child: A rare case report. Indian J Cancer. 2016;53:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Chen GX, Zhang XS, Li ZH, Gong ZQ. Clinicopathological Features and Minimal Invasive Management of Oncocytic Adrenal Neoplasm. Zhongguo Weichuangwaike Zazhi. 2018;18:694-698. [DOI] [Full Text] |
| 13. | Yordanova G, Iotova V, Kalchev K, Ivanov K, Balev B, Kolev N, Tonev A, Oosterhuis W. Virilizing adrenal oncocytoma in a 9-year-old girl: rare neoplasm with an intriguing postoperative course. J Pediatr Endocrinol Metab. 2015;28:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Pereira BD, Rios ES, Cabrera RA, Portugal J, Raimundo L. Adrenocortical oncocytoma presenting as Cushing's syndrome: an additional report of a paediatric case. Endocr Pathol. 2014;25:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Kolev NY, Ignatov VL, Tonev AY, Zlatarov AK, Encheva EP, Kirilova TN, VM Bojkov VM, Ivanov KD. Adrenal oncocytoma in children—Case report. J IMAB. 2013;19:470-472. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Agarwal S, Agarwal K. Rare pediatric adrenocortical carcinoma with oncocytic change: a cytologic dilemma. Endocr Pathol. 2011;22:40-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Bisceglia M, Ludovico O, Di Mattia A, Ben-Dor D, Sandbank J, Pasquinelli G, Lau SK, Weiss LM. Adrenocortical oncocytic tumors: report of 10 cases and review of the literature. Int J Surg Pathol. 2004;12:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Kakimoto S, Yushita Y, Sanefuji T, Kondo A, Fujishima N, Kishikawa M, Matsumoto K. Non-hormonal adrenocortical adenoma with oncocytoma-like appearances. Hinyokika Kiyo. 1986;32:757-763. [PubMed] |
| 19. | Ertan Y, Argon A, Özdemir M, Yürekli BPS, Dökümcü Z, Makay Ö. Oncocytic Adreno Cortical Tumors: Pathological Features of 16 Cases and Review of the Literature. J Environ Pathol Toxicol Oncol. 2017;36:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Gasparre G, Bonora E, Tallini G, Romeo G. Molecular features of thyroid oncocytic tumors. Mol Cell Endocrinol. 2010;321:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Kanitra JJ, Hardaway JC, Soleimani T, Koehler TJ, McLeod MK, Kavuturu S. Adrenocortical oncocytic neoplasm: A systematic review. Surgery. 2018;164:1351-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Geramizadeh B, Norouzzadeh B, Bolandparvaz S, Sefidbakht S. Functioning adrenocortical oncocytoma: a case report and review of literature. Indian J Pathol Microbiol. 2008;51:237-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Xiao GQ, Pertsemlidis DS, Unger PD. Functioning adrenocortical oncocytoma: a case report and review of the literature. Ann Diagn Pathol. 2005;9:295-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Li F, Wang JJ, Deng HY, Zhang LL, Ding Y, Liu Y, Zhang M, Liu YP. New classification of adrenal endocrine tumors by WHO. Linchuang Yu Shiyanbinglixue Zazhi. 2018;34:709-713. [DOI] [Full Text] |
| 25. | Liao WF, Ma LL. Eosinophiloma of the adrenal cortex: a case report. Oncology. 2012;39:179. [DOI] [Full Text] |
| 26. | Kasajima A, Nakamura Y, Adachi Y, Takahashi Y, Fujishima F, Chiba Y, Uehara S, Watanabe M, Sasano H. Oncocytic adrenocortical neoplasm arising from adrenal rest in the broad ligament of the uterus. Pathol Int. 2014;64:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Li H, Hes O, MacLennan GT, Eastwood DC, Iczkowski KA. Immunohistochemical distinction of metastases of renal cell carcinoma to the adrenal from primary adrenal nodules, including oncocytic tumor. Virchows Arch. 2015;466:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Henry JF, Sebag F, Iacobone M, Mirallie E. Results of laparoscopic adrenalectomy for large and potentially malignant tumors. World J Surg. 2002;26:1043-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Tanaka K, Kumano Y, Kanomata N, Takeda M, Hara I, Fujisawa M, Kawabata G, Kamidono S. Oncocytic adrenocortical carcinoma. Urology. 2004;64:376-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Ahmed MA, Sureshkannan KS, Raouf ZR, Koliyadan SV, Grant CS, Al-Habsi AH, Saparamadu PA, Al-Sajee D. Adrenal oncocytic neoplasm with uncertain malignant potential. Sultan Qaboos Univ Med J. 2013;13:E334-E338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Papathomas TG, Pucci E, Giordano TJ, Lu H, Duregon E, Volante M, Papotti M, Lloyd RV, Tischler AS, van Nederveen FH, Nose V, Erickson L, Mete O, Asa SL, Turchini J, Gill AJ, Matias-Guiu X, Skordilis K, Stephenson TJ, Tissier F, Feelders RA, Smid M, Nigg A, Korpershoek E, van der Spek PJ, Dinjens WN, Stubbs AP, de Krijger RR. An International Ki67 Reproducibility Study in Adrenal Cortical Carcinoma. Am J Surg Pathol. 2016;40:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Erickson LA. Challenges in surgical pathology of adrenocortical tumours. Histopathology. 2018;72:82-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |