Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5668
Peer-review started: March 5, 2021
First decision: April 29, 2021
Revised: May 7, 2021
Accepted: May 15, 2021
Article in press: May 15, 2021
Published online: July 16, 2021
Processing time: 124 Days and 0.2 Hours
Arterial perforation has inevitably increased as endovascular treatments have become more common for intracranial large vessel occlusions, and even distal, medium vessel occlusions. A distal, medium vessel has a tortuous course and thinner wall compared to large arteries, making it more susceptible to damage. Here, we review the treatment strategies for arterial perforation during mechanical thrombectomy, and we report the case of a patient treated with gelfoam embolization.
A 63-year-old woman presented to the emergency department with sudden neurologic symptoms of right hemiparesis and global aphasia. The initial National Institutes of Health Stroke Scale score was 15. Computed tomography (CT) and CT angiography revealed hyperacute infarction and emergent arterial occlusion of the left middle cerebral artery M2-3 portion. During endovascular mechanical thrombectomy, arterial rupture occurred. The patient’s vital signs were stable, but delayed angiography showed persistent active bleeding. Therefore, selective embolization of the injured artery was performed using gelfoam. Subsequent left vertebral and internal carotid angiography was performed to confirm hemostasis. A localized subarachnoid hemorrhage (SAH) was confirmed on a follow-up CT scan. A repeated CT scan after 12 d showed resolution of the SAH, and rebleeding did not occur.
Rescue embolization with gelfoam could be considered an additional option in distal, medium vessel perforation.
Core Tip: Arterial perforation has inevitably increased as endovascular treatments have become more common for intracranial large vessel occlusions, and even for distal, medium vessel occlusions (DMVOs). Perforation tends to occur particularly with DMVOs, increases mortality, and lowers the rate of good functional outcomes. The optimal rescue technique for vessel perforation during mechanical thrombectomy has not been established. This case report reviews the treatment strategy of rescue embolization for arterial perforation and presents the case of a patient treated with gelfoam embolization.
- Citation: Kang JY, Yi KS, Cha SH, Choi CH, Kim Y, Lee J, Cho BS. Gelfoam embolization for distal, medium vessel injury during mechanical thrombectomy in acute stroke: A case report. World J Clin Cases 2021; 9(20): 5668-5674
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5668.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5668
Endovascular treatments for acute ischemic stroke are becoming more common, and the associated technical complications are also inevitably increasing. Arterial perforation is one of the most serious procedural complications, with a reported incidence of 0.6% to 4.9%[1,2]. Perforation tends to occur particularly with distal, medium vessel occlusions (DMVOs), increases mortality, and lowers the rate of good functional outcomes[2]. Arterial perforation may resolve spontaneously without additional intervention due to hemostasis, but in cases of more severe injury or the concomitant use of intravenous tissue plasminogen activator (IV-tPA), extravasation tends to persist. However, the optimal rescue technique for vessel perforation during mechanical thrombectomy has not been established. Techniques that have been reported include permanently leaving a microcatheter in place or embolization with coil or glue. The use of gelfoam embolization for controlling extravasation in cases of arterial perforation has not been reported previously. Here, we review the treatment strategies for arterial perforation during mechanical thrombectomy, and we report a patient case involving gelfoam embolization.
A 63-year-old woman presented to the emergency department with a sudden onset of right hemiparesis and global aphasia beginning 30 min prior to arrival.
Neurologic examination revealed decreased level of consciousness, right hemiparesis, aphasia, and a score of 15 on the National Institutes of Health Stroke Scale (NIHSS).
The patient had a history of atrial fibrillation and was taking aspirin, but her pre-stroke modified Rankin scale score was 0.
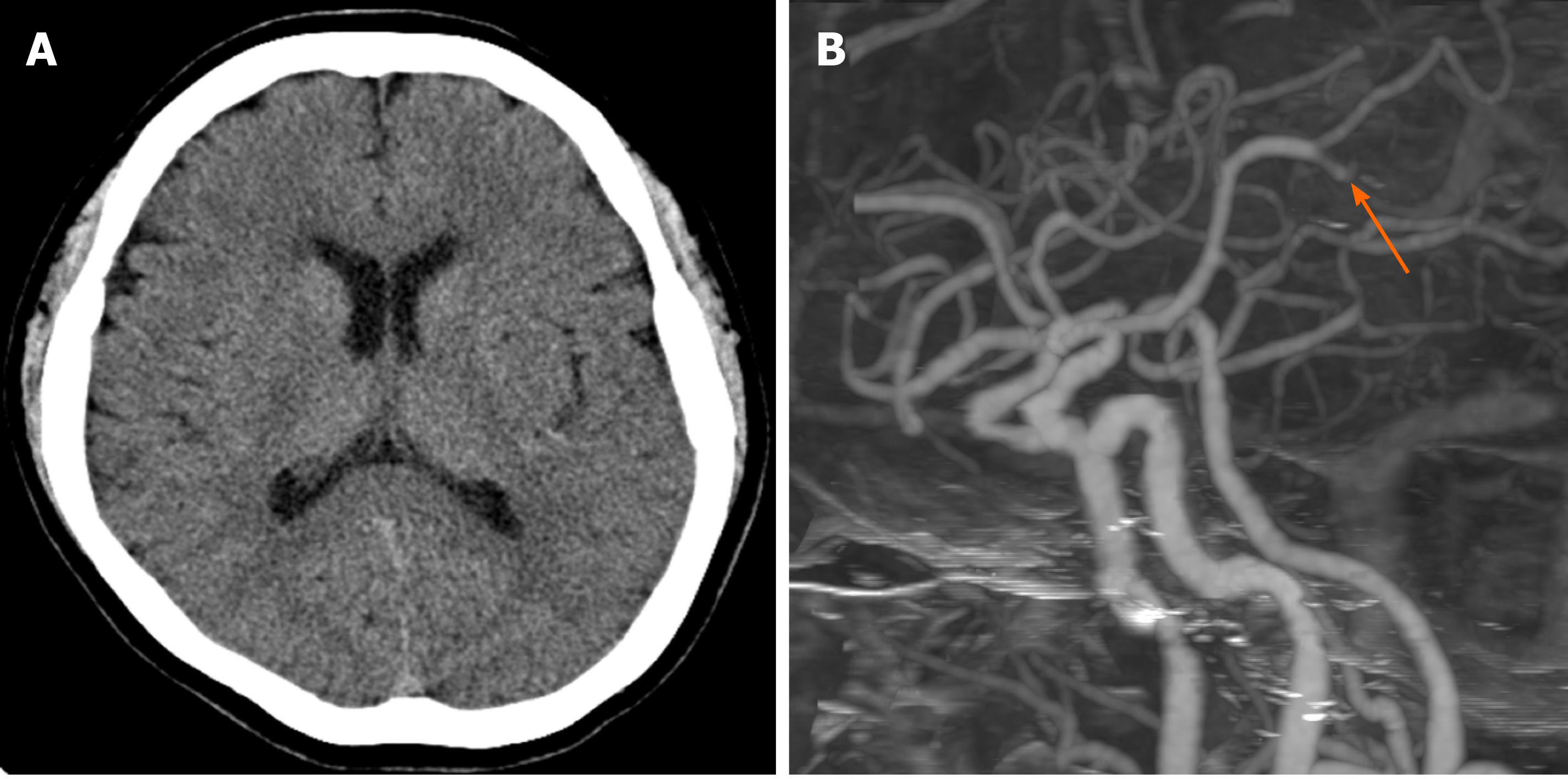
Brain computed tomography (CT) and CT angiography were performed. The Alberta stroke program early CT (ASPECT) score was 8 points, and occlusion of the left middle cerebral artery (MCA) M2-3 segment was demonstrated (Figure 1). Although the occlusion was in a distal branch of the left MCA, presentation within 6 h and NIHSS score of 15 were indications for mechanical thrombectomy according to the guidelines for healthcare professionals from the American Heart Association/American Stroke Association[3].
CT and CT angiography revealed hyperacute infarction and emergent arterial occlusion of the left MCA M2-3 portion.
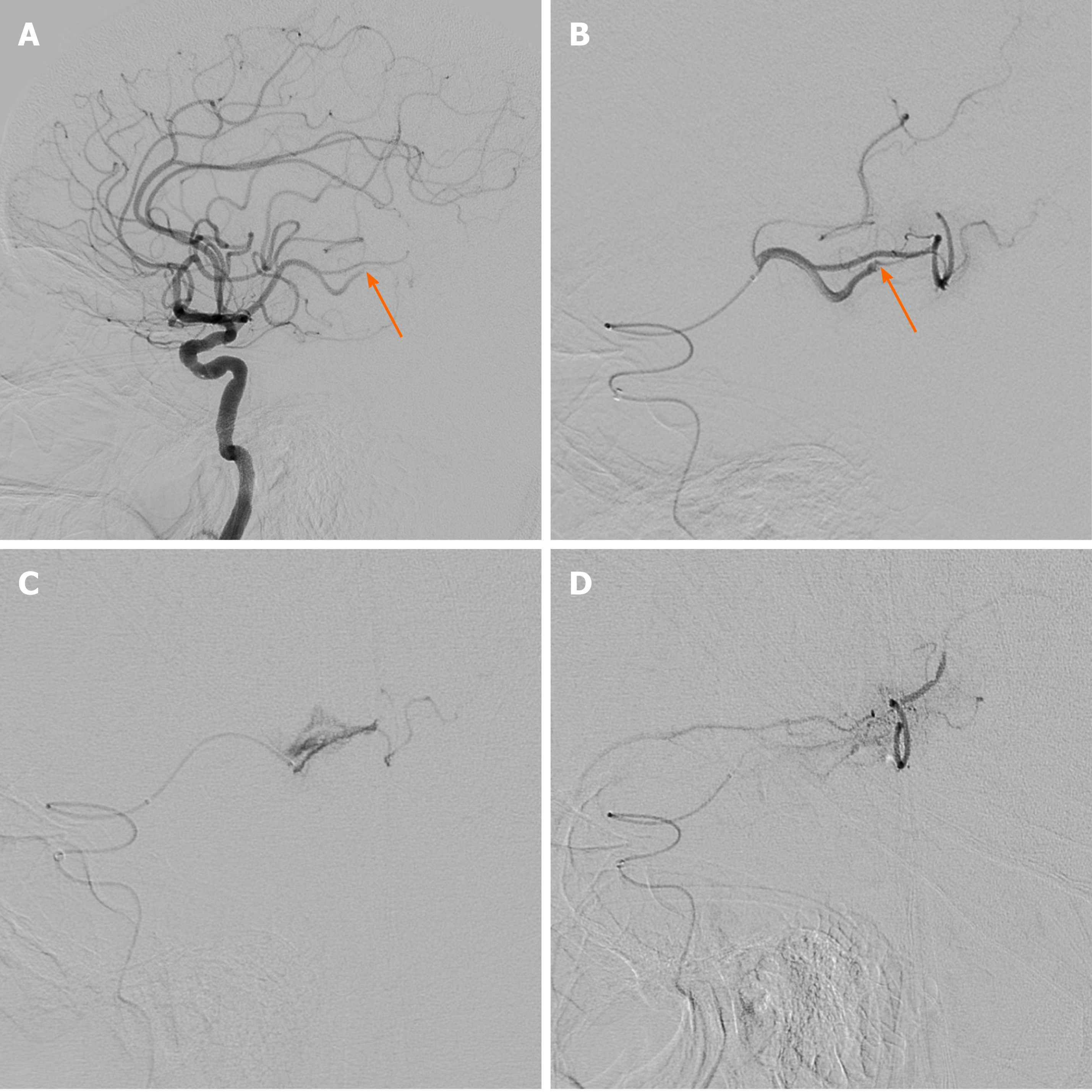
The patient was treated with intravenous alteplase immediately after the CT scan. Subsequent intra-arterial mechanical thrombectomy was planned, and written informed consent to conduct the procedure was obtained from the patient’s family. The procedure was performed with a transfemoral approach under conscious sedation. An 8-French balloon guide catheter (Optimo; Tokai Medical Products Inc., Kasugai, Japan) was advanced into the left internal carotid artery (ICA), then the tip of the guiding catheter was localized in the proximal ICA with a continuous flushing system. Initial cerebral angiography revealed occlusion of the inferior division of the MCA at the M2-3 segment (Figure 2A and B). A Rebar 18 microcatheter (ev3, Covidien, Irvine, CA, United States) with a 0.014-inch microguidewire (Synchro 14, Stryker, Kalamazoo, MI, United States) was advanced in a coaxial fashion through an intermediate catheter (Catalyst 6, Stryker Neurovascular, Mountain View, CA, United States) to reach the occluded MCA branch. Advancement of the microcatheter along the microguidewire was attempted, but some resistance was encountered at the occlusion point, and the microcatheter was not advanced further. After the tension on the microcatheter was released, cautious angiography was performed through the microcatheter. This revealed contrast extravasation at the M3 branch due to vascular injury (Figure 2C). After 5 min with the microcatheter in place, delayed angiography confirmed that the active bleeding persisted. Then, a mixture of 1400-2000 µm gelfoam (EGgel S Plus, Engain, Seongnam, South Korea) with contrast agent and normal saline was injected for selective embolization. A total of 3 mL of mixture was injected, and repeat angiography confirmed hemostasis of the vessel (Figure 2D). Subsequent left vertebral and ICA angiography confirmed hemostasis and leptomeningeal collaterals via the guiding catheter.
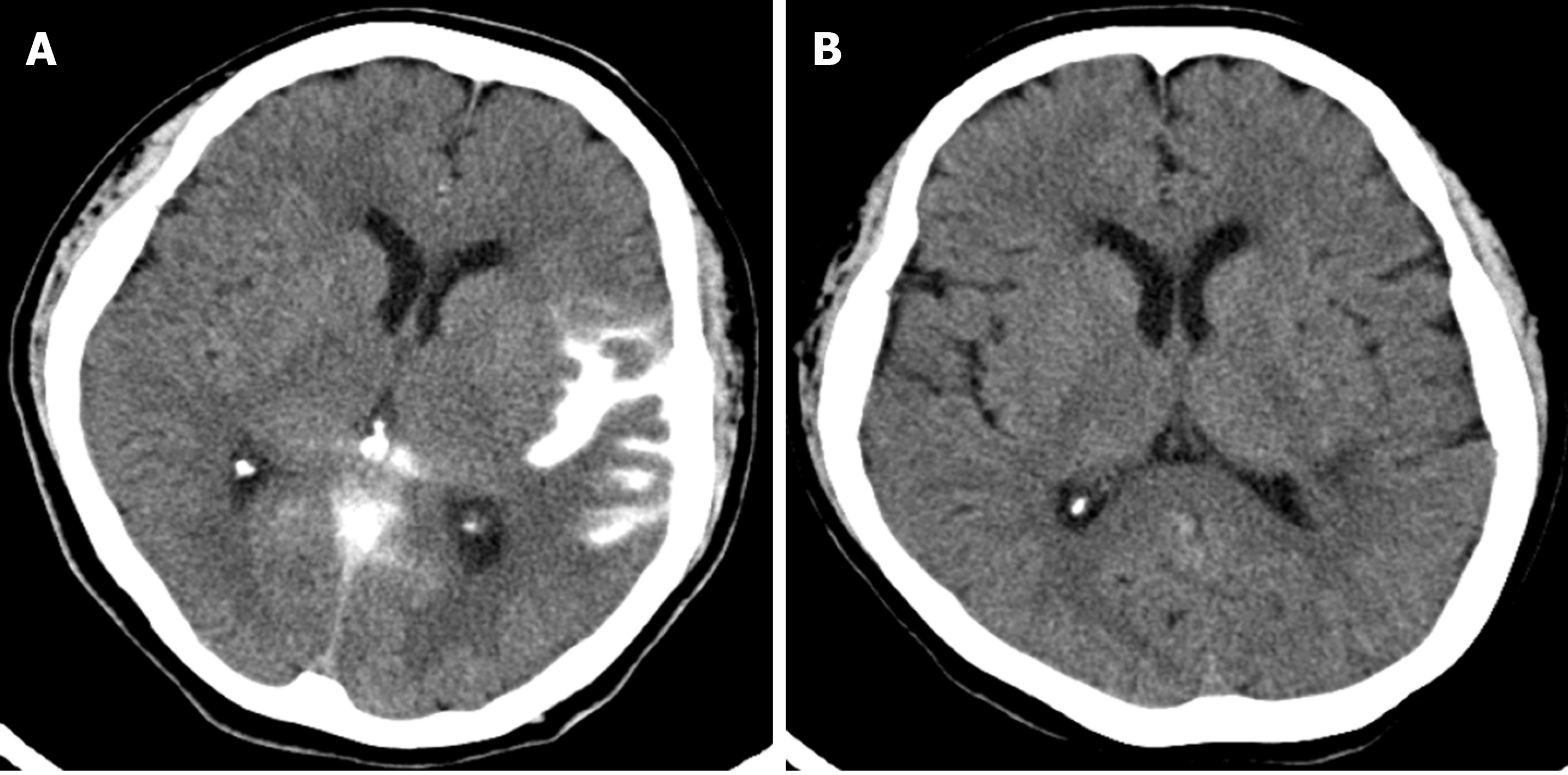
On the day following the procedure, the patient’s NIHSS score decreased to 5, and repeat brain CT imaging revealed a localized subarachnoid hemorrhage (SAH) at the left sylvian cistern and cerebral cortical sulci (Figure 3A). A follow-up brain CT 4 days after the procedure showed some resolution of the SAH, and the infarct had not increased in size compared to the initial preprocedural MRI. Twelve days after the procedure, follow-up brain CT imaging revealed complete resolution of the SAH (Figure 3B). The patient’s recovery progressed well, and she was discharged 2 wk after admission with an NIHSS score of 1.
Since mechanical thrombectomy has been proven superior to intravenous tissue plasminogen activator (IV-tPA) alone for treating acute stroke with emergent large vessel occlusion, endovascular treatment has evolved as the standard of care[3]. Since development of the 3rd generation thrombectomy devices such as Solitaire, Trevo, and Penumbra 5 Max, the success rate of endovascular treatment has also increased.[4] However, arterial injury during the endovascular procedure is still an inevitable and serious complication, and its incidence is reported to be 0.6% to 4.9%[1,2]. Although mechanical thrombectomy for DMVOs still requires a larger prospective controlled study, it may be reasonable for the recovery of important functional independence in selected patients, and it is now emerging as a promising endovascular treatment frontier with advances in catheter technology[3,5,6]. However, in performing mechanical thrombectomy of DMVOs, the smaller vessels tend to have a higher risk of vascular damage than do MCA M1 segments, as they have more tortuous courses and thinner walls compared to large arteries[2,7].
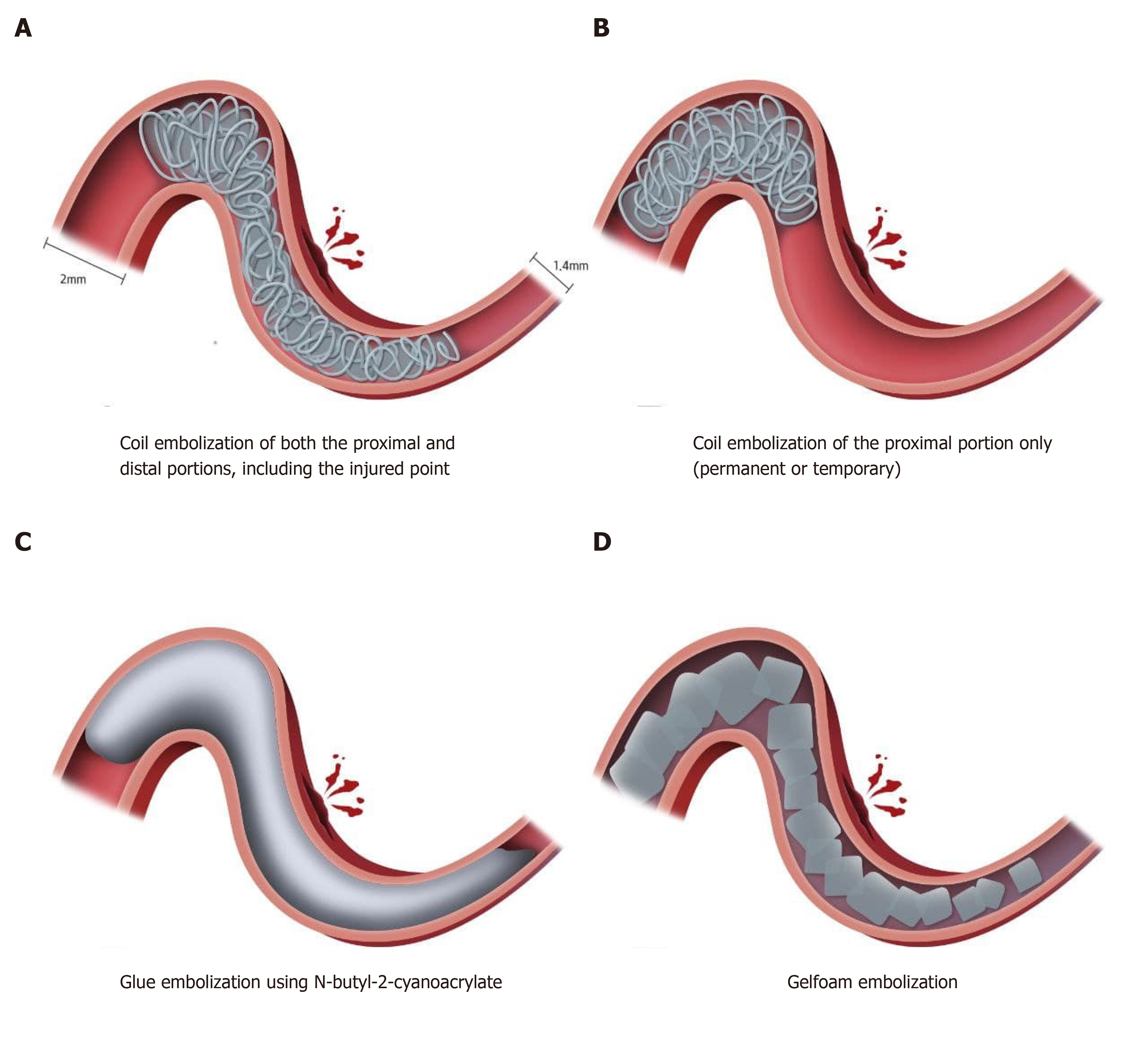
The optimal rescue technique for vessel perforation that occurs during mechanical thrombectomy has not been established[2]. Depending on the grade of injury, vessel perforation may resolve spontaneously without additional intervention due to hemostasis caused by the clot itself. However, in cases of more severe injury or the concomitant use of IV-tPA, extravasation tends to persist. In these instances, permanently leaving the microcatheter in place or embolization with coil or glue can be options for treatment (Figure 4)[8-10]. Among these treatment strategies, rescue embolization using coils can be easily applied permanently or temporarily. It is better to occlude both the proximal and distal portions, including the injured point, to control extravasation through distal collateral flow. In cases with little collateral flow, extravasation can be controlled with only proximal obstruction, but as in this case, it is better to evaluate for continuing extravasation due to collateral flow.
Glue embolization using N-butyl-2-cyanoacrylate (NBCA) can also be used for rescue embolization, but the use of NBCA requires special precautions and significant expertise to handle, especially for the distal, medium vessels. This is because embolization-related complications such as reflux of NBCA may hinder accurate occlusion of the intended site and may affect uninvolved vascular territory, and the microcatheter may become “glued” to the vessel wall[11]. NBCA was not chosen as an embolic agent in this case because of the possibility of triggering a secondary event in an already injured fragile vessel. Rescue embolization was performed using gelfoam particles to prevent persistent extravasation in this case. To the best of our knowledge, gelfoam embolization of arterial rupture during mechanical thrombectomy in patients with acute ischemic stroke has not been reported in the literature to-date. In fact, it is presumed that gelfoam is not widely used in neurointervention, because it is difficult to perform delicate procedures selectively occluding the target point, and rebleeding may occur because it is a temporary occlusive agent lasting about 2 wk[12]. However, the advantage is that occlusion can be performed in the proximal portion, as in glue embolization, for cases in which access to the injured point is difficult due to vascular tortuosity. In addition, it is expected that selective occlusion of the target point can be achieved by using gelfoam of an appropriate size.
To occlude a ruptured vessel, an appropriate size of gelfoam should be used, because larger particles occlude too proximally, and smaller particles occlude too far distally. In general, vessel diameters of the MCA M1 and MCA M2 are 3.1 ± 0.4 mm and 2.4 ± 0.4 mm, respectively[13]. In our case, the perforated arterial diameter was measured as 1.5-1.7 mm. Currently, ready-to-use gelfoam products in sizes ranging from 150-350 μm to 2000-4000 μm are commercially available, and we used gelfoam of 1400-2000 μm.
The advantage and strength of gelfoam embolization is that gelfoam products are mixed in a size range that broadly includes the proximal and distal portions of the injured vessel (Figure 4). Also, it is easy to handle and the incidence of fatal adverse events is lower than with NBCA glue embolization. However, as mentioned earlier, it is necessary to selectively occlude the target point while performing delicate procedures. As gelfoam is an embolic agent that causes temporary occlusion, it is necessary to check for rebleeding through follow-up CT or CT angiography after gelfoam embolization. For this reason, we performed a follow-up CT in our patient after about 2 wk, and confirmed that there was no rebleeding.
Intracranial vessel perforation during mechanical thrombectomy in acute ischemic stroke is a rare complication that can have a fatal outcome if not correctly managed. Rescue embolization with coils or glue is effective to deal with the arterial perforation. However, when the injured point cannot be covered due to vascular tortuosity in distal, medium vessel perforation, gelfoam embolization could be considered an additional option in the arsenal of neurointerventional methods.
Manuscript source: Unsolicited manuscript
Specialty type: Clinical neurology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME S-Editor: Gong ZM L-Editor: A P-Editor: Xing YX
| 1. | Balami JS, White PM, McMeekin PJ, Ford GA, Buchan AM. Complications of endovascular treatment for acute ischemic stroke: Prevention and management. Int J Stroke. 2018;13:348-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 2. | Mokin M, Fargen KM, Primiani CT, Ren Z, Dumont TM, Brasiliense LBC, Dabus G, Linfante I, Kan P, Srinivasan VM, Binning MJ, Gupta R, Turk AS, Elijovich L, Arthur A, Shallwani H, Levy EI, Siddiqui AH. Vessel perforation during stent retriever thrombectomy for acute ischemic stroke: technical details and clinical outcomes. J Neurointerv Surg. 2017;9:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344-e418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 4089] [Article Influence: 681.5] [Reference Citation Analysis (0)] |
| 4. | Yoo AJ, Andersson T. Thrombectomy in Acute Ischemic Stroke: Challenges to Procedural Success. J Stroke. 2017;19:121-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 5. | Saver JL, Chapot R, Agid R, Hassan A, Jadhav AP, Liebeskind DS, Lobotesis K, Meila D, Meyer L, Raphaeli G, Gupta R; Distal Thrombectomy Summit Group*†. Thrombectomy for Distal, Medium Vessel Occlusions: A Consensus Statement on Present Knowledge and Promising Directions. Stroke. 2020;51:2872-2884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 6. | Grossberg JA, Rebello LC, Haussen DC, Bouslama M, Bowen M, Barreira CM, Belagaje SR, Frankel MR, Nogueira RG. Beyond Large Vessel Occlusion Strokes: Distal Occlusion Thrombectomy. Stroke. 2018;49:1662-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 7. | Saber H, Narayanan S, Palla M, Saver JL, Nogueira RG, Yoo AJ, Sheth SA. Mechanical thrombectomy for acute ischemic stroke with occlusion of the M2 segment of the middle cerebral artery: a meta-analysis. J Neurointerv Surg. 2018;10:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Akpinar SH, Yilmaz G. Periprocedural complications in endovascular stroke treatment. Br J Radiol. 2016;89:20150267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Nguyen TN, Lanthier S, Roy D. Iatrogenic arterial perforation during acute stroke interventions. AJNR Am J Neuroradiol. 2008;29:974-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Xu H, Guan S, Liu C, Wang L, Yan B, Han H, Quan T. Rescue Glue Embolization of Vessel Perforation During Mechanical Thrombectomy for Acute Ischemic Stroke: Technical Note. World Neurosurg. 2019;121:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Niimi Y, Berenstein A, Setton A. Complications and Their Management During NBCA Embolization of Craniospinal Lesions. Interv Neuroradiol. 2003;9:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Abada HT, Golzarian J. Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol. 2007;10:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Rai AT, Hogg JP, Cline B, Hobbs G. Cerebrovascular geometry in the anterior circulation: an analysis of diameter, length and the vessel taper. J Neurointerv Surg. 2013;5:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |