Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5280
Peer-review started: February 9, 2021
First decision: February 28, 2021
Revised: March 11, 2021
Accepted: April 25, 2021
Article in press: April 25, 2021
Published online: July 6, 2021
Processing time: 134 Days and 21.3 Hours
Glycogen storage disease type Ib (GSD-Ib) is a glycogen metabolism disorder that leads to the manifestations of inflammatory bowel disease (IBD), especially Crohn’s disease (CD)-like colitis. Although biological agents are effective for treating CD, their application in the treatment of GSD-Ib with CD-like colitis has been rarely reported.
A 13-year-old Han male was diagnosed with GSD-Ib with CD. The patient was treated with granulocyte colony-stimulating factor. When he had symptoms of CD-like colitis, he was continuously pumped with enteral nutrition and administered oral mesalazine for 2 wk; however, the symptoms did not improve significantly. Hence, infliximab (IFX) was administered. Hitherto, the patient has been followed up for 1 year, and no clinical manifestations have been observed. After 6 mo of treatment (fifth IFX treatment), the disease activity index and all inflammatory indexes decreased, and a review of the colonoscopy data showed that the ulcers appeared smooth.
In this study, the patient was successfully treated with IFX. In cases of GSD-Ib, IBD should be highly considered.
Core Tip: Conventional treatment cannot alleviate symptoms of intestinal inflammation in glycogen storage disease type Ib (GSD-Ib). Although biological agents are effective for treating Crohn’s disease (CD), their application in the treatment of GSD-Ib with CD has been rarely reported. Infliximab was selected for this patient, and the intestinal symptoms were successfully alleviated. For cases with poor outcome using the granulocyte colony-stimulating factor treatment, infliximab can be used for therapy.
- Citation: Gong YZ, Zhong XM, Zou JZ. Infliximab treatment of glycogenosis Ib with Crohn's-like enterocolitis: A case report. World J Clin Cases 2021; 9(19): 5280-5286
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5280.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5280
Glycogen storage disease type Ib (GSD- Ib) is a glycogen metabolism disorder caused by mutation in the SLC37A4 gene that encodes glucose-6-phosphotransferase. As a result, glucose-6-phosphate cannot be transported into the microsome and be decomposed into glucose[1]. The clinical manifestations include abnormal glucose metabolism, which leads to fasting hypoglycemia, liver enlargement, growth retardation, count reduction, and granulocyte dysfunction.
The count reduction and granulocyte dysfunction induce intestinal mucosal ulcers, which lead to the manifestations of inflammatory bowel disease (IBD)[2], especially Crohn’s disease (CD-like colitis). The manifestations include abdominal pain, diarrhea, vomiting, growth retardation, poor nutrient absorption, repeated perianal abscess, as well as multiple ulcers in the intestinal mucosa. Several studies revealed that 77% of GSD-Ib disease cases could be concurrent with IBD-like manifestations[2]. GSD-Ib can be effectively treated by the regular administration of granulocyte colony-stimulating factor (G-CSF). Mesalazine can also relieve intestinal inflammation. However, conventional treatment cannot alleviate symptoms of intestinal inflammation[3]. Although biological agents are effective for treating CD, their application in the treatment of GSD-Ib with CD or CD-like colitis has been rarely reported.
Herein, a case of GSD-Ib combined with CD-like enterocolitis who received ineffective G-CSF treatment is reported. Owing to its demonstrated efficacy for the treatment of moderate to severe CD, infliximab (IFX) was selected, as it can suc-cessfully alleviate intestinal symptoms.
A 13-year-old Han male was admitted for intermittent abdominal pain and defecation for 1.5 years and aggravation with vomiting for half a month.
One and a half years ago, the patient presented tolerable upper abdominal pain without an obvious cause, with loose stool three to four times/d. In the past half month, the abdominal pain worsened, which affected eating; the patient vomited after eating. He did not have fever, hematochezia, articular pain, or other symptoms. The patient was injected with amoxicillin [50 mg/(kg.d), i.e. 1500 mg/d, three times a day for 7 d] intravenously for anti-infection, which was ineffective. The patient lost 2 kg since the onset of the condition.
At the age of 4 years, the patient was admitted to our hospital due to large liver and spleen, short stature, and repeated hypoglycemia. Finally, he was diagnosed with glycogen storage disease type Ib [SLC37A4 gene c.572C > T (p.T191L) (maternal) and c.359C > T (p.P120L) (parental) compound heterozygous mutation]. He was given raw corn starch regularly (2 g/kg, once every 4-6 h) and G-CSF (5 μg /kg each time) when he repeatedly showed low neutrophil counts (minimum 0.5 × 109/L) and respiratory infections that were not treated regularly. He suffered from recurrent oral ulcers in the past 5 years. In addition, recurrent perianal abscesses appeared in the past 4 years that underwent surgical drainage each time.
The birth history and feeding history were uneventful. There was no history of similar illness in the family.
Height: 138 cm (< P3), weight 32.3 kg (< P3), multiple ulcers in the oral mucosa, tenderness in the upper abdomen, and soft liver with dull edge at 2 cm below the ribs.
After admission, examination showed neutrophils of 0.71 × 109/L, and the patient was treated with G-CSF [5 μg/(kg.d) i.e. 160 mg/d]. The other data were as follows: Hemoglobin: 107 g/L (120-160 g/L), uric acid: 581 μmol/L (164-376 μmol/L), triglyceride: 2.3 mmol/L (0-1.69 mmol/L), normal blood sugar, erythrocyte sedimentation rate: 104 (normal: < 15) mm/h, C-reactive protein: 61 (normal: < 8) mg/L, cytokines of tumor necrosis factor (TNF)-alpha: 204 pg/mL (0-8.1 pg/mL), IL-6: 17.2 pg/mL (0-3.4 pg/mL), and normal interleukin (IL)-2R, IL-8, and IL-10. In addition, stool routine, stool parasites, and stool Clostridium difficile toxin were all negative. Also, the results of T cell spot test (T-spot) and purified protein derivative test were negative.
Chest computed tomography showed normal results, while abdominal computed tomography revealed hepatosplenomegaly and fatty liver.
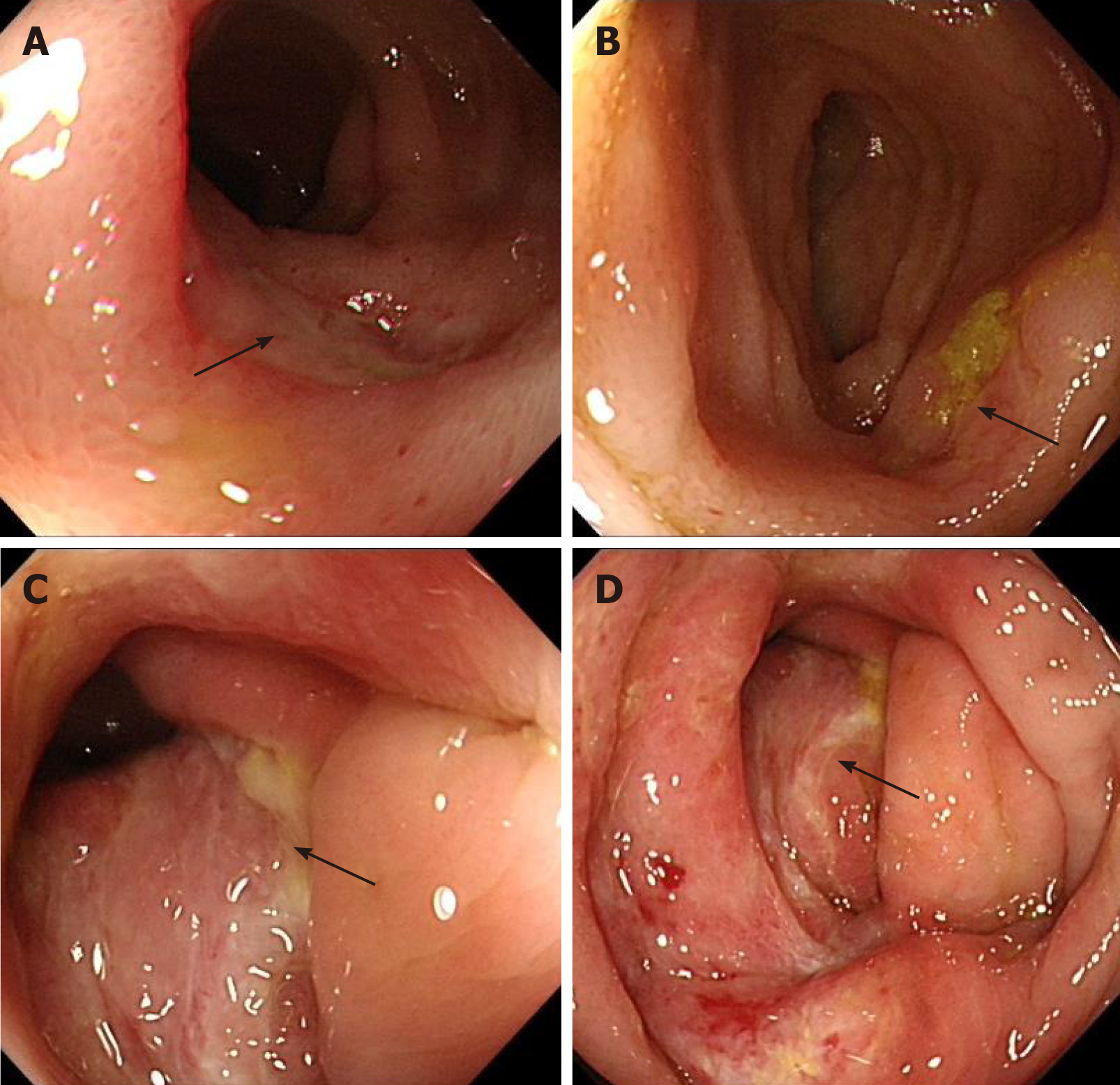
Esophagogastroduodenoscopy detected chronic gastritis and bile reflux, while colonoscopy showed three deep cyclic ulcers in the terminal ileum, with a maximum circumference of 1/4 (of intestinal circumference), covered with white fur. Moreover, the ileocecal valve was deformed and narrow, and deep ulcers, about 1 cm × 0.5 cm, were visible along the tissue; several large cyclic deep ulcers were visible in the ascending colon, with a maximum circumference of 1/2 (of intestinal circumference). Moreover, the ulcers were fused with each other, and several deep ulcers were scattered in the descending colon and rectum (Figure 1). The pathology of antral mucosa showed chronic inflammation.
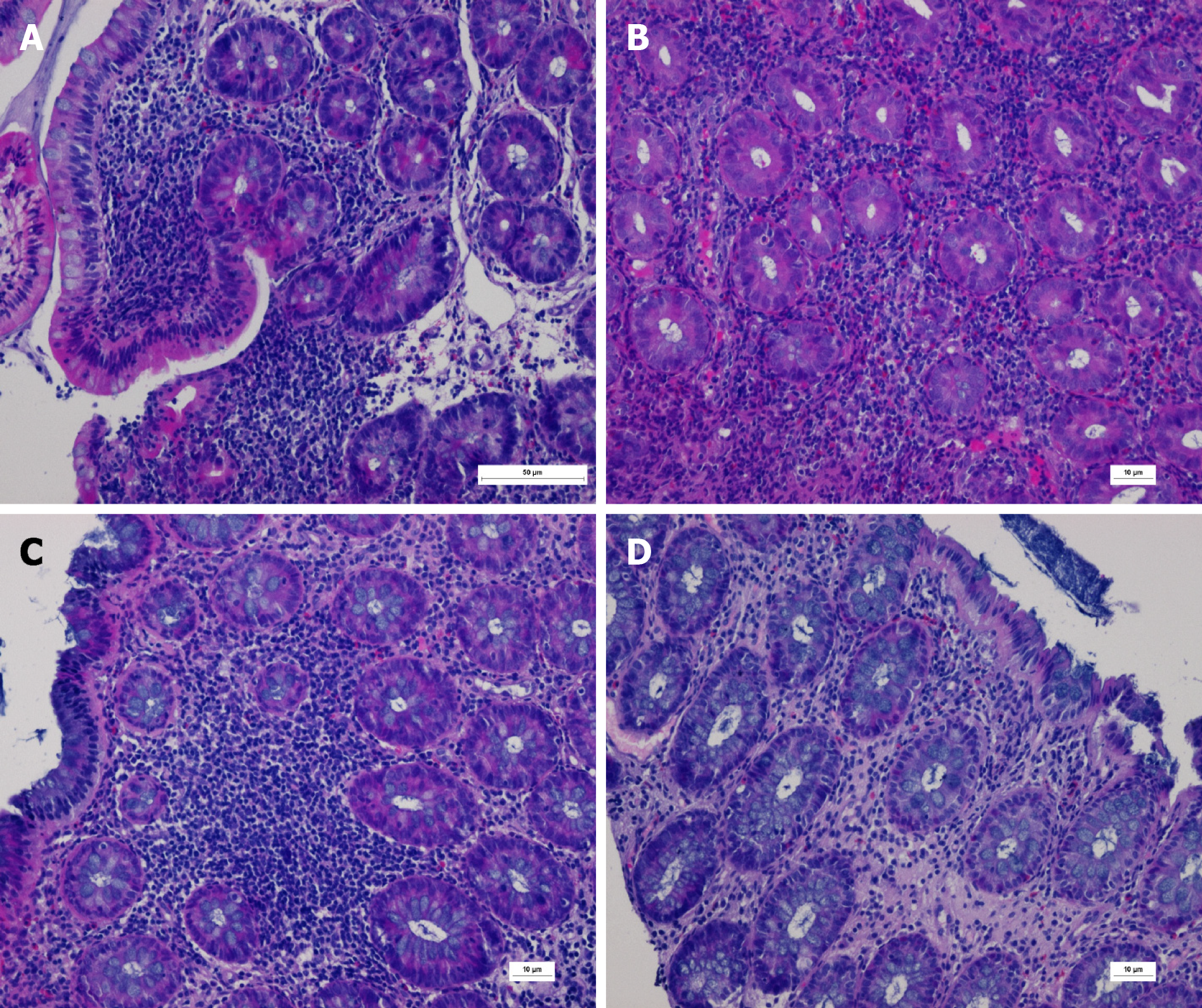
The pathology of the terminal ileum, ileocecal region, ascending colon, descending colon, and rectum prompted infiltration of diffuse inflammatory cells and scattered eosinophils with visible ulcer formation. Periodic acid-Schiff staining, immunohistochemical cytomegalovirus (CMV) staining, and Epstein-Barr virus encoded ribonucleic acid in situ hybridization were negative (Figure 2).
Capsule endoscopy showed several cyclic small ulcers in the terminal ileum.
Finally, the patient was diagnosed with glycogen storage disease type Ib with CD-like enterocolitis.
The patient was continuously pumped with enteral nutrition (Ensure, Abbott, Chicago, IL, United States) and administered mesalazine (Etiasa, 500 mg, three times per day, Ethypharm Pharmaceutical Co., Ltd, Saint-Cloud, France) orally for 2 wk; however, the symptoms did not improve significantly. Although the neutrophil count was normal, the gastrointestinal symptoms did not improve. Owing to the early onset of the symptoms, extensive lesions were detected in the small intestine and colon, with repeated perianal abscesses. Since IFX is effective in the treatment of CD, it was used at the initial dose of 5 mg/kg each time (actually 200 mg each time, administered at the 2nd and 6th wk after the first administration, and every 8 wk thereafter), while G-CSF, mesalazine, and enteral nutrition were applied continually.
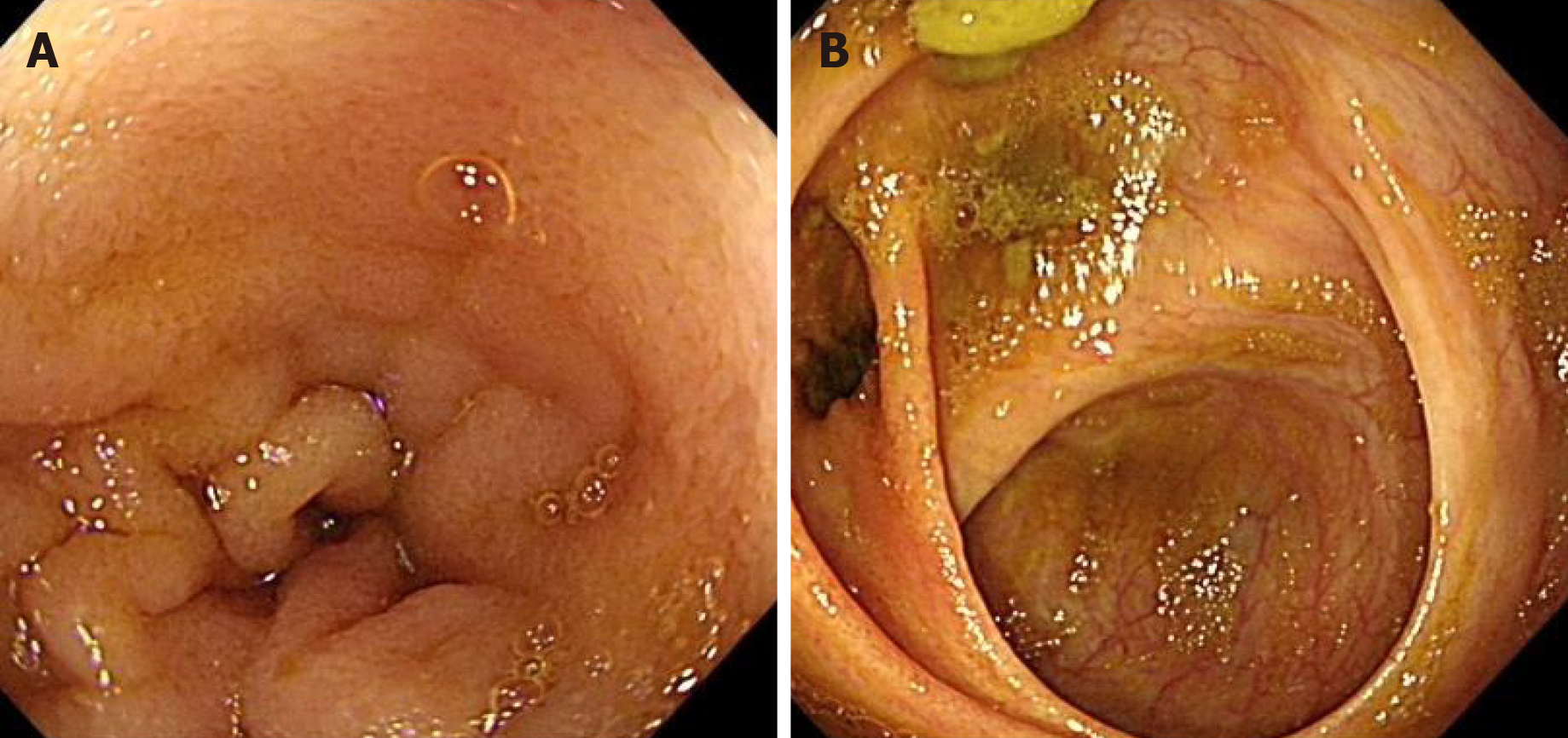
After the first administration of IFX (200 mg) for 1 wk, the abdominal pain and vomiting were significantly relieved, and the stool with one to two times per day was regular as compared to that before administration of the drug. After 6 mo of treatment (the 5th IFX treatment), the Pediatric Crohn’s disease activity index decreased to 5 points (Table 1), and all the inflammatory indexes decreased (Table 1). A review of the colonoscopy data showed that the ulcers in the terminal ileum and colon had healed, and the intestinal mucosa was smooth (Figure 3). The weight and height of the child increased significantly as compared to that before the treatment (Table 1).
| Indicator | Before treatment | 1 mo after treatment | 6 mo after treatment | 10 mo after treatment |
| PCDAI (points) | 47.5 | 12.5 | 5 | 5 |
| CRP (mg/L) | 61 | 1.2 | 2 | 3 |
| ESR (mm/h) | 104 | 93 | 44 | 28 |
| Height (cm) | 138 | 139 | 144 | 145 |
| Weight (kg) | 32.3 | 33.5 | 39.5 | 40 |
| Colonoscopy | Ulcers | Healed |
Hitherto, the patient has been followed up for 1 year, and no clinical manifestations, such as abdominal pain, diarrhea, vomiting, and perianal abscess, have been observed. During the treatment with IFX, no adverse reactions, such as infections and allergies, were noted. Also, the etiological indicators, such as T cell spot test, Epstein-Barr virus, and CMV, were negative, while liver and kidney functions were normal.
GSD-Ib results in quantitative or qualitative neutrophil dysfunction and is associated with an intestinal phenotype resembling CD[4]; these patients have a prolonged GSD-Ib, and the few neutrophils are dysfunctional. The bowel inflammation could result from chronic low infection of the gut mucosa[5]. Herein, a case with GSD-Ib that was not administered G-CSF, presented low neutrophil count and had CD-like manifestations at 9 years of age. The patient was first treated with traditional G-CSF and mesalazine, but the outcome was not satisfactory. Since the child exhibited typical CD-like manifestations, we selected IFX and achieved satisfactory results. Nonetheless, the drug has been rarely reported for the treatment of GSD-Ib with CD or CD-like colitis.
G-CSF has been used to treat neutropenia and colitis in some patients with GSD-Ib. Some studies demonstrated that the regular use of G-CSF could prevent or delay IBD while preventing infection[6]. In the present case, although the neutrophil count was normal after treatment with G-CSF, the clinical symptoms were not improved. This might be because despite the increase in neutrophil counts, G-CSF does not correct the neutrophil function[7]. Dysfunctional glucose-6-phosphatase-deficient neutrophils are less effective in dealing with inflammatory processes.
The primary mechanism of action of IFX is binding to TNF to inhibit its binding to TNF receptors and block its biological activity, thereby achieving the anti-inflammatory effects[8]. The early use of anti-TNF inhibitors in patients with moderate to severe CD might improve its efficacy and prevent penetrating complications of the disease[9]. IFX is especially useful in patients with severe perianal disease[10]. In the present study, the patient had severe Pediatric Crohn’s disease activity index score[11], combined with repeated perianal abscesses, which is a major indication for the application of IFX. Hence, the drug was selected after the traditional treatment was ineffective. After the first application of IFX, the patient’s gastrointestinal symptoms, such as abdominal pain and vomiting, were significantly improved, the nutritional status was gradually improved, and the level of inflammation indicators, such as C-reactive protein and erythrocyte sedimentation rate, was decreased significantly as compared to that before the treatment. A review of colonoscopy showed that the intestinal mucosal ulcers were healed. Only a few studies reported the use of biological agents for the treatment of GSD-Ib combined with CD or CD-like manifestations. Davis et al[12] reported a case of GSD-Ib with CD treated with adalimumab because of allergy to IFX.
Moreover, the adverse effects of IFX, especially infections and infusion reactions (anaphylaxis, fever, nausea, vomiting, convulsion, and rash), need to be evaluated. Hosoi et al[13] reported that 18.2% (31/181) of patients had infusion reactions. de Bie et al[14] reported that 3.3% of patients had severe or unusual infections, and one sepsis-related death occurred in a pediatric CD patient. The patient in this study did not experience any adverse reactions during the infusion of IFX; however, long-term monitoring is essential.
Furthermore, it is important to evaluate the incidence of non-responders. de Bie et al[14] reported approximately 30% of primary and secondary non-responders among Japanese pediatric CD patients. In the current study, we monitored the blood concentration of IFX (> 1.0 mg/mL) and the serum concentration of anti-IFX (< 30 ng/mL) once every 6 mo during the treatment. Hence, we were able to adjust the medication dose according to the drug concentration. For children with GSD-Ib, concurrent infections, such as tuberculosis, Epstein-Barr virus, and CMV, should be a focus during treatment with IFX as well as monitoring side effects.
In conclusion, the patient was successfully treated with IFX, as the drug relieved intestinal inflammation, promoted mucosal healing, and improved clinical symptoms. However, this is only a case report. For cases with poor outcome using the G-CSF treatment, IFX can be considered a first line of treatment. However, some children may present a loss of drug response, which affects the treatment effect. Therefore, the long-term efficacy should be monitored. Moreover, we can try to use immune checkpoint inhibitors such as ipilimumab[15] to improve enterocolitis.
In the case of children with GSD-Ib, attention should be given to monitoring symptoms, such as abdominal pain, diarrhea, hematochezia, weight loss, repeated oral ulcers, and perianal lesions, and for those presenting repeated neutropenia, IBD should be monitored vigilantly. Although the patient with GSD-Ib and CD-like was successfully treated with infliximab, more patients need to be studied to confirm that IFX could help patients with GSD-Ib.
The authors are grateful to the patient and his parents for allowing publication of this case report.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chuang WL, Kermenli T S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LL
| 1. | Gerin I, Veiga-da-Cunha M, Achouri Y, Collet JF, Van Schaftingen E. Sequence of a putative glucose 6-phosphate translocase, mutated in glycogen storage disease type Ib. FEBS Lett. 1997;419:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 147] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Visser G, Rake JP, Fernandes J, Labrune P, Leonard JV, Moses S, Ullrich K, Smit GP. Neutropenia, neutrophil dysfunction, and inflammatory bowel disease in glycogen storage disease type Ib: results of the European Study on Glycogen Storage Disease type I. J Pediatr. 2000;137:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Volz MS, Nassir M, Treese C, von Winterfeld M, Plöckinger U, Epple HJ, Siegmund B. Inflammatory bowel disease (IBD)-like disease in a case of a 33-year old man with glycogenosis 1b. BMC Gastroenterol. 2015;15:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Couper R, Kapelushnik J, Griffiths AM. Neutrophil dysfunction in glycogen storage disease Ib: association with Crohn's-like colitis. Gastroenterology. 1991;100:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Roe TF, Coates TD, Thomas DW, Miller JH, Gilsanz V. Brief report: treatment of chronic inflammatory bowel disease in glycogen storage disease type Ib with colony-stimulating factors. N Engl J Med. 1992;326:1666-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Dieckgraefe BK, Korzenik JR, Husain A, Dieruf L. Association of glycogen storage disease 1b and Crohn disease: results of a North American survey. Eur J Pediatr. 2002;161 Suppl 1:S88-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Dale DC, Bolyard AA, Marrero T, Kelley ML, Makaryan V, Tran E, Leung J, Boxer LA, Kishnani PS, Austin S, Wanner C, Ferrecchia IA, Khalaf D, Maze D, Kurtzberg J, Zeidler C, Welte K, Weinstein DA. Neutropenia in glycogen storage disease Ib: outcomes for patients treated with granulocyte colony-stimulating factor. Curr Opin Hematol. 2019;26:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Liang S, Dai J, Hou S, Su L, Zhang D, Guo H, Hu S, Wang H, Rao Z, Guo Y, Lou Z. Structural basis for treating tumor necrosis factor α (TNFα)-associated diseases with the therapeutic antibody infliximab. J Biol Chem. 2013;288:13799-13807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Zimmerman L, Bousvaros A. The pharmacotherapeutic management of pediatric Crohn's disease. Expert Opin Pharmacother. 2019;20:2161-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | de Zoeten EF, Pasternak BA, Mattei P, Kramer RE, Kader HA. Diagnosis and treatment of perianal Crohn disease: NASPGHAN clinical report and consensus statement. J Pediatr Gastroenterol Nutr. 2013;57:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Leach ST, Nahidi L, Tilakaratne S, Day AS, Lemberg DA. Development and assessment of a modified Pediatric Crohn Disease Activity Index. J Pediatr Gastroenterol Nutr. 2010;51:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Davis MK, Rufo PA, Polyak SF, Weinstein DA. Adalimumab for the treatment of Crohn-like colitis and enteritis in glycogen storage disease type Ib. J Inherit Metab Dis. 2008;31 Suppl 3:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Hosoi K, Ohtsuka Y, Fujii T, Kudo T, Matsunaga N, Tomomasa T, Tajiri H, Kunisaki R, Ishige T, Yamada H, Arai K, Yoden A, Ushijima K, Aomatsu T, Nagata S, Uchida K, Takeuchi K, Shimizu T. Treatment with infliximab for pediatric Crohn's disease: Nationwide survey of Japan. J Gastroenterol Hepatol. 2017;32:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | de Bie CI, Escher JC, de Ridder L. Antitumor necrosis factor treatment for pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:985-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275-5283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |