Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5547
Peer-review started: November 26, 2020
First decision: May 11, 2021
Revised: May 22, 2021
Accepted: May 25, 2021
Article in press: May 25, 2021
Published online: July 16, 2021
Processing time: 222 Days and 23 Hours
The Hartmann procedure is currently recognized as a common, safe, and feasible surgical procedure. However, its reversal rate is low, and the optimal timing for Hartmann reversal surgery is controversial.
A 65-year-old man came to our hospital with a complaint of an intestinal fistula next to the stoma. The patient had undergone a Hartmann procedure 13 years prior. We performed colonoscopy, computed tomography, and other diagnostics before successfully reversing the stoma.
Although the optimal time for Hartmann procedure reversal is controversial, time may ultimately not be a factor in the success of reversal.
Core Tip: First, for acute intestinal obstruction in the left colon or rectum, Hartmann’s surgery is a safe and feasible treatment. Second, there is controversy about the time to reversal after Hartmann’s procedure. This case is the longest successful reversal in the world.
- Citation: Huang W, Chen ZZ, Wei ZQ. Successful reversal of ostomy 13 years after Hartmann procedure in a patient with colon cancer: A case report. World J Clin Cases 2021; 9(20): 5547-5555
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5547.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5547
Currently, colorectal cancer is one of the most common malignant tumors, having the third highest incidence among all malignancies and being the second leading cause of cancer-related mortality. Many colorectal cases are diagnosed as acute onset due to intestinal obstruction and/or perforation. The majority of the reported figures are approximately 30% for such presentation[1]. However, colorectal obstruction accounts for approximately 80% of emergency cases related to colorectal cancer[1]. The most common site of colorectal cancer obstruction is the sigmoid colon, and 75% of such tumors are located in the distal splenic flexure[2].
When colon cancer is complicated by intestinal obstruction, emergency surgery is often required, and Hartmann's procedure[3] (proximal fistula, distal closure) is usually preferred. Currently, the Hartmann procedure is recognized as a common, safe, and feasible surgical method[4]. However, after Hartmann, the long-term stoma state leads to a lower quality of life for the patient, and there are also complications such as parastomal hernia[5], stoma prolapse[6], stoma stenosis[6], and stoma infection[6]. However, due to the locally advanced nature of most colorectal tumors, the possibility of tumor recurrence and metastasis after surgery is relatively high. Therefore, it is necessary to fully evaluate the characteristics of the tumor before considering the reversal surgery. Additionally, Hartmann procedure reversal is extremely difficult due to severe abdominal adhesions, difficulty in finding stumps, and changes in distal disuse, which have caused the time to perform reversal to be delayed. Moreover, the patient's bowel function may be affected after the reversal procedure, so often no such surgery is considered. Currently, the time to reversal after Hartmann's operation is the focus of controversy.
In this case study, we report a patient with a successful fistula reversal 13 years after undergoing Hartmann’s procedure.
A 65-year-old man was admitted to our hospital for “discovering a nodule adjacent to the stoma in April that had enlarged for 1 wk, and then ruptured 1 d prior”.
Four months ago, the patient accidentally found a small nodule on the right side of the colostomy that was approximately 0.5 cm in size without redness, swelling, or tenderness, and did not go to the doctor. Two months ago, the nodule enlarged, accompanied by redness, swelling, heat, and pain. After oral administration of cefdinir, he improved and did not take antibiotics regularly. One week ago, the patient had an enlarged nodule on the right side of the stoma, accompanied by a mass on the left side of the stoma that was approximately 4 cm × 3 cm in size, with redness, swelling, heat, pain, and a black mass of pus in the middle; intravenous infusion of cefoxitin did not alleviate symptoms. Three days ago, the nodule on the right side of the stoma ruptured, and a fistula of approximately 1.5 cm in size appeared, which caused a large amount of overflowing stool. A day ago, the mass on the left side of the fistula ruptured and released pus, so he went to our hospital.
Thirteen years ago, the patient underwent Hartmann surgery due to a sigmoid colon tumor with acute obstruction. Postoperative pathological diagnosis was stage II sigmoid colon adenocarcinoma (T4N0M0). Due to the patient's own reasons, he did not undergo regular treatment and not reverse the stoma in time. The patient was admitted to our hospital for paraostomy hernia and right inguinal hernia 3 years ago, and underwent tension-free inguinal repair and paraostomy hernia repair via an open operation. The abscess was cut for drainage 1 year ago due to a paraostomy abscess.
There was a 10-cm surgical scar (from the Hartmann surgical incision) on the right abdomen, a 15-cm surgical incision scar (paraostomy hernia repair incision) in the middle abdomen, and a 5-cm surgical scar on the right inguinal area. The colostomy port was located in the left lower abdomen. On the right side of the colostomy port, there was an abscess of approximately 5 cm × 5 cm in size. The skin was red, swollen, and hot. A middle ulcer was also found. The place where the mass overflowed with pus was located at the anterior upper pole of the iliac and was approximately 1.5 cm × 1.5 cm, at a depth of approximately about 2 cm, and there was pus outflow. On the left side of the stoma, there was a fistula of approximately 1.5 cm × 1.5 cm in size, located next to the belly button that had much stool overflow. A mass of approximately 5 cm × 3 cm was palpable in the left groin area, which was obvious when standing, soft in quality, and did not protrude into the scrotum.
Routine blood markers and tumor markers were normal, but Calcitonin original was 0.53.
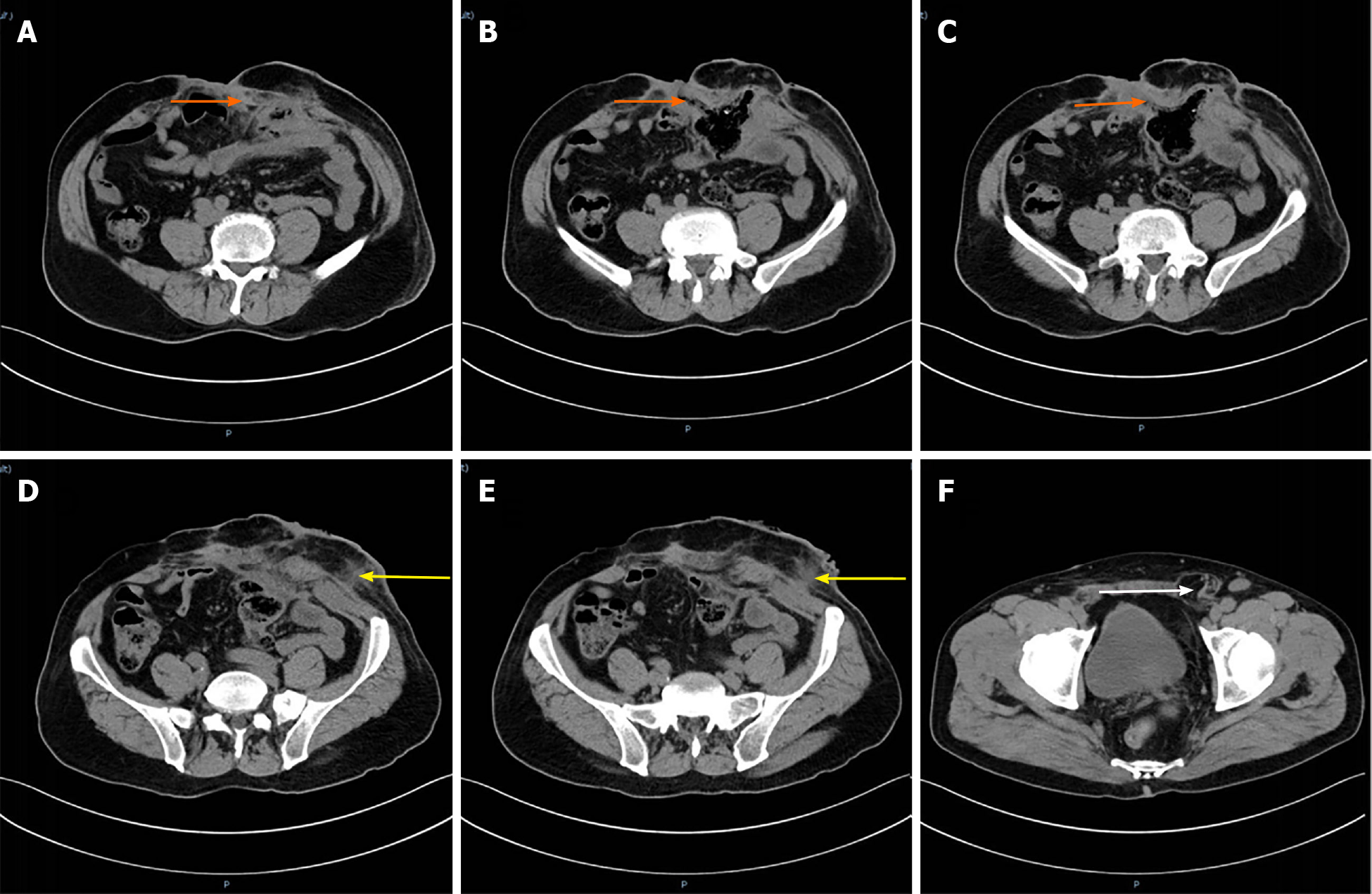
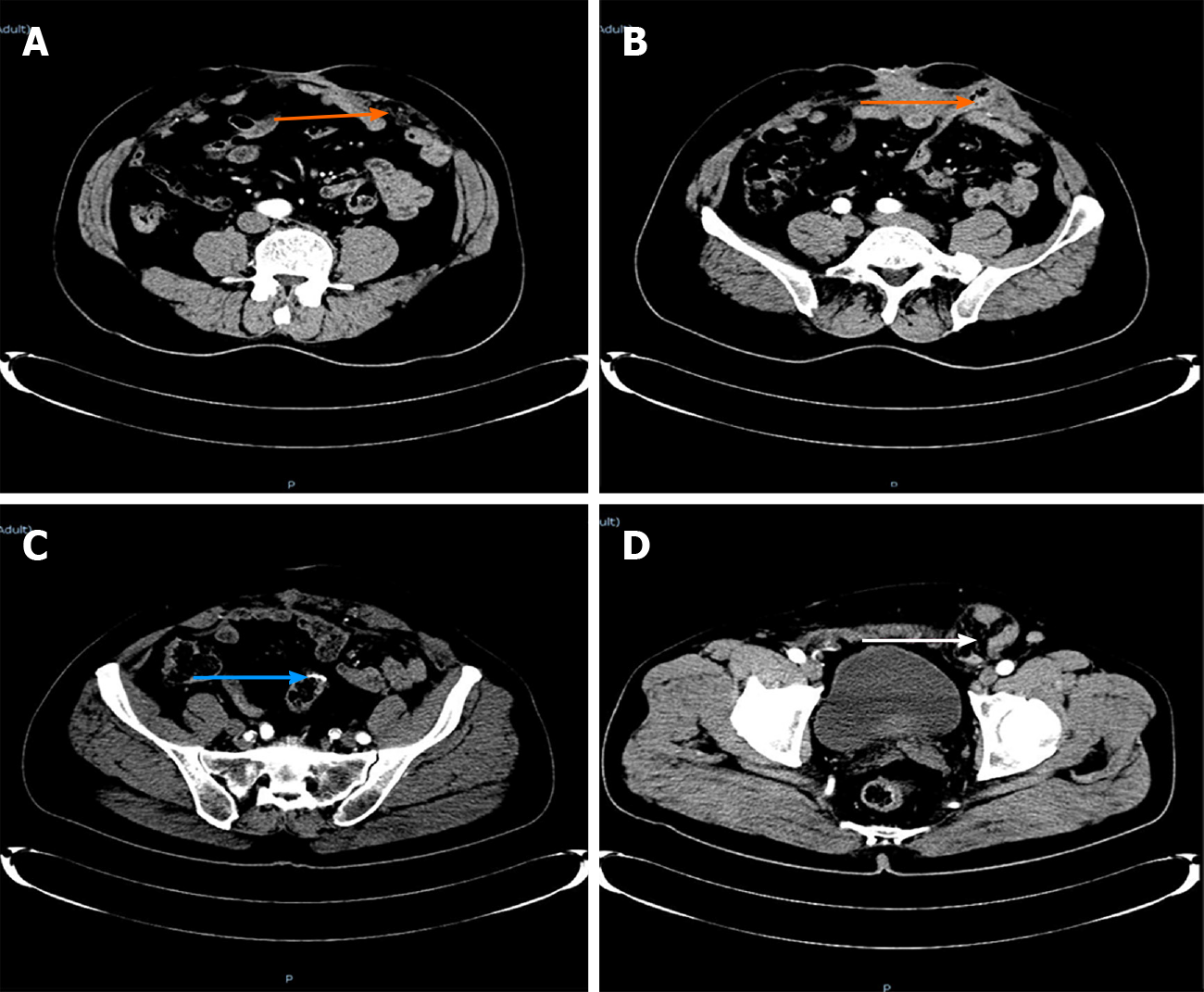
Enhanced computed tomography: The right sinus of the stoma was formed, which was the same as the intestinal cavity (orange arrow), and the parastomal hernia can be seen (Figure 1A-C). The left side of the stoma had a suspicious sinus formation (yellow arrow), with surrounding soft tissue swelling (Figure 1D and E) and left inguinal hernia (white arrow). No tumor recurrence was seen (Figure 1F).
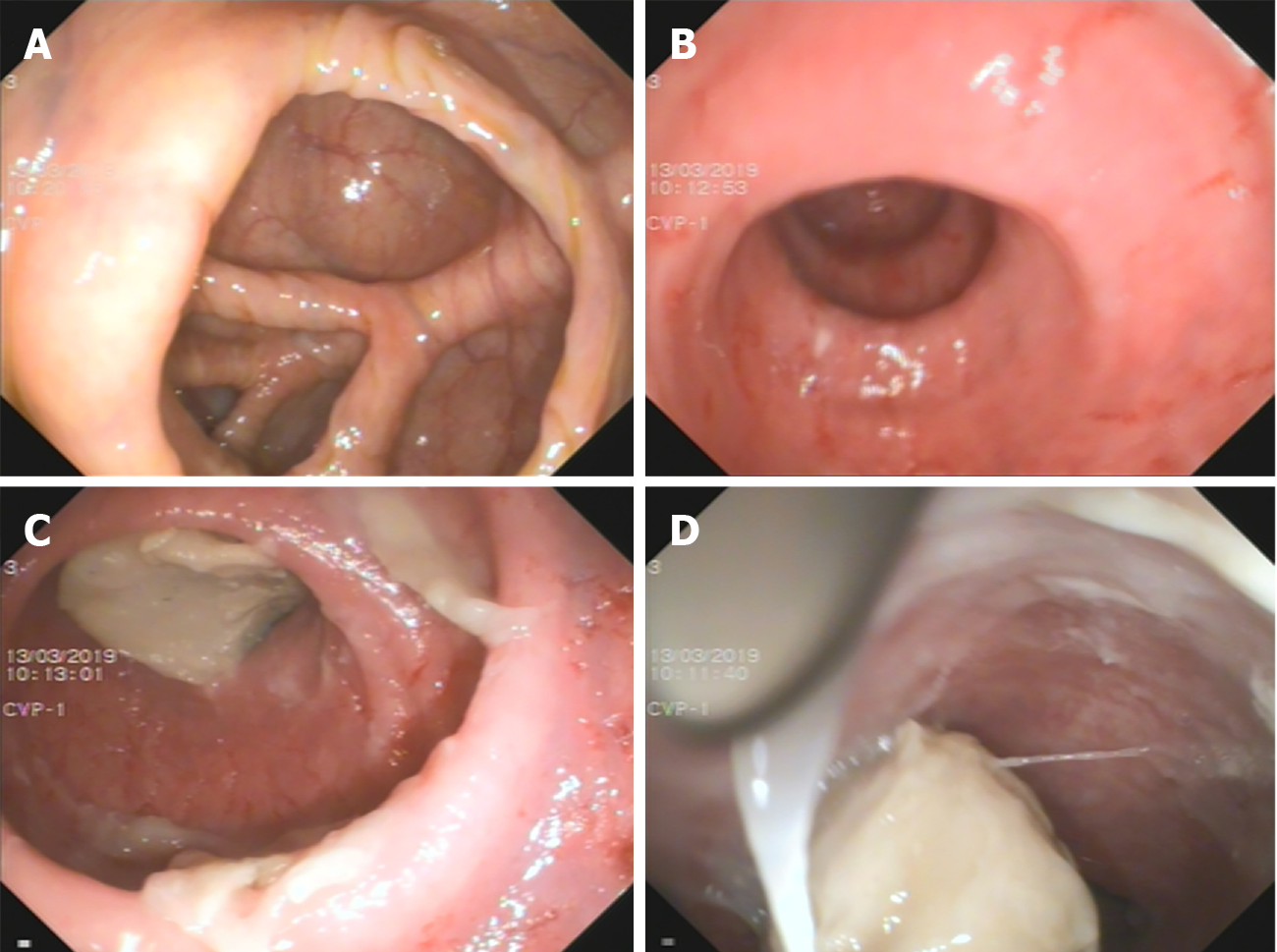
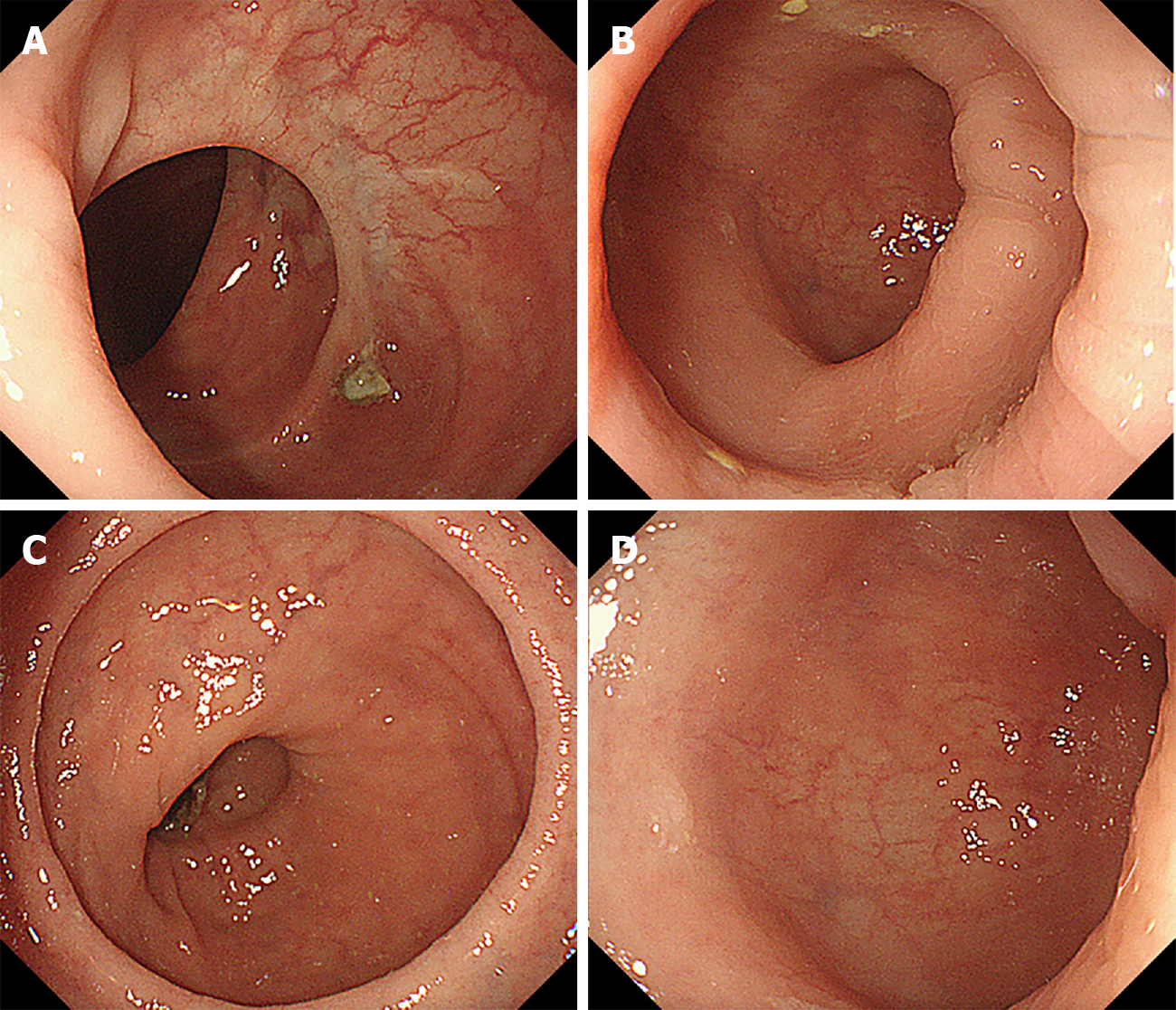
Colonoscopy: The proximal bowel (Figure 2A and B) was not significantly abnormal; the distal bowel (Figure 2C and D) was approximately 25 cm, and showed changes from disuse, with a large number of white mucus masses (The white mucus masses were formed by the absorption of water by intestinal fluid secreted by the colon).
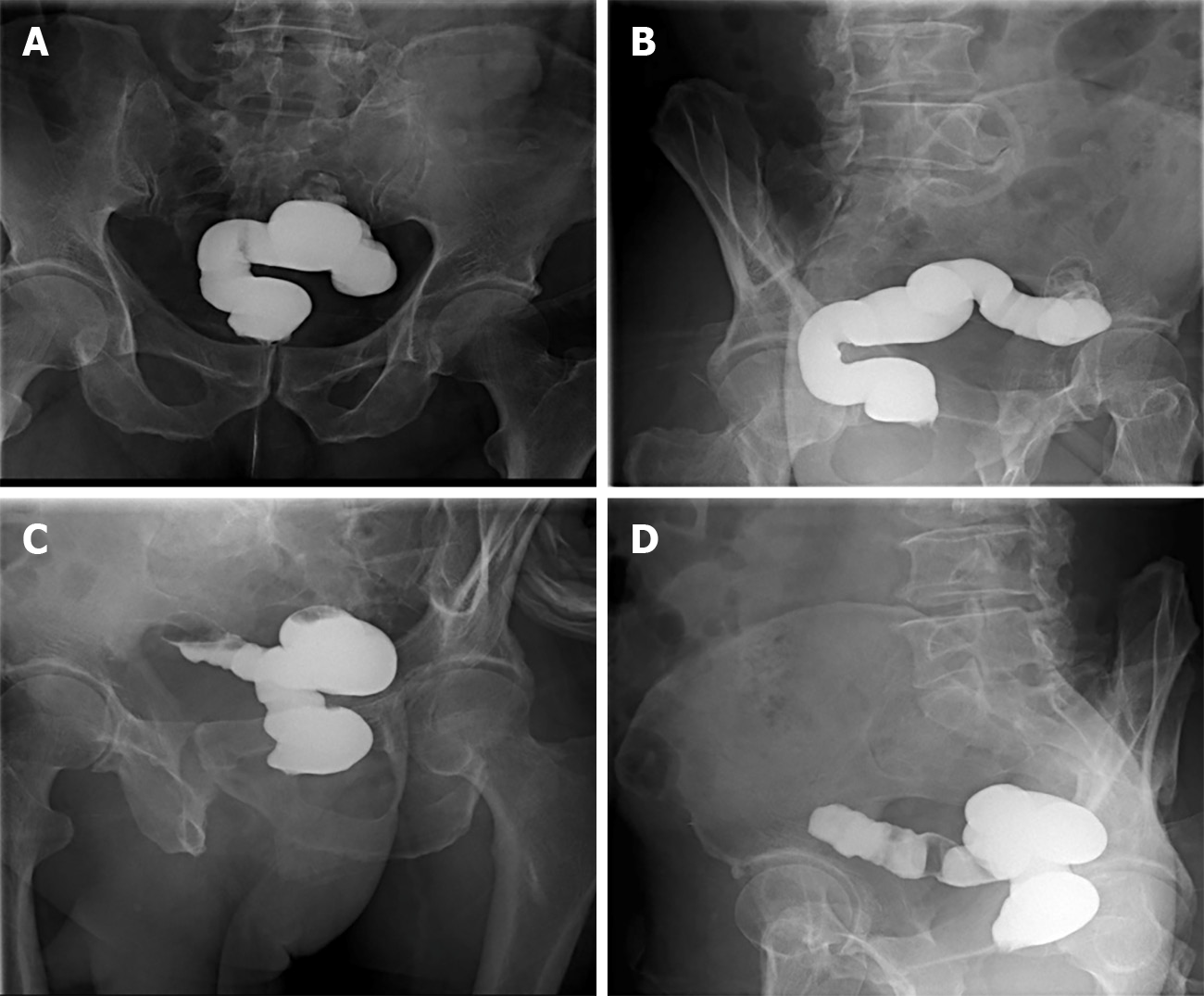
Retrograde transanal digestive tract radiography: Front view (Figure 3A and B) and lateral view (Figure 3C and D) showed that the distal bowel was approximately 25 cm, with no bowel stenosis and a smooth bowel mucosa.
(1) Colostomy with infection; (2) Parastomal hernia; (3) Intestinal fistula; and (4) Inguinal hernia.
Although the patient had no fever after admission and the infection index was not high, the patient’s local infection was severe, and the left abscess was treated with incision and drainage. According to the results of drug sensitivity, ceftizoxime was given as an anti-inflammatory with nutritional support. With general care, including changing the dressing on the fistula, the patient's infection was gradually controlled and improved.
The patient has a large amount of leakage due to the intestinal fistula, which was a high-flow fistula[7] that seriously affects the patient’s quality of life. According to the patient’s condition, the following treatment options were available: (1) Stoma displacement and stoma reconstruction; (2) Proximal colostomy to address the abdominal wall where two stomas had formed; (3) Hartmann reversal; and (4) Waiting and observation. After full communication, the patient, the patient's family, and the doctor unanimously decided to perform Hartmann's reversal. The patient underwent laparoscopic exploration under general anesthesia, lysis of intestinal adhesions, partial removal of the parastomal mesh, bowel resection and anastomosis, and abdominal sinus resection. A small incision in the right upper abdomen was first made. During the operation, it was found that abdominal adhesion was serious, no tumor recurrence was found, the distal bowel was located in the left paracolic sulcus (approximately 25 cm in length), and the distal bowel was changed due to disuse with obvious atrophy. There was also a mucus mass in the stump. To ensure a successful anastomosis, a 5-cm intestinal tube was removed from the stump and end-to-end anastomosis was performed. The inflation test was negative, so preventive stoma was not performed. Intraoperative bleeding was 200 mL, and the operation took 420 min.
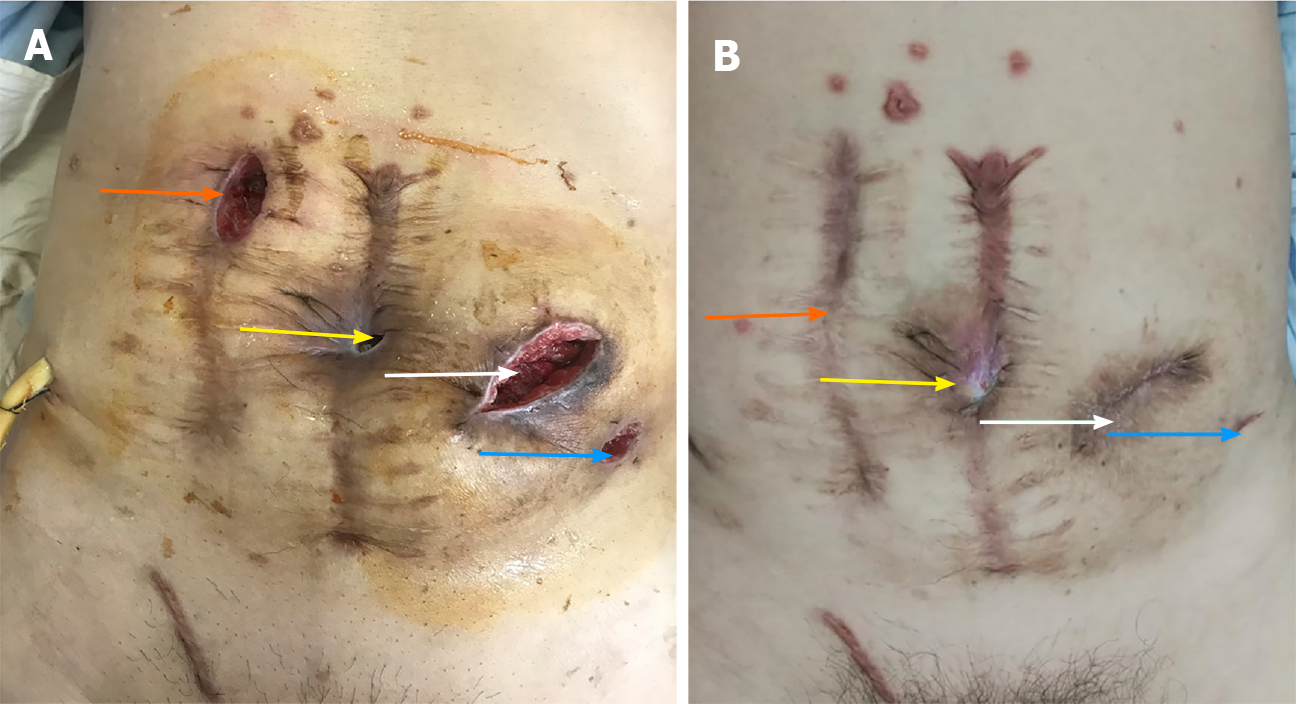
After the operation, Escherichia coli and Enterococcus faecium were cultured in the abdominal drainage fluid of the patient, who had signs of peritonitis. According to the drug sensitivity test, piperacillin sodium and tazobactam sodium 4.5 g every 8 h (total 14 d) were given for anti-inflammatory. An incision infection occurred on the 6th day (Figure 4A), and the dressing was changed. On the 6th day after the operation, he developed exhaust and defecated on the 8th day. After active anti-infection treatment, he was discharged smoothly after the operation.
In the first 6 mo after surgery, regular follow-up visits were made every 3 mo in the outpatient clinic, and the patient’s intestinal function was assessed through the Ligament Augmentation and Reconstruction System (LARS) scale. Tumor markers were evaluated every 6 mo after surgery. Colonoscopy and computed tomography (CT) examinations were completed 1 year after surgery. Three months after surgery, bowel movements occurred 4 to 7 times a day, with stool irregularity. The LARS score was 21 points, which is considered mild LARS. Six months after surgery, the patient had a bowel movement once a day, with stool formation. The LARS score was 11 points, meaning no LARS. There were also normal tumor markers and CT prompts for incisional hernia and inguinal hernia formation (Figure 5), abdominal wounds (Figure 4B), abdominal wall hernia repair, and inguinal hernia repair. One year after the operation, the patient had a bowel movement once a day, with stool formation. The LARS score remained at 11 points, with no LARS, normal tumor markers, and normal colonoscopy results (Figure 6). Over the past year, the patient's psychological pressure has significantly decreased, as he can travel, visit friends, and have a bright mood, which has improved his quality of life.
According to reports in the literature, the incidence of colorectal cancer is increasing annually, with up to 40% of colorectal cancers developing acutely. Among them, most are caused by obstruction. The current common processing methods[3] are: (1) Loop colostomy; (2) Primary resection with end colostomy: Hartmann’s procedure; (3) Endoscopic colonic stenting with self-expanding metallic stents; and (4) Primary resection and anastomosis. Currently, Hartmann’s procedure is one of the main methods for treating left colorectal cancer with acute intestinal obstruction[2]. In this case, the patient started with acute mechanical obstruction. Since colonic stent implantation was immature in China 13 years ago, doctors chose this safe and feasible method, which not only cured the intestinal obstruction, but was also more successful. The tumor was removed, and the patient had no tumor recurrence after the operation.
The rate of complications after receiving Hartmann’s procedure is as high as 51%[8]. Common complications include incision infection, distal rectal stump dysfunction, and parastomal complications. In this case, the patient experienced stoma-related complications such as parastomal hernia[6], parastomal abscess, and intestinal fistula, which seriously affected the patient's quality of life and increased psychological pressure. According to the literature, the rate of parastomal hernia is as high as 58%. Stoma patients have poor mental health, body image, and physical and emotional function[5]. In addition to the above-mentioned complications, the survival rate following Hartmann’s operation is lower[9]; but fortunately, the patient in this case achieved R0 resection and no metastasis occurred after the operation.
In this case, the patient underwent Hartmann reversal, which decreased his psychological pressure and allowed him to better integrate into society. It is also because this case had two parastomal hernias and bilateral inguinal hernias on the abdominal wall. The patient’s abdomen was weak. If the stoma is displaced or two stomas are performed, the patient will still have stoma-related complications and the abdominal wall will be worse. Therefore, the team chose stoma reversal.
The reversal rate of Hartmann surgery is very low. As reported in the literature, the Hartmann reversal rate is 25.9%-61%[4,10-14], and most of the literature reports are less than 50%. According to the 2015 United States bowel cancer audit report, 95% of patients who received Hartmann surgery still had a stoma 18 mo later. Currently, the intervening time before performing Hartmann reversal is controversial. It has been reported in the literature that the average time interval between Hartmann surgery and Hartmann reversal is 11 mo[4], of which the longest fistula reversal time is 96 mo[4]. The reversal time in this case was 156 mo, much longer than the time reported in the literature.
Previous studies have reported increased rates of complications with increased time to the reversal operation[12,14-16]. They speculated that this was related to atrophy of the distal stump, and it is recommended to reverse it as soon as possible. Others have noted that complications can be reduced by clinical treatment and nutrition support[12,17-20]. Finally, some believe that the length of reversal time does not affect postoperative complications or reversal rate[5,14,21,22]. In this case, the reversal time was 156 mo, which proves that the interval length does not increase the risk of reversal. At the same time, the incidence of complications after Hartmann reversal is quite high. According to reports in the literature, the total incidence of complications after Hartmann reversal is 16.3% (3.6%-50%)[11]. The most common postoperative complication is wound infection, with incidence rates ranging from 5% to 30% (average 12.5%)[12], followed by anastomotic failure, whose incidence rate is 4.2%[22]. In this case, the complication was wound infection, but no serious complications were present such as cardiopulmonary complications, anastomotic leakage, or other related complications.
Technical difficulties for Hartmann reversal include pelvic adhesions[4], chronic pelvic infections[4], difficulty in identifying short rectal stumps[4], and failure of rectal stump anastomosis[11]. Currently, the methods for Hartmann's reversal include: Traditional open surgery, laparoscopic surgery, and transostomy reversal. Studies have shown that using laparoscopic surgery for reversal is more beneficial than using traditional approaches[11]. Additionally, laparoscopic surgery can reduce post
First, for acute intestinal obstruction in the left colon or rectum, Hartmann’s surgery is a safe and feasible treatment. Second, there is controversy about the time to reversal after Hartmann’s procedure. It is not possible to use conventional experience or treatment methods to make the patient lose the opportunity to reverse the fistula. As in this case, the longer interval did not increase reversal complications; of course, the opinions of the patients and the surgical team should also be integrated. This successful case is a boon for patients after Hartmann’s procedure, as it offers more choices.
We would like to express our gratitude to all those who contributed to the study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wetwittayakhlang P S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li X
| 1. | Pisano M, Zorcolo L, Merli C, Cimbanassi S, Poiasina E, Ceresoli M, Agresta F, Allievi N, Bellanova G, Coccolini F, Coy C, Fugazzola P, Martinez CA, Montori G, Paolillo C, Penachim TJ, Pereira B, Reis T, Restivo A, Rezende-Neto J, Sartelli M, Valentino M, Abu-Zidan FM, Ashkenazi I, Bala M, Chiara O, De' Angelis N, Deidda S, De Simone B, Di Saverio S, Finotti E, Kenji I, Moore E, Wexner S, Biffl W, Coimbra R, Guttadauro A, Leppäniemi A, Maier R, Magnone S, Mefire AC, Peitzmann A, Sakakushev B, Sugrue M, Viale P, Weber D, Kashuk J, Fraga GP, Kluger I, Catena F, Ansaloni L. 2017 WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg. 2018;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 2. | Frago R, Ramirez E, Millan M, Kreisler E, del Valle E, Biondo S. Current management of acute malignant large bowel obstruction: a systematic review. Am J Surg. 2014;207:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Ansaloni L, Andersson RE, Bazzoli F, Catena F, Cennamo V, Di Saverio S, Fuccio L, Jeekel H, Leppäniemi A, Moore E, Pinna AD, Pisano M, Repici A, Sugarbaker PH, Tuech JJ. Guidelenines in the management of obstructing cancer of the left colon: consensus conference of the world society of emergency surgery (WSES) and peritoneum and surgery (PnS) society. World J Emerg Surg. 2010;5:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Hallam S, Mothe BS, Tirumulaju R. Hartmann's procedure, reversal and rate of stoma-free survival. Ann R Coll Surg Engl. 2018;100:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Näsvall P, Dahlstrand U, Löwenmark T, Rutegård J, Gunnarsson U, Strigård K. Quality of life in patients with a permanent stoma after rectal cancer surgery. Qual Life Res. 2017;26:55-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Ambe PC, Kurz NR, Nitschke C, Odeh SF, Möslein G, Zirngibl H. Intestinal Ostomy. Dtsch Arztebl Int. 2018;115:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 7. | Kumpf VJ, de Aguilar-Nascimento JE, Diaz-Pizarro Graf JI, Hall AM, McKeever L, Steiger E, Winkler MF, Compher CW; FELANPE; American Society for Parenteral and Enteral Nutrition. ASPEN-FELANPE Clinical Guidelines. JPEN J Parenter Enteral Nutr. 2017;41:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Ermolov AS, Rudin EP, Novruzov NG, Upyrev AV, Mironov AS. [Complications after Hartman's operation]. Khirurgiia (Mosk). 2007;11-14. [PubMed] |
| 9. | Biondo S, Kreisler E, Millan M, Fraccalvieri D, Golda T, Martí Ragué J, Salazar R. Differences in patient postoperative and long-term outcomes between obstructive and perforated colonic cancer. Am J Surg. 2008;195:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Horesh N, Lessing Y, Rudnicki Y, Kent I, Kammar H, Ben-Yaacov A, Dreznik Y, Tulchinsky H, Avital S, Mavor E, Wasserberg N, Kashtan H, Klausner JM, Gutman M, Zmora O. Considerations for Hartmann's reversal and Hartmann's reversal outcomes-a multicenter study. Int J Colorectal Dis. 2017;32:1577-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | van de Wall BJ, Draaisma WA, Schouten ES, Broeders IA, Consten EC. Conventional and laparoscopic reversal of the Hartmann procedure: a review of literature. J Gastrointest Surg. 2010;14:743-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Banerjee S, Leather AJ, Rennie JA, Samano N, Gonzalez JG, Papagrigoriadis S. Feasibility and morbidity of reversal of Hartmann's. Colorectal Dis. 2005;7:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Tokode OM, Akingboye A, Coker O. Factors affecting reversal following Hartmann's procedure: experience from two district general hospitals in the UK. Surg Today. 2011;41:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Roque-Castellano C, Marchena-Gomez J, Hemmersbach-Miller M, Acosta-Merida A, Rodriguez-Mendez A, Fariña-Castro R, Hernandez-Romero J. Analysis of the factors related to the decision of restoring intestinal continuity after Hartmann's procedure. Int J Colorectal Dis. 2007;22:1091-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Tan WS, Lim JF, Tang CL, Eu KW. Reversal of Hartmann's procedure: experience in an Asian population. Singapore Med J. 2012;53:46-51. [PubMed] |
| 16. | Schmelzer TM, Mostafa G, Norton HJ, Newcomb WL, Hope WW, Lincourt AE, Kercher KW, Kuwada TS, Gersin KS, Heniford BT. Reversal of Hartmann's procedure: a high-risk operation? Surgery. 2007;142:598-606; discussion 606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Pearce NW, Scott SD, Karran SJ. Timing and method of reversal of Hartmann's procedure. Br J Surg. 1992;79:839-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 170] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Keck JO, Collopy BT, Ryan PJ, Fink R, Mackay JR, Woods RJ. Reversal of Hartmann's procedure: effect of timing and technique on ease and safety. Dis Colon Rectum. 1994;37:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 94] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Khan AL, Ah-See AK, Crofts TJ, Heys SD, Eremin OJ. Reversal of Hartmann's colostomy. J R Coll Surg Edinb. 1994;39:239. |
| 20. | Fleming FJ, Gillen P. Reversal of Hartmann's procedure following acute diverticulitis: is timing everything? Int J Colorectal Dis. 2009;24:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Takahashi-Monroy T, Velasco L, Morales-Olivera JM. [Morbi-mortality of Hartmann's reversal procedure]. Cir Cir. 2006;74:329-333. [PubMed] |
| 22. | Guerra F, Coletta D, Del Basso C, Giuliani G, Patriti A. Conventional Versus Minimally Invasive Hartmann Takedown: A Meta-analysis of the Literature. World J Surg. 2019;43:1820-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Kwak HD, Kim J, Kang DW, Baek SJ, Kwak JM, Kim SH. Hartmann's reversal: a comparative study between laparoscopic and open approaches. ANZ J Surg. 2018;88:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Celentano V, Giglio MC, Bucci L. Laparoscopic vs open Hartmann's reversal: a systematic review and meta-analysis. Int J Colorectal Dis. 2015;30:1603-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Fiscon V, Portale G, Mazzeo A, Migliorini G, Frigo F. Laparoscopic reversal of Hartmann's procedure. Updates Surg. 2014;66:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |