Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5556
Peer-review started: December 8, 2020
First decision: March 27, 2021
Revised: April 10, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: July 16, 2021
Processing time: 211 Days and 6.6 Hours
With an increased number of surgical procedures involving the mitral annular region, the risk of mitral valve prolapse (MVP) has also increased. Previous studies have reported that worsening of MVP occurred early after radiofrequency catheter ablation (RFCA) at papillary muscles in ventricular tachycardia (VT) patients with preoperative MVP.
We report a case where MVP and papillary muscle rupture occurred 2 wk after RFCA in a papillary muscle originated VT patient without mitral valve regurgitation or prolapse before. The patient then underwent mitral valve replacement with no premature ventricular contraction or VT. During the surgery, a papillary muscle rupture was identified. Pathological examination showed necrosis of the papillary muscle. The patient recovered after mitral valve replacement.
Too many ablation procedures and energy should be avoided.
Core Tip: The risk of early mitral valve prolapse (MVP) increases after radiofrequency catheter ablation (RFCA). However, the delayed MVP after RFCA was not reported before. We report the first case of delayed MVP after RFCA and analyze the reason.
- Citation: Sun ZW, Wu BF, Ying X, Zhang BQ, Yao L, Zheng LR. Delayed papillary muscle rupture after radiofrequency catheter ablation: A case report. World J Clin Cases 2021; 9(20): 5556-5561
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5556.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5556
Previous studies have reported cases where mitral valve prolapse (MVP) occurred early after cardiac interventional surgery[1,2] due to mechanical injury and radio frequency (RF) energy. In these cases, patients had mild-to-moderate mitral valve regurgitation before and the MVP occurred early after the operation and was a result of ruptured chordae tendineae. However, there are no reports investigating the delayed rupture of papillary muscles. Here, we report a rare case of delayed rupture of papillary muscles occurring 2 wk after radiofrequency catheter ablation (RFCA) of ventricular tachycardia that originated from papillary muscles. Pathological examination revealed necrosis of the papillary muscle. The patient underwent mitral valve replacement and fully recovered.
A 58-year-old man who received aortic valve replacement 4 years ago due to aortic regurgitation was referred to our hospital in March 2020 since he experienced syncope 1 d before.
The patient experienced syncope 1 d before when he was watching TV on bed. And the consciousness recovered after 5 min and the patient visited our emergency center. In our emergency center, the patient received amiodarone at a dose of 200 mg three times per day until hospitalization.
The patient received aortic valve replacement 4 years ago due to aortic regurgitation. The patient had no other illness in the past, such as arterial hypertension, diabetes, dyslipidemia, chronic heart failure, and renal dysfunction.
The patient had a long history of heavy smoking.
The physical examination revealed no significant abnormalities.
The laboratory examinations revealed no significant abnormalities. The detailed results of the laboratory examinations can be seen in Table 1.
| Parameter | Result |
| White blood cell count | 8.2 × 109 |
| Troponin I | 0.059 ng/mL |
| Nt-Pro Bnp | 32 pg/mL |
| Low density lipoprotein cholesterol | 2.82 mmoml/L |
| Fasting blood glucose | 5.34 mmol/L |
| C-reactive protein | 0.5 mg/L |
| International normalized ratio | 0.94 |
| Potassium | 3.77 mmol/L |
| Magnesium | 0.82 mmol/L |
| Creatinine | 83 μmol/L |
| eGFR | 88.51 mL/min |
| T4 | 62.88 nmol/L |
| T3 | 1.86 nmol/L |
| TSH | 0.68 mIU/L |
The Holter monitor showed paired ventricular premature contraction (Supplementary Figure 1A) and paroxysmal ventricular tachycardia (Supplementary Figure 1B). A computed tomography scan of the head showed no signs of cerebral hemorrhaging. Echocardiography revealed no mitral stenosis or regurgitation present at that time (Supplementary Figure 1C). The LVEF was 65% and the function of the aortic bioprosthetic valve was normal.
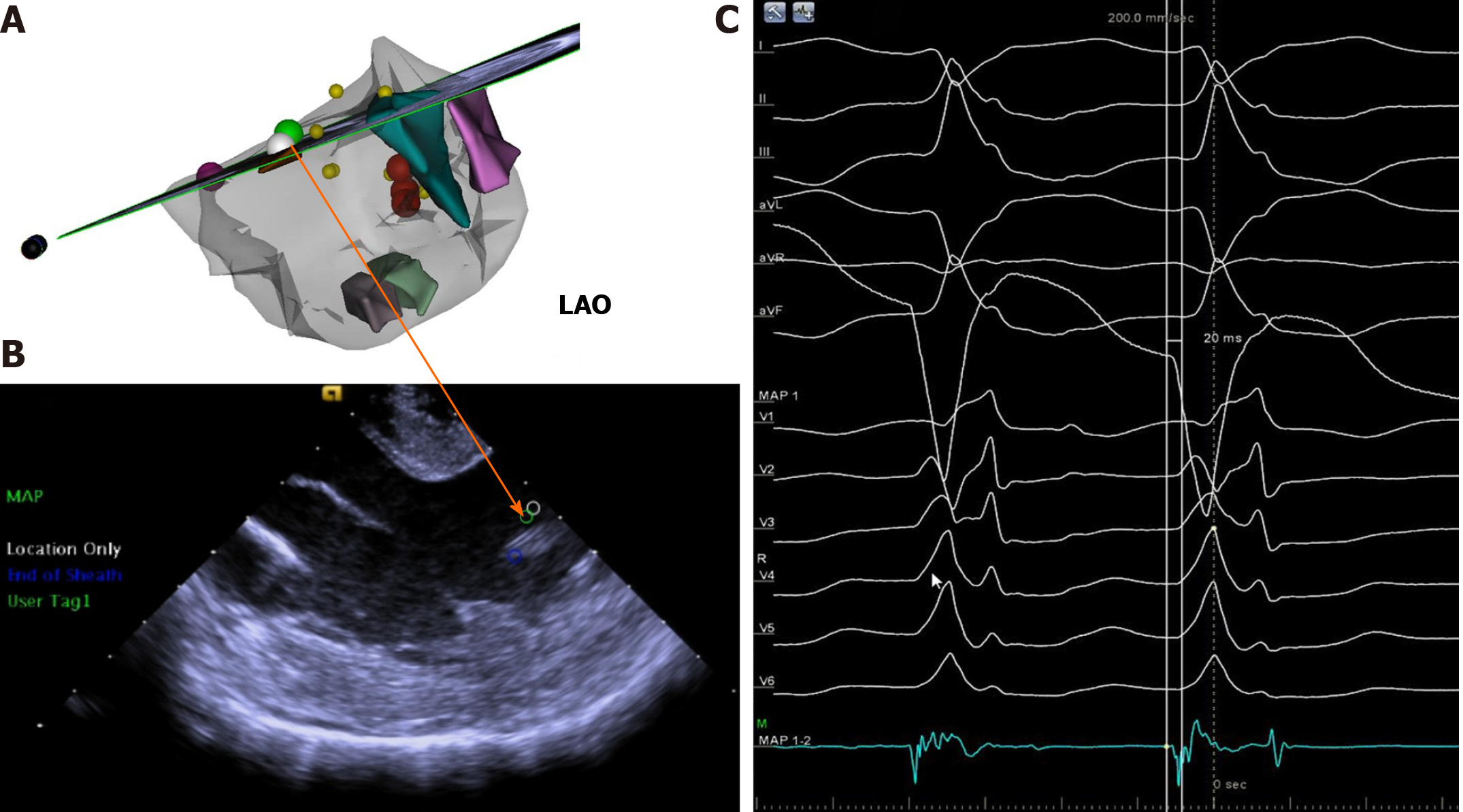
The electrophysiology study and RFCA were performed using a 3-dimensional mapping system (CARTO UNIV; Biosense Webster, Diamond Bar, CA). The three-dimensional geometries of the left ventricle (LV) and the papillary muscles were reconstructed by intracardiac echocardiography (ICE) (SOUNDSTAR; Biosense Webster). An ablation catheter (NAVISTAR Thermocool Smart Touch, Biosense Webster) was introduced into the aortic valve, where no potential was recorded on it. The transseptal approach was used to place the ablation catheter into the LV since it was difficult to do so by the transaortic approach. Detailed activation and pace mapping were performed in the anterolateral papillary muscle (ALPM) area of the LV. A 0.05-Hz high-pass filter and a 500-Hz low-pass filter were used with virtual unipolar electrograms while a 30-Hz high-pass filter and a 500-Hz low-pass filter were used with virtual bipolar electrograms. The local fractional potential at the base of ALPM preceding the onset of surface QRS at 10 ms was recorded with a “QS” pattern in the unipolar mode. Pace morphologies were not satisfactory at the septal sides of the APLM (Supplementary Figure 2). The most optimal pace score of these three sites with pace mapping software (PASO) was 0.891 (Supplementary Figure 2A, pace 1). Tentative RFCA at these sites was performed using 40 W with an irrigation flow rate of 30 mL/min. When acceleration or reduction of ventricular tachycardia (VT) was observed at the beginning of the application, RFCA current delivery was continued for an additional 180 s. The VT turned into bigeminy of premature ventricular contra
The extensive pace and activation mapping were performed as a next step. The perfect pace-map site with a 0.995 PASO score was identified at another top of ALPM (Supplementary Figure 4). The ablation catheter on this site also recorded a complex fractional prepotential preceding the onset of surface QRS complex at 20 ms. And a QS pattern was recorded by a local unipolar lead during the PVCs at this site (Figure 1). Therefore, ablation on this site of the papillary muscle was performed under electroanatomic map guidance combined with real-time ICE. The RFCA current was delivered in power control mode, starting at 40 W and gradually raised to 45 W with an irrigation flow rate of 30 mL/min. The VT rate slowed down and was successfully eliminated during ablation at this site (Supplementary Figure 5). Three times of additional RFCA was performed for consolidation with a total RF time of 6 min.
After RFCA, the patient was monitored for 20 min. During the monitoring period, no ventricular arrhythmias were induced even after programmed electrical stimulation with isoproterenol infusion. No complications such as steam pop, pericardial effusion, or mitral valve injury were observed (Video 1). We reanalyzed using a Holter monitor and found no presence of ventricular tachycardia after the operation. The patient was then discharged from the hospital 1 wk after the operation with no symptoms.
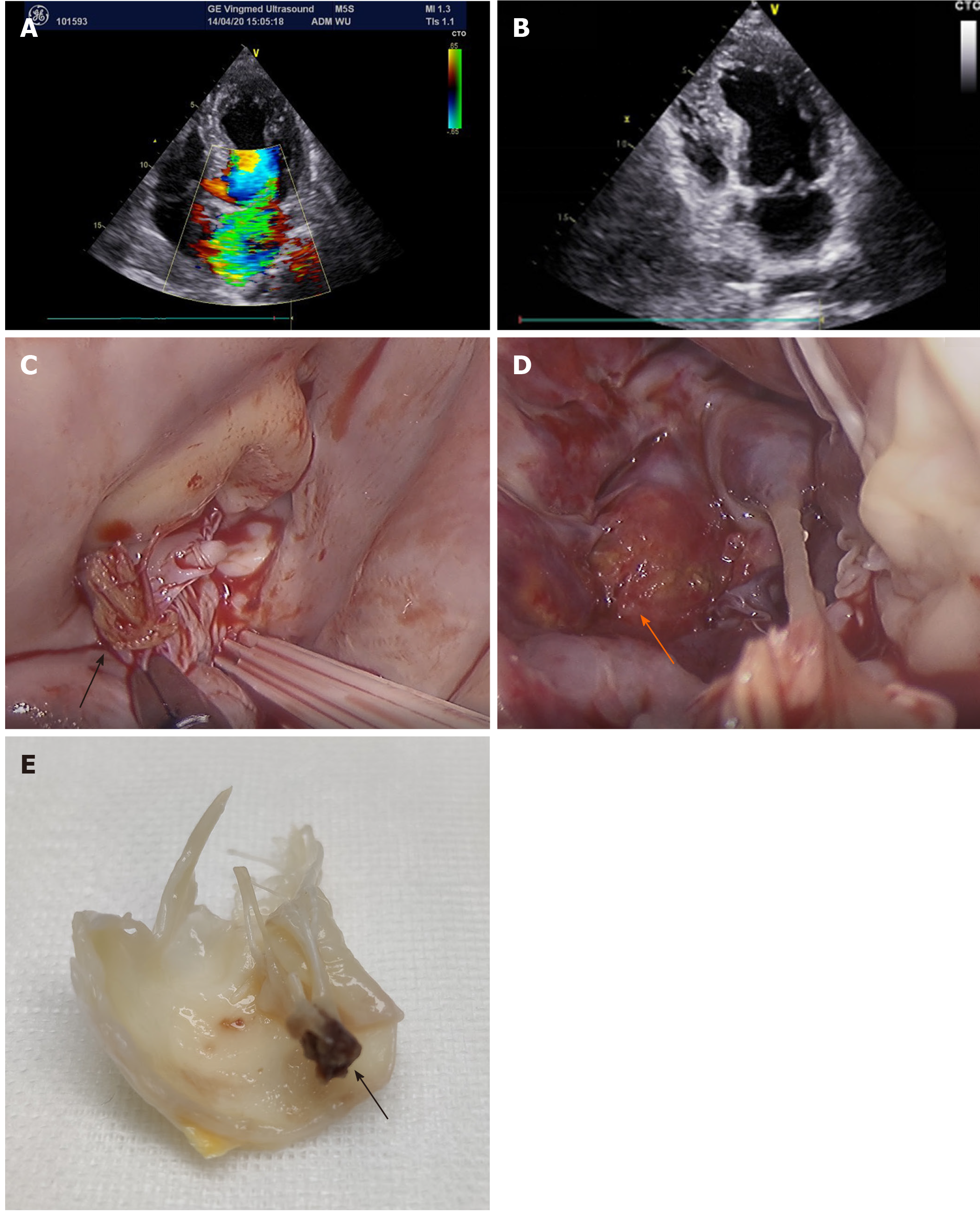
Approximately 1 wk later (2 wk after RFCA), the patient experienced dyspnea and revisited the hospital. Echocardiography showed severe mitral valve prolapse (Figure 2A and B).
The final diagnosis of the presented case was delayed MVP after RFCA.
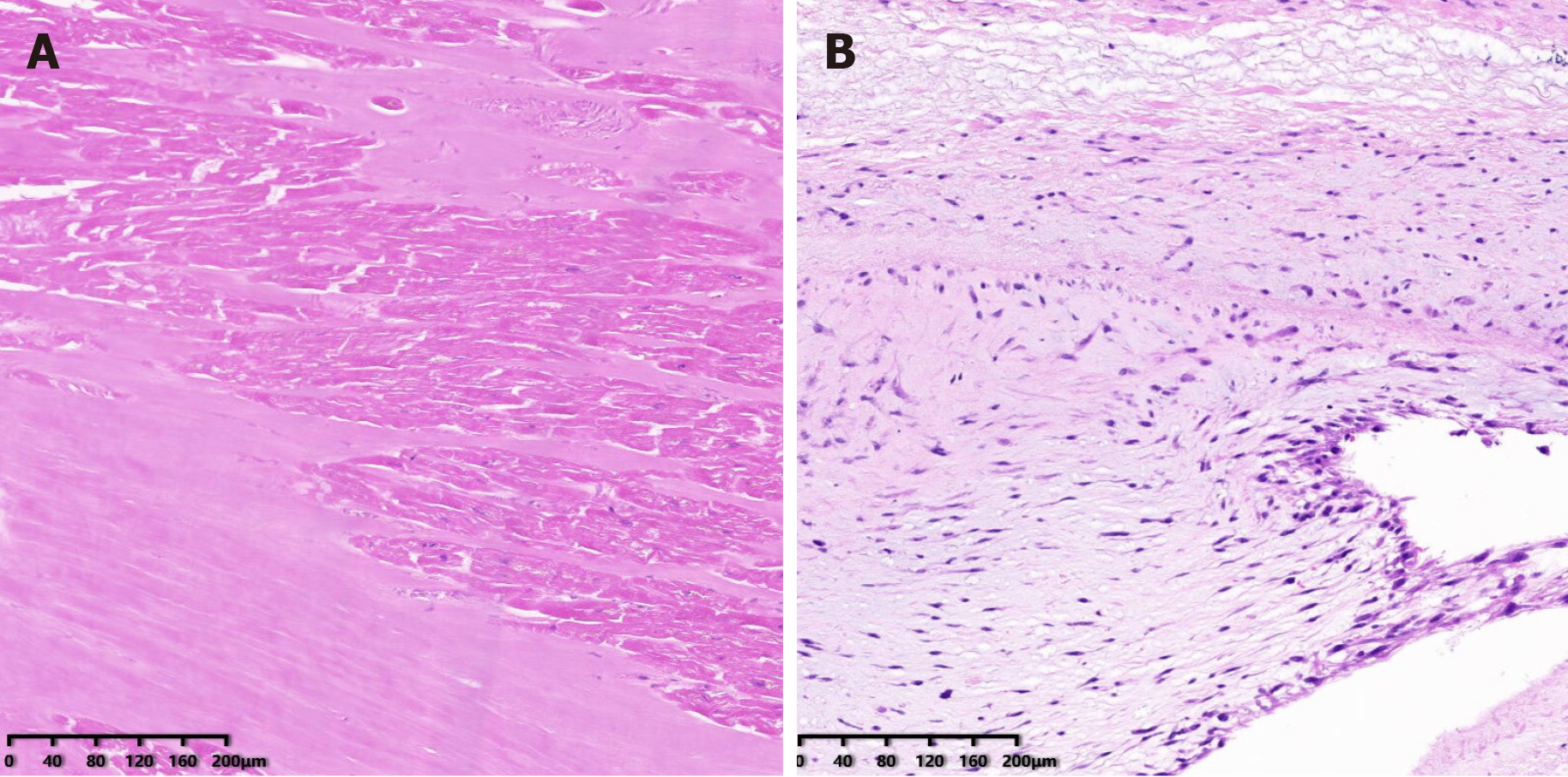
The patient received mitral valve replacement. During the operation, the papillary muscle was found to be ruptured and was entering into the left atrium (Figure 2C), with another end in the left ventricle (Figure 2D). The mitral valve was removed (Figure 2E) and replaced with a bioprosthetic valve. Echocardiography was performed after the operation to find the normal function of the bioprosthetic mitral valve. Pathological examination revealed that the papillary muscle underwent necrosis and karyolysis (Figure 3A). There was no necrosis present in the chordae tendineae (Figure 3B). After the operation, the symptom of dyspnea resolved.
The patient was followed at 1, 3, and 6 mo after the surgery. And the function of the bioprostheticmitral valve was normal and there was no symptom of dyspnea after the surgery.
In this paper, we report a case where delayed rupture of a papillary muscle occurred after RFCA. The patient showed no mitral valve regurgitation before RFCA and the rupture of papillary muscle occurred 2 wk after RFCA of ventricular tachycardia originating from anterolateral papillary muscle (ALPM). Pathological examination showed necrosis of the papillary muscles. To our knowledge, this is the first case report on delayed rupture of papillary muscles after RFCA.
Several mitral valve prolapse cases occurring after catheter operations have been reported[1,2]. However, our case differed from previously reported cases. For instance, four patients with atrial fibrillation reported by Desimone et al[2] developed MVP due to mechanical injury caused by a circular mapping catheter. Two patients with accessory pathways developed MVP due to mechanical injury or ablation of the mitral valve annulus. Desimone et al[2] also reported three VT cases where two cases developed MVP due to ablation at mitral valve annulus or mitral isthmus. Only one case was reported with ablation at papillary muscles; however, this patient showed MVP before RFCA and the prolapse became worse immediately after the operation. The reason for the worsening of the MVP in this case was not clear, since the pathological examination was not performed. Mochizuki et al[1] also reported a VT case with MVP before ablation at papillary muscles. In this case, the worsening of the MVP was because of the attenuated contraction of the posterior papillary muscles and its discoordination with the anterior papillary muscles. The papillary muscles and chordae tendineae were not ruptured. Since the patient in our case had normal MV before RFCA and prolapse occurred 2 wk after the operation and the reason for MVP was necrosis of the papillary muscles, our case differed from cases previously reported. Thus, this is the first reported cases showing delayed MVP after ablation at papillary muscles.
We believe that MVP was caused by repeated ablations in ALPM at too high of energy, in the light of the facts that MVP occurred 2 wk after the operation and necrosis of the papillary muscles appeared in pathological analysis. There was no mechanical injury since no pulling of the MV apparatus occurred during RFCA.
There are several proposed methods to prevent MVP after RFCA. First, the exact target site should be found and there should be a limited number of ablations with high energy during RFCA. Second, an intracardiac echocardiography and contact force should be implemented to guide the ablation.
In conclusion, we have reported a case for the first time in which delayed MVP occurred after RFCA at papillary muscles. The reason for this complication was necrosis of the papillary muscles caused by the RF energy. Thus, too many times and too high energy should be avoided in the ablation in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheungpasitporn W, Lakusic N S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Mochizuki A, Nagahara D, Takahashi H, Saito R, Fujito T, Miura T. Worsening of mitral valve regurgitation after radiofrequency catheter ablation of ventricular arrhythmia originating from a left ventricular papillary muscle. HeartRhythm Case Rep. 2017;3:215-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Desimone CV, Hu T, Ebrille E, Syed FF, Vaidya VR, Cha YM, Valverde AM, Friedman PA, Suri RM, Asirvatham SJ. Catheter ablation related mitral valve injury: the importance of early recognition and rescue mitral valve repair. J Cardiovasc Electrophysiol. 2014;25:971-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |