Published online Jan 16, 2021. doi: 10.12998/wjcc.v9.i2.445
Peer-review started: August 23, 2020
First decision: October 18, 2020
Revised: October 28, 2020
Accepted: November 13, 2020
Article in press: November 13, 2020
Published online: January 16, 2021
Processing time: 138 Days and 5.4 Hours
Malignant solitary fibrous tumors (SFTs) account for 15%-20% of all SFTs, and malignant SFTs arising from the greater omentum are extremely rare. Most malignant SFTs of the greater omentum are diagnosed via pathological examinations after surgery. In this study, we report a case of malignant omental SFT and review the published literature on this rare malignancy.
A 64-year-old female presented with an abdominal mass, and underwent exploratory surgery, during which a huge tumor originating from the greater omentum and intraperitoneal implants were identified and resected. The results of the pathological examination, immunohistochemistry staining, and gene sequencing led to the diagnosis of malignant SFT of the greater omentum. The patient died one and a half years later due to tumor recurrence and metastasis.
This is the first report of the application of gene sequencing in the diagnosis of malignant SFTs of the greater omentum.
Core Tip: In this study, we present the rare case of a huge malignant solitary fibrous tumor (SFT) of the greater omentum. At presentation, the patient complained of an abdominal mass. After routine imaging examination, she underwent an exploratory laparotomy with a suspected diagnosis of gastrointestinal stromal tumor. Post-operative histological examination and gene sequencing indicated a malignant SFT of the greater omentum. We reviewed and discussed the pre-operative diagnosis, surgical options, and post-operative treatment of reported cases of malignant SFTs of the greater omentum. We suggest the application of gene sequencing in the diagnosis of malignant SFTs.
- Citation: Guo YC, Yao LY, Tian ZS, Shi B, Liu Y, Wang YY. Malignant solitary fibrous tumor of the greater omentum: A case report and review of literature. World J Clin Cases 2021; 9(2): 445-456
- URL: https://www.wjgnet.com/2307-8960/full/v9/i2/445.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i2.445
A solitary fibrous tumor (SFT) is a mesenchymal tumor, which was initially reported as an intra-pleural neoplasm by Klemperer and Coleman[1]. Increasing interest in SFTs and their revised definition has led to the additional diagnosis of SFTs in a wide variety of extra-pleural sites, such as the orbit, thyroid gland, head, neck, and extremities. This conforms to the theory that as the tumor is derived from mesenchymal cells, SFT can occur anywhere within the human body. SFTs are generally benign; only one-fifth of all SFTs are invasive, with poor prognoses[2]. In the abdomen, primary SFTs are mostly observed in the retroperitoneal space, and malignant SFTs arising from the greater omentum are not sufficiently documented[3], which results in limited knowledge on SFTs and their management. We herein report a case of malignant SFT of the greater omentum in a female. For a better understanding of malignant SFTs, we also collected and summarized the data in existing reports on malignant SFTs originating from the greater omentum.
A 64-year-old female was admitted to our center due to an incidental mobile abdominal mass.
Prior to admission, the patient noticed an increase in her abdominal size, which she construed as weight gain.
On physical examination, a large non-tender mobile mass was palpated in the right abdomen.
Laboratory examinations showed a cancer antigen 125 (CA125) level of 540.6 U/mL (normal range < 35 U/mL).
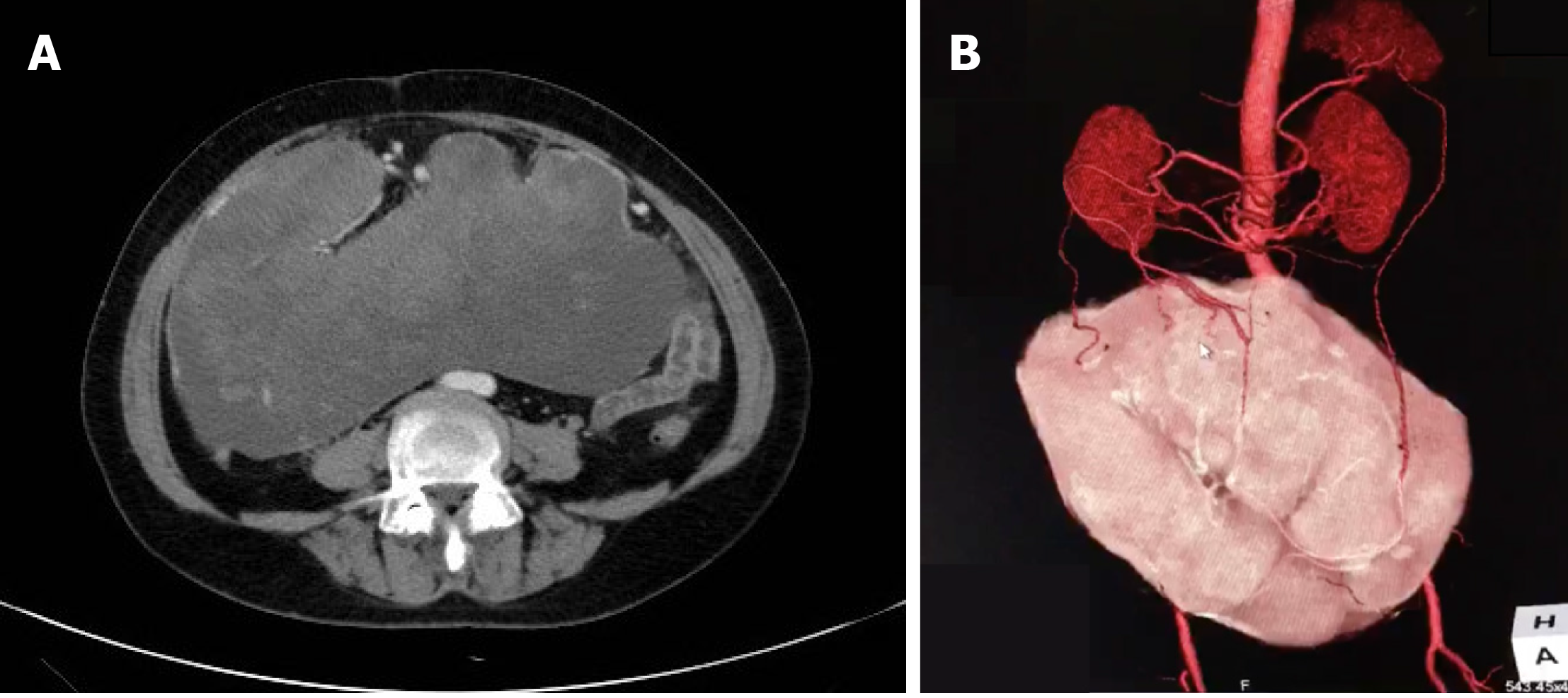
Contrast-enhanced abdominal computed tomography (CT) revealed a huge mass measuring 25.4 cm × 23.0 cm with a mixed density and heterogeneous enhancement (Figure 1A). CT three-dimensional (3D) reconstruction showed that the feeding arteries were from the splenic artery and celiac axis (Figure 1B). From the imaging findings, we suspected a gastrointestinal stromal tumor (GIST).
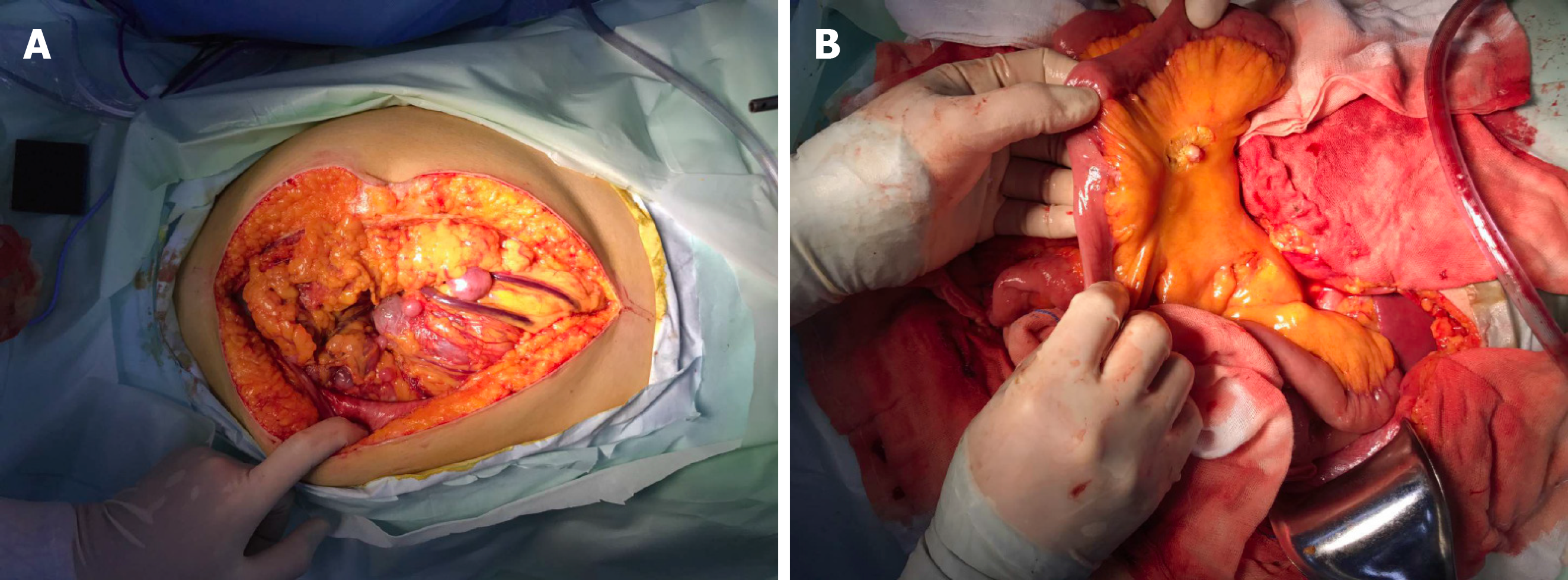
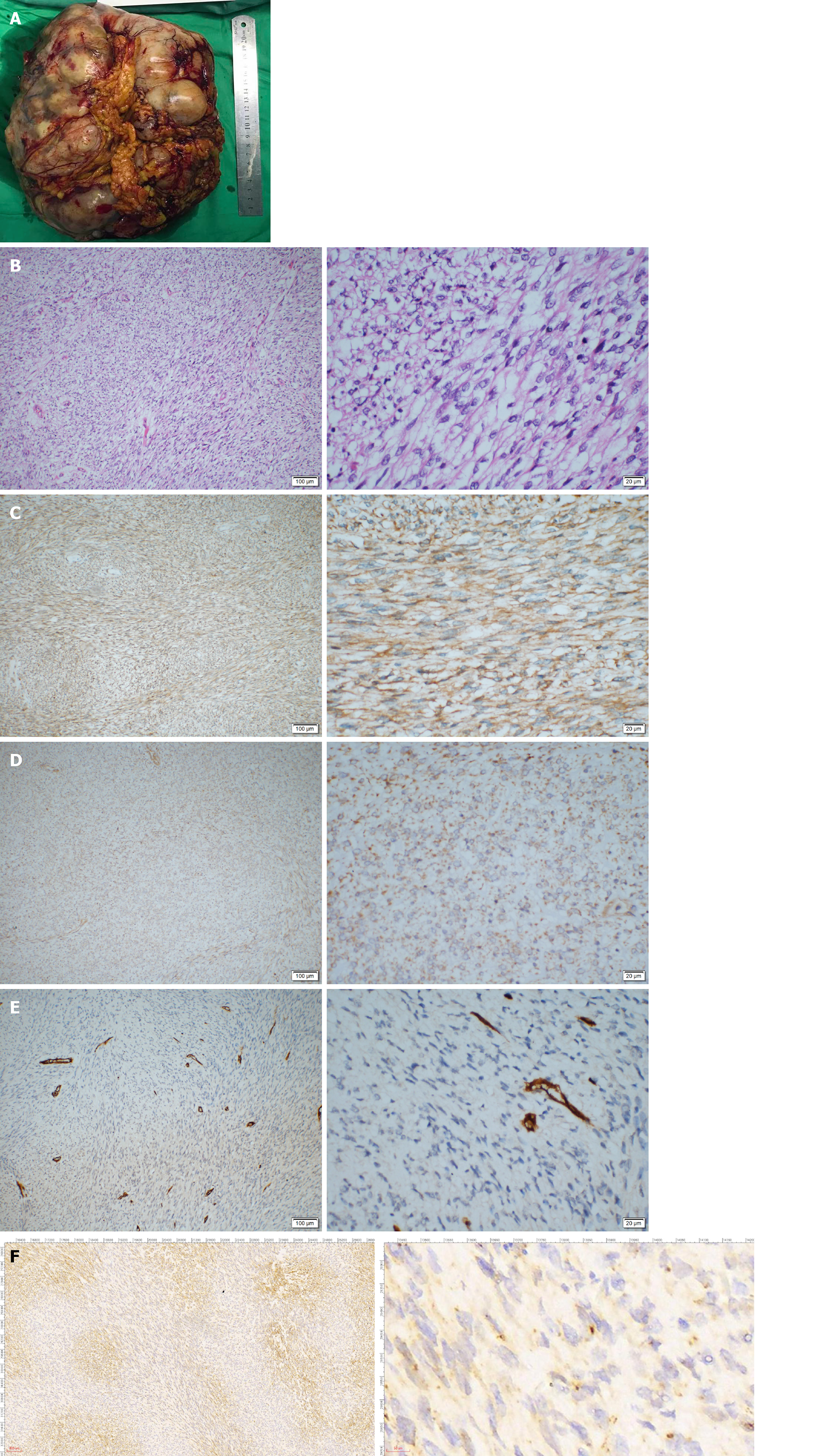
Guo YC, MD, PhD, Attending, Department of Gastrointestinal Surgery: The patient then underwent an exploratory laparotomy. Intraoperatively, a huge tumor originating from the greater omentum with several implant nodules on the omentum and intestinal mesentery were identified (Figure 2A and B). Tumorectomy and omentectomy with excision of the adjacent parietal peritoneum were performed, and the implanted nodules on the mesentery were also resected. The tumor weighed 4.32 kg and measured 27 cm × 21 cm × 9 cm in size (Figure 3A).
On pathological examination of the resected specimen, hypercellularity with spindle cells (Figure 3B) was observed, and the mitotic rate was 30/10 on high power field (HPF). Immunohistochemistry revealed positivity for Vimentin; weak positivity for BCL-2, DOG-1, CD99, and Ki-67 (+ 40%); negativity for CD34, Desmin, SMA, CD117, S-100, STAT6, and CK, suggesting the diagnosis of malignant SFT or GIST (Figure 3C-F).
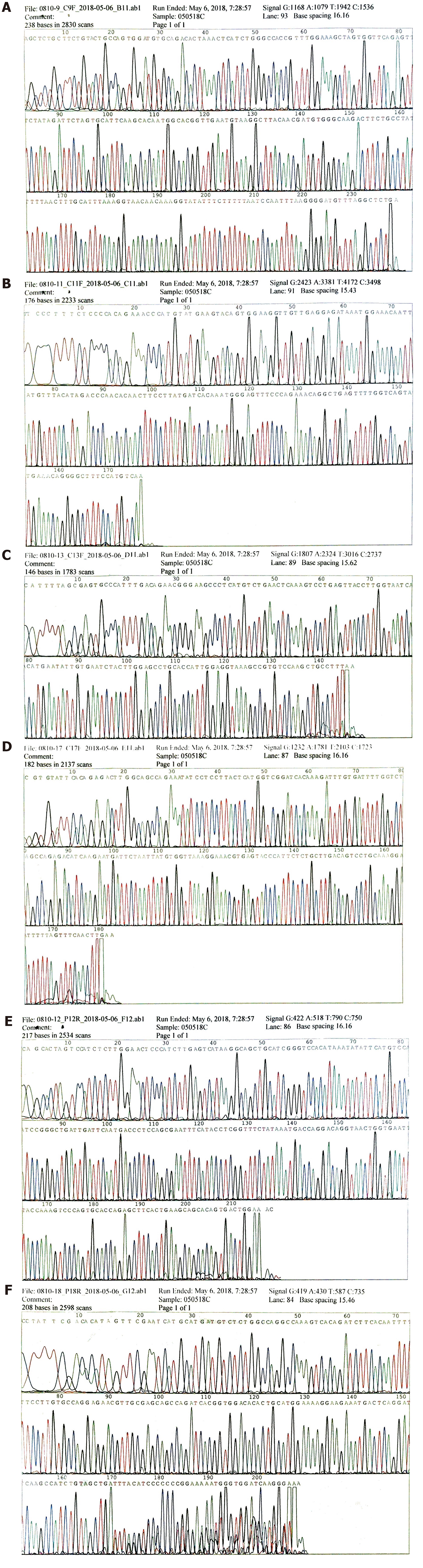
To make an exact diagnosis, gene sequencing was performed using a paraffin section of the tumor tissue obtained during surgery. The Sanger sequencing method was used. The tumor cell content was 90%. The nucleic acid content was 152.3 ng/μL, and the ratio of OD260/OD280 was 1.84. We examined 4 exons of c-KIT including 9, 11, 13, and 17; and 2 exons of PDGFRA including 12 and 18. As a result, neither c-KIT nor PDGFRA had a gene mutation in their exons (Figure 4A-4F which further excluded the probability of GIST and supported the diagnosis of a malignant SFT.
The final diagnosis was malignant SFT of the greater omentum.
Additional treatment was not provided after the tumorectomy and omentectomy.
The post-operative course was unremarkable, and the patient was discharged from the hospital 8 d after surgery. However, the patient refused further medical therapy after surgery and died one and a half years later due to tumor recurrence and disseminated metastasis.
SFT accounts for approximately 5% of all sarcomas, and SFTs originating from the greater omentum are extremely rare[4]. SFTs mainly occur at the age of 50-70 years, without gender predilection[2]. Typically, the histological features of SFTs are characterized by a submesothelial mesenchymal neoplasm with a combination of hemangiopericytoma-like spindle cells and collagenous fibroma-like collagen bundles[5,6]. According to the third World Health Organization classification of sarcomas, several morphologically similar tumors including extra-meningeal SFT, hemangiopericytoma (HPC), lipomatous HPC, and giant cell angiofibroma were grouped under the umbrella of extra-pleural SFT[4]. To completely review malignant SFTs of the greater omentum, we searched all the subtypes that were classified as SFTs. As a result, we found that besides SFTs, cases were also described in patients with HPC, and the other subtypes of SFTs did not occur in the greater omentum. Since 1963, a total of 13 cases of malignant SFTs of the greater omentum have been reported, and our patient is the fourteenth (Table 1)[2,7-17]. The patients (6 males and 7 females, 1 unknown) had an average age of 55 ± 10.3 years, and the shortest follow-up time was 4 mo. Of these 14 cases, 10 were recorded as HPC, which is a tumor of Zimmermann’s pericyte in capillary walls and post capillary venule walls[18]; it was confirmed as one of the SFTs in 2002, since HPCs do not encompass the origins of all HPC-like tumors[19]. Compared with other reported malignant omental SFT cases, our case is unique. Firstly, the tumor was huge; it was the third largest malignant SFT resected from the greater omentum. Secondly, our case is the first in which satellite lesions were detected during the primary exploratory surgery. Among the reported cases, abdominal carcinomatosis at presentation was reported once by Prakash et al[15], who defined the implants via contrast-enhanced CT and did not perform surgery. Lastly, this is the first case in which gene sequencing was used in the diagnosis.
| Ref. | Age/gender | Chief complaint | Suspected diagnosis | Size | Mitosis/ HPF | Immunohistochemical staining | Pathological diagnosis | Surgical treatment | Chemotherapy | Follow-up | Recurrence and metastasis |
| Forman et al[7], 1952 | 40/M | Abdominal pain, rectal and vaginal pressure | Ovarian cyst | 5 cm | NA | NA | HPC | Tumor resection and partial omentectomy | No | Recurrence and metastasis in 1 year | Rectum invasion after 11 mo |
| Stout et al[8], 1963 | 57/F | NA | NA | 11 cm × 7 cm × 5 cm | 2/50 | NA | HPC | NA | No | NA | NA |
| Stout et al[8], 1963 | 64/M | Pain, swelling of the abdomen | NA | 28 cm × 20 cm × 15 cm | 11/50 | NA | HPC | Tumor resection, omentectomy, transverse colon and the gastrocolic omentum | No | Died of disease | Distant metastasis on lung and liver |
| Imachi et al[9], 1990 | 62/F | Abdominal distension and pain, increasing abdominal girth, diarrhea, and weight loss | Malignant ovarian tumor | 24 cm × 20 cm × 12 cm | 12/10 | Positive for vimentin, negative for S-100 protein, myoglobin, desmin, actin, and factor VIII | HPC | Tumor resection, omentectomy, and hysterectomy | Applied | Recurrence after 1 yr | Implant in the peritoneum and the mesentery were found after 1 yr |
| Schwartz et al[10], 1991 | 40/Unknown | Abdominal pain, abdominal mass, early satiety, weight loss | NA | NA | NA | NA | HPC | Tumor resection | Applied | Tumor spread after 18 mo. Died in 2 mo | Tumor wide spread |
| Cajano et al[11], 1995 | 49/F | Left hypochondrial abdominal pain | NA | 7 cm | NA | NA | HPC | Tumor resection and omentectomy | Applied | Recurrence after 9 mo | Intraperitoneal and liver metastases after 9 mo |
| Ahmad et al[12], 2004 | 74/F | Abdominopelvic mass | Malignant ovarian tumor | NA | NA | NA | HPC | Tumor resection | NA | Died of disease | NA |
| Slupski et al[13], 2007 | 61/M | Left lumbar pain | NA | 5, 1, and 12 cm | NA | NA | HPC | Tumor and liver segment resection, diaphragm clearance | No | Recurrence after 18 yr | Recurrence after 18 yr, 3 metastases were found |
| Salem et al[14], 2008 | 60/M | Intermittent periumbilical pain, weight loss, abdominal distension | NA | 24 cm × 19 cm × 10 cm | 25 | Positive for CD34 and CD99, negative for SMA, desmin, S-100 protein and C-kit | SFT | Tumor resection | No | Uneventful recovery | No |
| Prakash et al[15], 2009 | 45/F | Lower abdominal pain and dysuria | Hemangiopericytoma | 21 cm × 16 cm × 13 cm | NA | NA | HPC | No | Applied | Symptoms decreased | No |
| Rodriguez Tarrega et al[2], 2016 | 34/F | Unremarkable | NA | 6 cm | 13/10 | Positive for CD34, CD99, negative for SMA, desmin, kit and DOG-1 | SFT | Tumor resection and omentectomy | No | Disease-free in 32-mo follow-up | No |
| Vasdeki et al[16], 2018 | 72/M | Recurrent mass of the anterior abdominal wall | NA | 11 cm × 10.4 cm × 10.7 cm, 8 cm × 6.5 cm and 7.5 cm × 5.7 cm | < 4/10 | Positive for vimentin, CD34 and CD99 | HPC | Tumor resection and omentectomy | No | Recurrence twice in 19 yr | 2 lesions in the omentum in 2011, 3 lesions in the omentum in 2018 |
| Jung et al[17], 2019 | 57/M | Asymptomatic | NA | 18 cm × 11 cm × 6.2 cm | 5-6/HPF | Positive for STAT6 and CD34 | SFT | Tumor resection andomentectomy | No | Uneventful recovery | No |
| Current case | Abdominopelvic mass | GIST | 27 cm × 21 cm × 9 cm | 30/10 | Positive for vimentin, weakly positive for DOG-1, CD99, and bcl-2; negative for STAT6, SMA, and CD34 | SFT | Tumor resection, omentectomy, mesentery clearance | No | Died in 1.5 yr | Tumor recurrent and metastasis in 1.5 yr |
SFT is difficult to diagnose. SFT in the abdominal cavity induces various abdominal symptoms, and the most frequent complaints are different degrees of abdominal pain with a palpable abdominal mass, and a sudden alteration of the tumor state, such as rupture, may cause a lethal acute abdomen[20]. On CT, malignant SFTs may present with a cystic area of low attenuation, consistent with necrosis, calcification, and external invasion[21]. Contrast-enhanced CT usually shows a highly vascularized mass. In ultrasonography, the malignancy evaluation is based on the morphological index and Doppler index together with CT findings[22]. Although the internal structures of the mass can be assessed by CT, magnetic resonance imaging, and ultrasonography, the precise origin, type of mass, and benign-malignant nature cannot be ascertained prior to surgery, resulting in a difficult diagnostic scenario. Of the reported cases with malignant SFT of the greater omentum, some were initially diagnosed with GISTs or ovarian tumors, which to an extent affected the treatment strategy. For instance, a patient had a benign SFT mimicking an ovarian tumor and underwent gynecologic surgery instead of gastrointestinal surgery[22]. Therefore, some clinicians recommend preoperative needle aspiration biopsy, which can provide preliminary pathological information.
Although SFTs are generally benign, 12-22% of SFTs are malignant. In 1976, Enzinger and Smith suggested HPC malignancy criteria by analyzing 106 cases[23]. Later in 1989, England et al[24] proposed histological malignant features of malignant fibrous tumors localized in the pleura after reviewing 223 cases. However, the diagnostic criteria of malignant SFT remains controversial. For a conclusive diagnosis, pathologists must assess all aspects of the tumor’s histology. This is why in many reported cases, pathologists have only described the histological features instead of giving a clear diagnosis of benignity or malignancy. Kaneko et al[25] concluded that SFT/HPC tumors ≥ 20 cm in size and a mitotic figure > 4 HPF had a poor prognosis. In the study by Demicco et al[26], besides large size and high mitotic activity, they added age ≥ 55 years as another risk factor for metastasis and death. Zong et al[5] suggested mitotic activity, pleomorphism, cellularity, and tumor size as predictors of SFT behavior, and they also recommended applying risk grade instead of the definitive diagnosis of benignity or malignancy, due to the limited number of cases which is insufficient to draw conclusive criteria. In this case, the external phase, histological morphology, and invasiveness resulted in the diagnosis of a malignant entity.
Immunohistochemical staining is another useful method for establishing diagnosis. SFT is generally positive for CD34, bcl2, STAT6, CD99, and vimentin[17]. Among these markers, CD34 and bcl2 are more frequently used. It is reported that 82%-95% and 88%-100% of SFTs are positive for CD34 and bcl2, respectively. However, the markers seem ineffective in predicting tumor behavior. The resected specimen from this patient was positive for vimentin and weakly positive for bcl2, CD99, and DOG-1. Remarkably, it was negative for CD34 and STAT6, which made it difficult to differentiate between SFTs and stromal tumors such as GISTs. Approximately 94%-98% of GISTs are positive for CD117, which is rarely expressed in other abdominal tumors. In addition, oncogenic mutation in c-KIT can occur in 80%-85% of GISTs. In this case, the patient was CD117-negative and had no c-KIT gene mutation. Therefore, malignant SFT was the more probable diagnosis[27]. This is the first reported case of a malignant SFT of the greater omentum, diagnosed by a combination of immunohistochemical staining and DNA sequencing, based on the negativity of the typical markers, CD34 and STAT6. Along with the comprehensive knowledge of genetic information acquired by clinicians, more genetic loci could be discovered as significant markers of diagnosis and prognosis of SFTs. Moreover, genetic targets of SFTs might be identified, which would alter the current treatment strategy. Therefore, gene sequencing might be more practical, helpful, and frequently used in the future.
Metastasis and recurrence are both typical features of malignant SFT. It mainly metastasizes via hematogenous and lymphogenous routes[13]. In 2009, Prakash et al[15] first reported peritoneal carcinomatosis caused by SFT tumor implants at the time of presentation, which is a rare metastasis route and is mostly observed in recurrent SFTs[13,25]. Several extra-pleural SFT cases with metastases after surgical excision in the greater omentum have been reported; 8 of the 14 cases reported in the literature involved metastasis or recurrence, and recurrence was observed even as long as 18 years after remission, or several times after resection[13,16]. Hence, long-term follow-up is necessary for patients who have SFT with malignant potential. Patients affected by recurrence within 2 years had a poor prognosis, and three-quarters of the cases died 2-15 mo after diagnosis of recurrence. In our case, omental and mesentery implants were confirmed during surgery. Such extensive metastases are extremely rare in SFT and indicate aggressive malignancy, which led to recurrence and death after 18 mo. As a result, close follow-up is required for the remainder of the patient's life.
Surgical excision is the only definite treatment for SFT[17]. Despite complete resections, local recurrences of SFT have been reported[13]. Considering the malignant and recurring potential of SFT, mass excision with a tumor-negative margin is recommended[28]. The greater omentum is a supportive vascular fatty fold, which protects against tumor growth and infection[6], and the decision to perform omentectomy with tumor resection is debatable. In reported omental malignant SFT cases, 8 patients underwent total or partial omentectomy. Of these 8 patients, 6 had recurrence. The high recurrence rate among the patients who underwent omentectomy explains why surgeons such as Shiba et al[3] questioned the significance of omental resection. Post-operative systematic treatment, adjuvant chemotherapy and radiotherapy were performed in several malignant cases[29]. However, routine usage of adjuvant therapies is questioned by clinicians due to little evidence supporting improvement in aggressive tumors with adjuvant chemotherapy[17,30]. Only 3 patients received adjuvant chemotherapy; all 3 patients had poor outcomes caused by recurrence and metastasis (Table 1). Moreover, the Tumor-Node-Metastasis staging system is not applicable to SFT, resulting in the lack of a standard guidance for the systematic treatment of malignant SFTs. Gene sequencing might be a potential solution for the current dilemma. It may provide evidence in order to decide which chemotherapy approach is more beneficial.
In managing this patient, our main limitation was the unavailability of sufficient knowledge on pre-operative diagnosis and post-operative treatment. In the treatment of our patient, omentectomy and tumorectomy with nodule clearance were provisional intraoperative treatment decisions due to the diffuse tumor implants. The limited knowledge on the pathology, origin, and metastasis status impeded the surgical plan, which resulted in an exploratory surgery instead of a specific procedure; therefore, there is an urgent requirement for accurate specific diagnostic work-up. In addition, the present systemic therapy lacks convincing clinical evidence and requires further confirmation before application in SFT patients with metastasis. Lastly, as a rare disease with a consistently low number of reports, cases of malignant SFTs of the greater omentum were not documented in a consistent manner; instead, there were a lot of variations in the completeness of the information contained in the reports. Such inconsistency does not allow a better understanding of the disease. For improvement, the records may be taken into a database of a centralized tumor registry, which is able to provide more comprehensive and consistent data for further research.
In this study, we report a rare case of a malignant SFT of the greater omentum, which was diagnosed via immunohistochemical staining and gene sequencing. Although the patient underwent tumorectomy and clearance, the patient died due to recurrence after 18 mo. In this case, gene sequencing was used to diagnose the SFT when the results of immunohistochemical staining were not able to distinguish between an SFT and GIST. In addition, more comprehensive records are necessary when reporting such a rare malignancy, which is able to stimulate the establishment of more effective management guidelines for omental malignant SFT cases and improve outcomes.
The authors thank Yang XG, who provided most of the immunohistochemical figures.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Verran DJ S-Editor: Gao CC L-Editor: Webster JR P-Editor: Xing YX
| 1. | Klemperer P, Coleman BR. Primary neoplasms of the pleura. A report of five cases. Am J Ind Med. 1992;22:1-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 195] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Rodriguez Tarrega E, Hidalgo Mora JJ, Paya Amate V, Vega Oomen O. Solitary fibrous tumor of the greater omentum mimicking an ovarian tumor in a young woman. Gynecol Oncol Rep. 2016;17:16-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Shiba H, Misawa T, Kobayashi S, Yokota T, Son K, Yanaga K. Hemangiopericytoma of the greater omentum. J Gastrointest Surg. 2007;11:549-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Pasquali S, Gronchi A, Strauss D, Bonvalot S, Jeys L, Stacchiotti S, Hayes A, Honore C, Collini P, Renne SL, Alexander N, Grimer RJ, Callegaro D, Sumathi VP, Gourevitch D, Desai A. Resectable extra-pleural and extra-meningeal solitary fibrous tumours: A multi-centre prognostic study. Eur J Surg Oncol. 2016;42:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Zong L, Chen P, Wang GY, Zhu QS. Giant solitary fibrous tumor arising from greater omentum. World J Gastroenterol. 2012;18:6515-6520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Cazejust J, Wendum D, Bourrier A, Chafai N, Menu Y. Solitary fibrous tumor of the greater omentum. Diagn Interv Imaging. 2015;96:959-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | FORMAN I, CAMPBELL WN. Hemangiopericytoma; an unusual pelvic tumor. Am J Obstet Gynecol. 1952;63:929-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | STOUT AP, HENDRY J, PURDIE FJ. Primary solid tumors of the great omentum. Cancer. 1963;16:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Imachi M, Tsukamoto N, Tsukimori K, Funakoshi K, Nakano H, Shigematsu T, Tanimura A. Malignant hemangiopericytoma of the omentum presenting as an ovarian tumor. Gynecol Oncol. 1990;39:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Schwartz RW, Reames M, McGrath PC, Letton RW, Appleby G, Kenady DE. Primary solid neoplasms of the greater omentum. Surgery. 1991;109:543-549. [PubMed] |
| 11. | Cajano P, Heys SD, Eremin O. Haemangiopericytoma of the greater omentum. Eur J Surg Oncol. 1995;21:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Ahmad GF, Athavale R, Hamid BN, Davies-Humphreys J. Pelvic malignant hemangiopericytoma mimicking an ovarian neoplasm; a case report. J Reprod Med. 2004;49:404-407. [PubMed] |
| 13. | Slupski M, Piotrowiak I, Wlodarczyk Z. Local recurrence and distant metastases 18 years after resection of the greater omentum hemangiopericytoma. World J Surg Oncol. 2007;5:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Salem AM, Bateson PB, Madden MM. Large solitary fibrous tumor arising from the omentum. Saudi Med J. 2008;29:617-618. [PubMed] |
| 15. | Prakash M, Mumtaz HA, Sodhi KS, Kapoor R, Khandelwal N. Hemangiopericytoma: an unusual cause of peritoneal carcinomatosis. Cancer Imaging. 2009;9:32-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Vasdeki D, Bompou E, Diamantis A, Anagnostou A, Tepetes K, Efthimiou M. Haemangiopericytoma of the greater omentum: a rare tumour requiring long-term follow-up. J Surg Case Rep. 2018;2018:rjy087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Jung CY, Bae JM. Primary omental malignant solitary fibrous tumour, an extremely rare malignancy: A case report and review of the literature. Arab J Gastroenterol. 2019;20:114-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 18. | Nickerson TP, Fahy AS, Bingener J. Laparoscopic resection of intra-abdominal metastasis from intracranial hemangiopericytoma. Int J Surg Case Rep. 2015;15:50-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology. 2006;48:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 400] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 20. | Küçük HF, Gülmez S, Kaptanoğlu L, Akyol H, Kurt N, Yavuzer D. Acute abdomen due to rupture of hemangiopericytoma of the greater omentum: case report. Ulus Travma Acil Cerrahi Derg. 2009;15:611-613. [PubMed] |
| 21. | Crusco F, Chiodi M, Pugliese F, Mosca S, Fischer MJ, Lupattelli L. Benign omental hemangiopericytoma presenting with hemoperitoneum: radiologic findings. AJR Am J Roentgenol. 2005;184:S67-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Moszynski R, Szubert S, Tomczak D, Saad A, Samulak D, Sajdak S, Szpurek D. Solitary fibrous mass of the omentum mimicking an ovarian tumor: case report. Eur J Gynaecol Oncol. 2016;37:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Enzinger FM, Smith BH. Hemangiopericytoma. An analysis of 106 cases. Hum Pathol. 1976;7:61-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 557] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 24. | England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 953] [Cited by in RCA: 845] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 25. | Kaneko K, Shirai Y, Wakai T, Hasegawa G, Kaneko I, Hatakeyama K. Hemangiopericytoma arising in the greater omentum: report of a case. Surg Today. 2003;33:722-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE, Lazar AJ, Wang WL. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 361] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 27. | Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A, Hirota S, Zheng J, Zhang B, Tzen CY, Yeh CN, Nishida T, Shen L, Chen LT, Kang YK. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat. 2016;48:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22:1501-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 436] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 29. | van Houdt WJ, Westerveld CM, Vrijenhoek JE, van Gorp J, van Coevorden F, Verhoef C, van Dalen T. Prognosis of solitary fibrous tumors: a multicenter study. Ann Surg Oncol. 2013;20:4090-4095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Bovino A, Basso L, Di Giacomo G, Codacci Pisanelli M, Basile U, De Toma G. Haemangiopericytoma of greater omentum. A rare cause of acute abdominal pain. J Exp Clin Cancer Res. 2003;22:649-650. [PubMed] |