Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5540
Peer-review started: January 27, 2021
First decision: February 11, 2021
Revised: February 22, 2021
Accepted: April 20, 2021
Article in press: April 20, 2021
Published online: July 16, 2021
Processing time: 160 Days and 19.4 Hours
Chemotherapy and radiotherapy followed by durvalumab is currently the standard treatment for locally advanced node-positive non-small-cell lung cancer (NSCLC). We describe the case of a patient with locally advanced node-positive NSCLC (LA-NSCLC) treated in a phase II prospective protocol with chemothe
A 69-year-old male, ex-smoker (20 PY), with a Karnofsky performance status of 90, was diagnosed with locally advanced squamous cell lung carcinoma. He was staged by total body computed tomography (CT) scanning, and integrated 18F-fluorodeoxyglucose positron emission tomography/CT scan [cT4 cN3 cM0, stage IIIC according to TNM (tumor-node-metastasis) 8th edition] and received AHRT between chemotherapy cycles, in accordance with the study protocol (EudractCT registration 2008-006525-14). At the end of the study the patient underwent surgery, which was not part of the protocol, and showed a complete pathological response.
This case report confirms that AHRT can be used successfully to treat primary LA-NSCLC with bilateral mediastinal lymph node involvement. Our case is of particular interest because of the pathological response after AHRT and the lack of surgical complications. We hypothesize that this radiotherapeutic approach, with its proven efficacy, could be delivered as a short course reducing treatment costs, increasing patient compliance and reducing toxicity. We are currently investigating the possibility of combining hypofractionation, chemotherapy and immunotherapy for patients with LA-NSCLC.
Core Tip: Non-small-cell lung cancer (NSCLC) represents the most common cause of death from cancer worldwide. The majority of patients present with locally advanced or metastatic disease at diagnosis. Concurrent chemoradiotherapy and immunotherapy is actually the gold standard treatment for this patient setting, with better long-term survival data than previously. As concurrent chemoradiotherapy is characterized by high-grade toxicity, a limited number of lung cancer patients is able to undergo or complete the treatment in clinical practice. We present a patient with NSCLC who was enrolled in a protocol using accelerated hypofractionated radiotherapy and who, after surgery, was staged as no residual oncological disease ypT0ypN0.
- Citation: Parisi E, Arpa D, Ghigi G, Micheletti S, Neri E, Tontini L, Pieri M, Romeo A. Complete pathological response in locally advanced non-small-cell lung cancer patient: A case report. World J Clin Cases 2021; 9(20): 5540-5546
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5540.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5540
Non-small-cell lung cancer (NSCLC) is a malignant tumor commonly and frequently diagnosed and represents an important cause of cancer death worldwide[1]. The majority of patients initially present with advanced or metastatic stage disease[2]. The current gold standard treatment for locally advanced NSCLC (LA-NSCLC) is chemotherapy, conventional fractionation radiotherapy (RT) and durvalumab for fit patients who have responded to definitive concurrent chemoradiotherapy (CRT)[3]. The integration of chemotherapy and RT is characterized by a high rate of potential side-effects. Indeed, comparison studies of concomitant and sequential CRT have shown the advantages of the former, albeit concomitant treatment has an important rate of acute and late toxicity[4,5].
Radiobiological studies[6] suggest that locoregional control is improved with higher radiation doses[7]. Some authors have studied the efficacy of accelerated hypofractionated RT (AHRT), reporting interesting results in early-stage peripheral lung cancer[8]. Hypofractionation with ablative doses is currently used for the stereotactic treatment of peripheral early-stage lung cancer thanks to improved imaging and RT technology. However, the scenario is completely different for LA-NSCLC in which dose fractionation has changed very little over the past 10 years[9]. Despite this, improved survival rates have been registered for patients with LA-NSCLC. The most significant improvement in survival in this patient setting has been achieved through the addition of immunotherapy (IO).
A 69-year-old Caucasian male, ex-smoker (20 PY), with a Karnofsky performance status of 90 presented with a cough and fever.
He reported an unintentional 3 kg weight loss in 1 mo.
He had no significant past medical history or surgical history. He was not taking any medication.
No family member had a history of cancer.
His temperature was 36.3°C, heart rate was 70 bpm, and SaO2 was 97%. Thorax examination revealed decreased breath sounds in the left lung.
Laboratory testing including complete blood count, were all within normal limits.
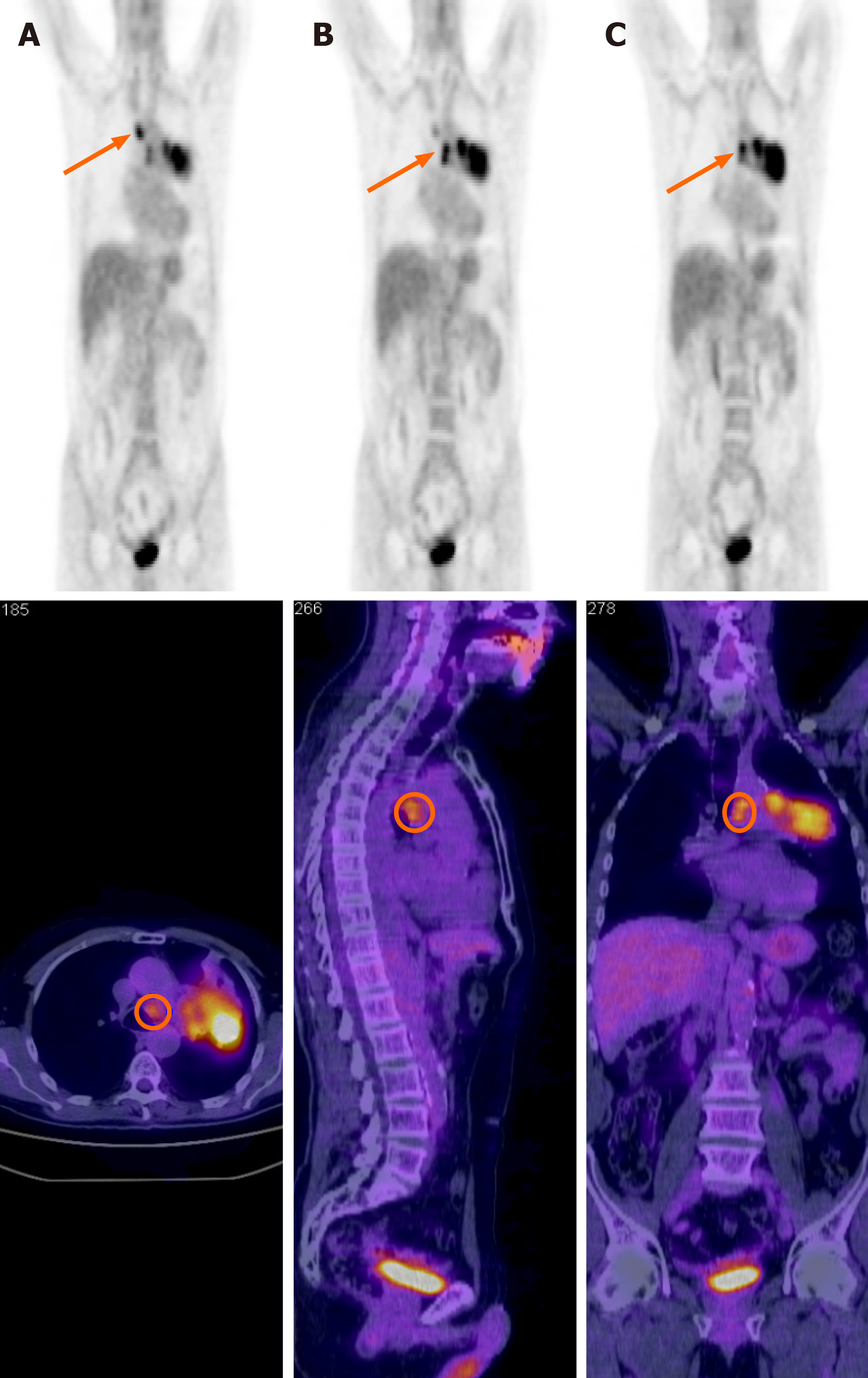
He was diagnosed in April 2009 with squamous cell lung carcinoma by endoscopic bronchial ultrasound and submitted to transbronchial nodal aspiration for suspected mediastinal node involvement (4 left and 2 right). Before RT, the patient underwent a lung function study, the results were in the normal range for FEV1 (forced expiratory volume in 1 s), FVC (forced vital capacity) and DLCO (diffusing lung capacity for carbon monoxide). The patient underwent staging by total body contrast-enhanced computed tomography (CT) scanning and 18F-fluorodeoxyglucose positron emission tomography/CT (PET-CT) scanning (Figure 1).
The final diagnosis was squamous cell lung carcinoma, cT4 cN3 cM0, stage IIIC according to TNM (tumor-node-metastasis) 8th edition.
He was enrolled in a prospective phase II clinical trial of CRT using AHRT in helical TomoTherapy (TomoTherapy Inc., Madison, WI, United States) for patients with LA-NSCLC (EudractCT registration 2008-006525-14)[10].
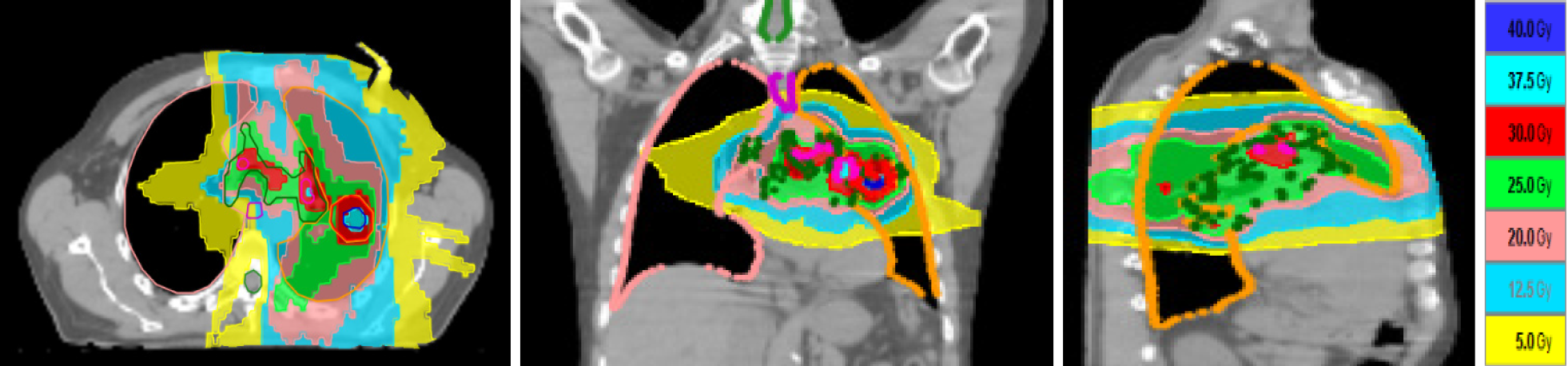
From April 30 to July 29, 2009, the patient underwent chemotherapy according to the following scheme: Cisplatin 75 mg/m2 and docetaxel 75 mg/m2 on day 1 every 21 d for two cycles. From the 15th to the 19th day after the second cycle of chemotherapy, he also underwent AHRT. The dose prescribed to the clinical target volume (CTV) was 30 Gy/5 daily fractions with an internal dose of up to 40 Gy in order to create a dose distribution[11,12] similar to brachytherapy; 25 Gy/5 fractions were prescribed to the lymph node CTV, with an internal dose of up to 37.5 Gy. The aim of the internal dose to the CTV was to increase the heterogeneity of dose distribution as in stereotactic body radiotherapy. Fifteen days after RT, the patient underwent endoscopic ultrasonography, with no evidence of mediastinal nodes. He thus completed the protocol with the 2 remaining courses of CT with the same cisplatin dosage and reduced dosage of docetaxel, as per the study protocol.
After the first chemotherapy cycle, the patient showed grade 4 leukopenia and neutropenia according to National Cancer Institute common terminology criteria for adverse events version 3.0[13]. He was hospitalized for treatment with antibiotics and granulocyte-colony stimulating factors. The 3 remaining chemotherapy cycles were administered with a dosage reduction. To plan RT, the patient underwent a multi
The patient then started the follow-up program according to the protocol schedule. Three months after the end of treatment, approval was obtained for the patient to undergo surgery comprising left pneumonectomy and mediastinal lymphadenectomy. The histological referral was as follows: Presence of numerous sections of peri
In this report we presented the case of a patient with NSCLC, stage IIIC according to TNM 8th edition. The tumor was initially defined as inoperable and the patient was enrolled in a clinical study. The novelty in this case was the RT schedule, i.e., AHRT to the tumor site (cT4 for mediastinal involvement) and bilateral mediastinal lymph nodes (cN3). To the best of our knowledge, this was the first protocol in the pre-IO era to use AHRT in only 5 fractions plus chemotherapy to treat locally advanced NSCLC in terms of both the primary tumor and involved mediastinal nodes. Our patient unexpectedly obtained a complete pathological response after treatment. Although surgery was not indicated because of the baseline stage of disease, radiological response before surgical intervention is nonetheless considered as a partial response according to RECIST criteria 1.1. This was the case in our patient. However, a stereotactic study on lung cancer by Bradley et al[8] reported the practicability of AHRT but potential late toxicity as the most important limit of its general clinical use. In their systematic review, Kaster et al[14] underlined better outcomes in stage III NSCLC used concurrently with systematic chemotherapy. The authors analyzed RT dose schedules with a maximum dose per fraction of 3.5 Gy and a fraction range of 15-35, concluding that AHRT increases regional control by the use of a high biologically effective dose (BED) value. In this case report, the dose prescribed to the primary tumor ranged from 6 Gy to 8 Gy per fraction and the dose prescribed to the involved mediastinal nodes ranged from 5 Gy to 7.5 Gy per fraction. Despite a high dose per fraction to the mediastinum, our patient did not report any RT-related toxicity during the later years of follow-up. Some studies have reported that toxicity, in the majority of cases, is correlated with the dose to the OaR[14,15]. Bradley et al[8], reported, in their protocol, the same grade toxicity for patients who underwent 60 Gy or 74 Gy of radiotherapy treatment but the death rate was different, and was higher in the 74 Gy arm. In a phase I study of AHRT, Cannon et al[16] underlined that the fractionation used was well tolerated when strict normal tissue constraints were maintained. In our patient, we obtained two different OaR DVHs (dose-volume histograms), one in hypofractionation and the other with the conversion into 2 Gy equivalent dose. All dose constraints recommended were met[17]. The use of intensity modulated arch therapy in TomoTherapy, of contrast-enhanced CT simulation, and of a daily megavoltage setup helped us to save the OaR. Furthermore, we applied non-isotropic expansion to the gross tumor volume. This aspect led to a reduction in the volume of irradiated lung.
Using BED10 at the isocenter, the mean dose to the tumor resulted in 74 Gy and the mean dose to the involved mediastinum resulted in 54 Gy, as reported in the protocol.
Despite the improvements in RT in recent years, no advantages in survival have been reported from its concomitant use with chemotherapy in LA-NSCLC. Although the addition of durvalumab to radiochemotherapy has improved overall survival, this immunotherapeutic drug is reserved for fit patients who respond to CRT. The question thus remains: What hope can we offer in terms of treatment to elderly or unfit patients with IIIB-C NSCLC?
In conclusion, concomitant CRT followed by IO is the treatment choice for LA-NSCLC. Our experience, reported in the previous protocol, confirms that short course radiotherapy can decrease treatment costs and increase patient compliance. It is thus time to design studies that contemplate chemotherapy, AHRT and IO in this patient setting.
The authors thank Tierney G for editorial assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fu T, Zeng Y S-Editor: Gao CC L-Editor: Webster JR P-Editor: Yuan YY
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20481] [Article Influence: 2048.1] [Reference Citation Analysis (19)] |
| 2. | Ishida K, Hirose T, Yokouchi J, Oki Y, Kusumoto S, Sugiyama T, Ishida H, Shirai T, Nakashima M, Yamaoka T, Ohnishi T, Ohmori T, Kagami Y. Phase II study of concurrent chemoradiotherapy with carboplatin and vinorelbine for locally advanced non-small-cell lung cancer. Mol Clin Oncol. 2014;2:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379:2342-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 2045] [Article Influence: 292.1] [Reference Citation Analysis (0)] |
| 4. | Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, Machtay M, Sause W, Cox JD. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 915] [Article Influence: 65.4] [Reference Citation Analysis (1)] |
| 5. | Fournel P, Robinet G, Thomas P, Souquet PJ, Léna H, Vergnenégre A, Delhoume JY, Le Treut J, Silvani JA, Dansin E, Bozonnat MC, Daurés JP, Mornex F, Pérol M; Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol. 2005;23:5910-5917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 397] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 6. | Martel MK, Ten Haken RK, Hazuka MB, Kessler ML, Strawderman M, Turrisi AT, Lawrence TS, Fraass BA, Lichter AS. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer. 1999;24:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 257] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2673] [Cited by in RCA: 2431] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 8. | Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, Kavadi V, Garces YI, Narayan S, Iyengar P, Robinson C, Wynn RB, Koprowski C, Meng J, Beitler J, Gaur R, Curran W Jr, Choy H. Standard-dose vs high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1197] [Cited by in RCA: 1523] [Article Influence: 152.3] [Reference Citation Analysis (0)] |
| 9. | Perez CA, Stanley K, Rubin P, Kramer S, Brady L, Perez-Tamayo R, Brown GS, Concannon J, Rotman M, Seydel HG. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45:2744-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Parisi E, Genestreti G, Sarnelli A, Ghigi G, Arpa D, Burgio MA, Gavelli G, Rossi A, Scarpi E, Monti M, Tesei A, Polico R, Romeo A. Accelerated hypofractionated radiotherapy plus chemotherapy for inoperable locally advanced non-small-cell lung cancer: final results of a prospective phase-II trial with a long-term follow-up. Radiat Oncol. 2019;14:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Polico R, Stea L, Antonello M, Princivalli M, Marchetti C, Busetto M, Schiavon S, Pizzi G. [The polycentric multiple arc complanar technic, or telebrachytherapy. A 4-year experience (an innovative way for the local control of solid neoplasms)]. Radiol Med. 1995;90:113-123. [PubMed] |
| 12. | Hennequin C, Tredaniel J, Chevret S, Durdux C, Dray M, Manoux D, Perret M, Bonnaud G, Homasson JP, Chotin G, Hirsch A, Maylin C. Predictive factors for late toxicity after endobronchial brachytherapy: a multivariate analysis. Int J Radiat Oncol Biol Phys. 1998;42:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Cancer Therapy Evaluation Program. Common terminology criteria for adverse events, version 3.0, DCTD, NCI, NIH, DHHS, March 31 2003. [cited 9 August 2020]. In: Cancer Therapy Evaluation Program [Internet]. Available from: http://ctep.cancer.gov. |
| 14. | Kaster TS, Yaremko B, Palma DA, Rodrigues GB. Radical-intent hypofractionated radiotherapy for locally advanced non-small-cell lung cancer: a systematic review of the literature. Clin Lung Cancer. 2015;16:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Wurstbauer K, Deutschmann H, Dagn K, Kopp P, Zehentmayr F, Lamprecht B, Porsch P, Wegleitner B, Studnicka M, Sedlmayer F. DART-bid (Dose-differentiated accelerated radiation therapy, 1.8 Gy twice daily)--a novel approach for non-resected NSCLC: final results of a prospective study, correlating radiation dose to tumor volume. Radiat Oncol. 2013;8:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Cannon DM, Mehta MP, Adkison JB, Khuntia D, Traynor AM, Tomé WA, Chappell RJ, Tolakanahalli R, Mohindra P, Bentzen SM, Cannon GM. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2013;31:4343-4348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Grills IS, Yan D, Martinez AA, Vicini FA, Wong JW, Kestin LL. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2003;57:875-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |