Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5535
Peer-review started: November 8, 2020
First decision: April 29, 2021
Revised: May 10, 2021
Accepted: May 17, 2021
Article in press: May 17, 2021
Published online: July 16, 2021
Processing time: 240 Days and 19.2 Hours
Primary cardiac tumors are uncommon, of which cardiac myxoma accounts for 50%-80%. Left ventricular myxoma has been rarely reported, accounting for only 3%-4% of all cardiac myxomas. Multiple left ventricular myxomas are, relatively, even rarer.
In this report, we present a case of multiple left ventricular myxomas combined with severe rheumatic valve lesions. Symptomatically, the patient presented with fatigue, shortness of breath, and palpitation after activities. The patient underwent complete surgical resection of multiple left ventricular myxomas combined with mechanical replacement of the mitral and aortic valves, tricuspid valvuloplasty. The patient recovered well after the operation, with no obvious related complications.
Multiple left ventricular myxomas may coexist with severe rheumatic valve disease. Operation is an effective treatment.
Core Tip: The patient in our case complained of fatigue, shortness of breath, and palpitation after activities. Based on a series of examinations, he was diagnosed with multiple left ventricular myxomas combined with severe rheumatic valve lesions; clinically, this is a relatively rare case.
- Citation: Liu SZ, Hong Y, Huang KL, Li XP. Multiple left ventricular myxomas combined with severe rheumatic valvular lesions: A case report. World J Clin Cases 2021; 9(20): 5535-5539
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5535.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5535
Cardiac myxoma is the most common primary cardiac benign tumor, with about 75%-80% occurring in left atrium, and only 3%-4% in left ventricle[1]. Multiple left ventricular myxomas are extremely rare. In this report, we present a case of multiple left ventricular myxomas combined with severe rheumatic valve lesions.
Patient complained of a 20-year history of fatigue after activities, which was aggrava
A 53-year-old male was admitted to hospital with complaints of a 20-year history of fatigue after activities, which was aggravated over the previous 2 mo.
He had a more than 2-year history of diabetes.
There was no history of rheumatic fever and family history of cardiac tumor or sudden death.
On admission, there was a diastolic rumbling pathological murmur of grade III/VI in the apical region. Grade IV/VI systolic ejection murmur and III/VI diastolic sighing murmur in the second intercostal space near the right margin of sternum were heard. Electrocardiogram showed atrial fibrillation with rapid ventricular rate.
Laboratory data revealed slightly elevated glutamic oxaloacetic transaminase (45 U/L, normal level < 40 U/L) and total bilirubin (56 μmol/L, normal range < 23 μmol/L) and remarkably increased brain natriuretic peptide (692.7 pg/mL, normal range 0-100 pg/mL). Erythrocyte sedimentation rate was 6 mm/h. There were unremarkable abnormalities in circulation levels of serum tumor markers.
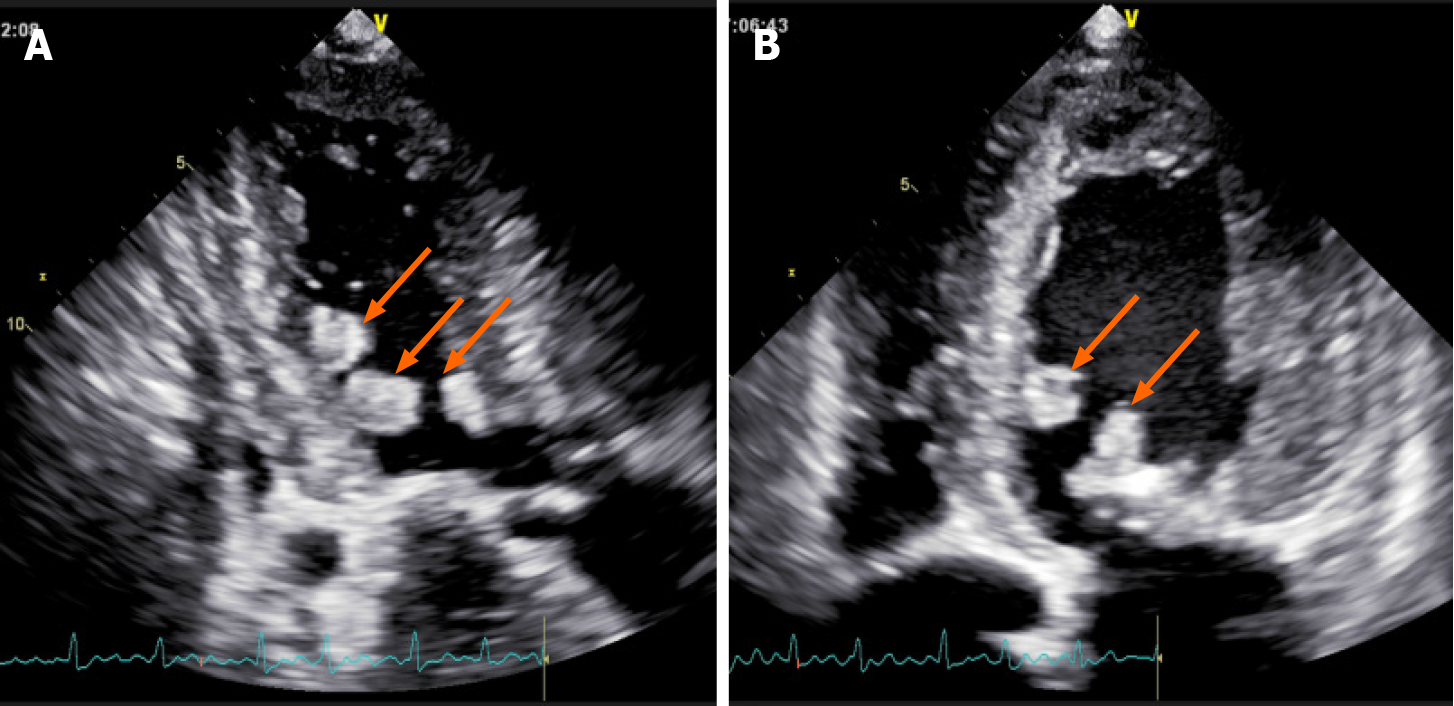
Chest computed tomography revealed bilateral emphysema with mild pulmonary edema, generally enlarged heart, and calcification of the aortic and mitral valves. Transesophageal echocardiography (TEE) demonstrated enlarged left atrium (50 mm), left ventricle (60 mm), and right atrium (64 mm × 57 mm). Mitral, aortic, and tricuspid valves were thickened and adhered, similar to rheumatic valve lesions. The mitral and aortic valve orifices exhibited severe stenosis, and the areas were 0.64 cm2 and 0.87 cm2, respectively. Multiple abnormal echo masses were found in the left ventricle (Figure 1), with the largest mass (about 23 mm × 15 mm in diameter) located in the left ventricular cavity at the junction of the anterior septum and the posterior papillary muscle.
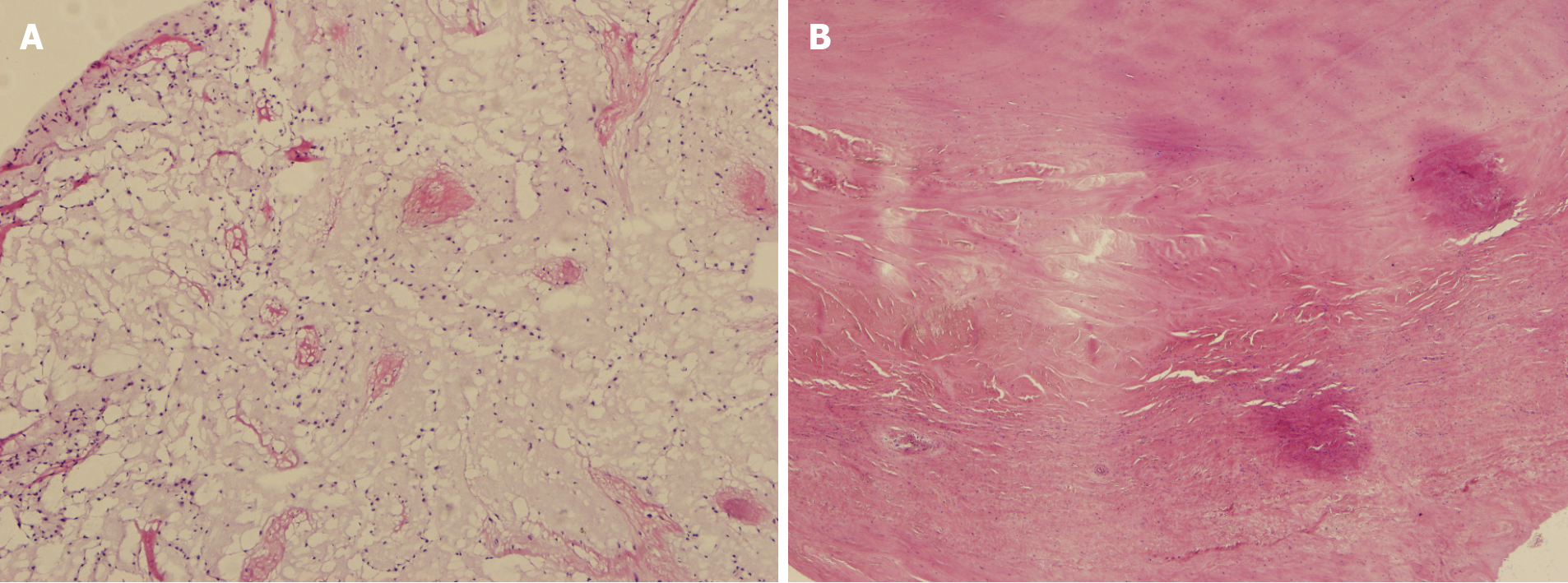
Postoperative histopathological examination showed that the tumor cells were irregular, surrounded by empty halos, and scattered with sparse stroma, which confirmed the cardiac myxoma (Figure 2A). Mitral valve and aortic valve exhibited very obvious rheumatic lesions. The main manifestations of the microscopic exami
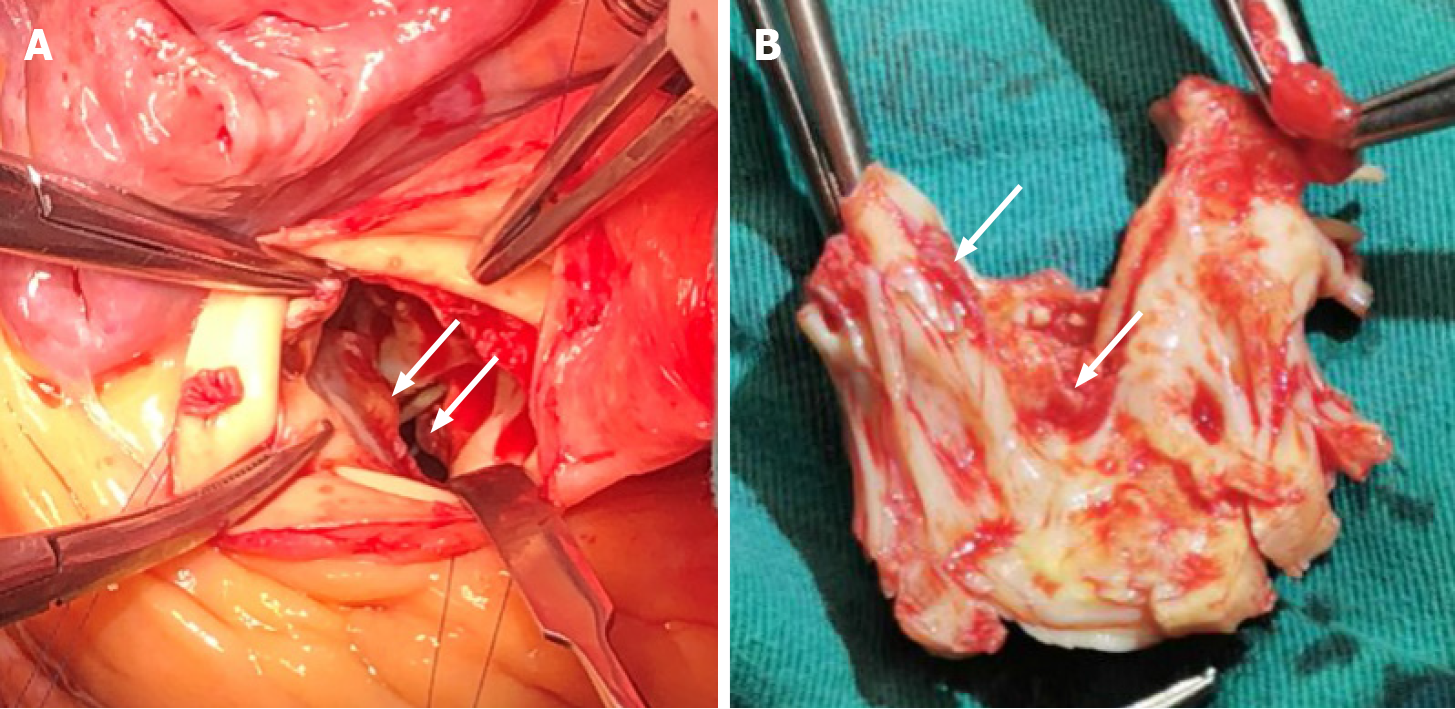
The patient underwent surgical treatment through median sternotomy under cardiopulmonary bypass. Multiple masses were found in the left ventricle, which existed in the left ventricular outflow tract, anterior interventricular septum, anterior and posterior papillary muscle roots, and anterior mitral valve. The diameter of masses ranged from 5 mm to 20 mm; they had a crisp texture and were gelatinous with high mobility (Figure 3). The masses in the left ventricle were completely resected via the mitral and aortic valve orifices. The thickened and adhered mitral and aortic valves were replaced with mechanical valves. The tricuspid valvuloplasty was performed by incising the junction of anterior and septal valve and the junction of posterior and anterior valve. Then, the thickened leaflet was thinned to increase the activity of leaflet. Meanwhile, a tricuspid annuloplasty ring was used. The left atrial appendage was ligated during operation.
The patient recovered well after the operation, and his condition improved considerably; there were no obvious related complications.
Myxoma, the most common primary cardiac tumor, mainly arises from the left atrium, with barely 3%-4% arising in left ventricle. Cardiac myxoma typically occurs as a single mass and originates from the subendocardial interlobular tissue and occasio
Surgery is an effective treatment for cardiac myxoma. Left ventricular myxoma is usually removed through the mitral orifice or aortic valve orifice or via left ventricular incision[4]. Due to severe rheumatic valve lesions, the patient required simultaneous mitral and aortic valve replacement, tricuspid valvuloplasty. Thus, the multiple left ventricular myxomas were completely removed through combination of mitral and aortic valve orifices approaches. In addition, considering the patient’s financial difficulties, the radiofrequency ablation for atrial fibrillation was not performed. However, we ligated the left atrial appendage to avoid the possibility of embolism caused by left atrial appendage thrombosis.
In conclusion, we describe here a unique case with multiple left ventricular myxomas combined and severe rheumatic valve lesions. Surgical treatment was effective.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nisi F, Xue L S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Natale E, Minardi G, Casali G, Pulignano G, Musumeci F. Left ventricular myxoma originating from the interventricular septum and obstructing the left ventricular outflow tract. Eur J Echocardiogr. 2008;9:84-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Kuyama N, Hamatani Y, Fukushima S, Ikeda Y, Nakai E, Okada A, Takahama H, Amaki M, Hasegawa T, Sugano Y, Kanzaki H, Fujita T, Ishibashi-Ueda H, Yasuda S, Anzai T, Kobayashi J. Left ventricular myxoma with Carney complex. ESC Heart Fail. 2018;5:713-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | El Sabbagh A, Al-Hijji MA, Thaden JJ, Pislaru SV, Pislaru C, Pellikka PA, Arruda-Olson AM, Grogan M, Greason KL, Maleszewski JJ, Klarich KW, Nkomo VT. Cardiac Myxoma: The Great Mimicker. JACC Cardiovasc Imaging. 2017;10:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Liu J, Guo HM, Xie B, Guo HJ, Chen JM, Zhuang J. Giant Left Ventricular Myxoma With Obstruction of the Left Ventricular Outflow Tract. Ann Thorac Surg. 2016;101:e63-e64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |