Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5238
Peer-review started: January 7, 2021
First decision: February 11, 2021
Revised: February 15, 2021
Accepted: March 8, 2021
Article in press: March 8, 2021
Published online: July 6, 2021
Processing time: 167 Days and 16.3 Hours
Adenoid cystic carcinoma (ACC) is a common malignant tumor of salivary gland. The lung and liver are frequent sites of distant metastasis. Liver metastasis as the initial clinical manifestation of sublingual gland ACC is very rare.
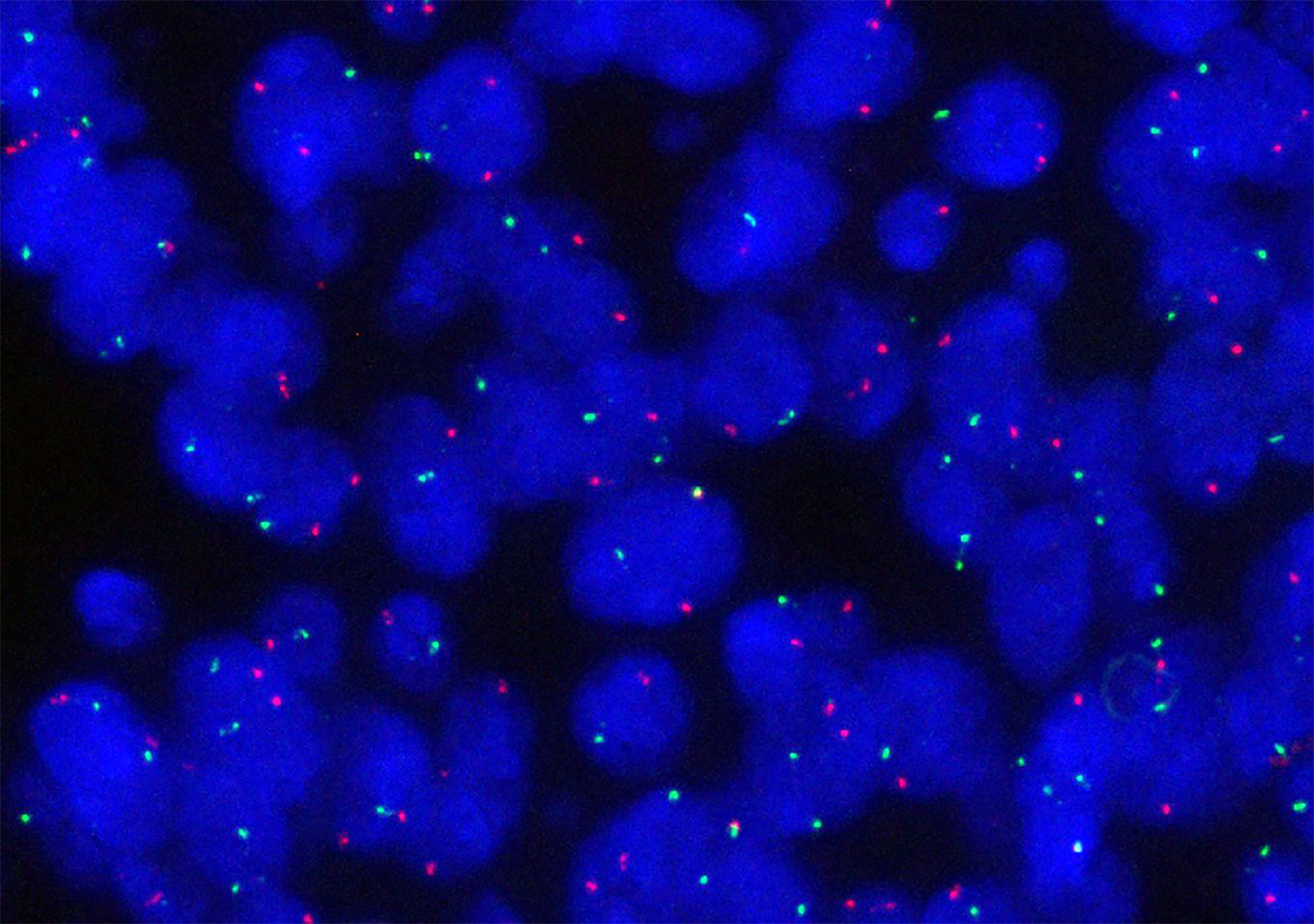
A 51-year-old Chinese woman presented with a painless mass in the right lobe of liver. The tumor was composed of ductal cells and myoepithelial cells with a morphology including tubiform and cribriform structures. Immunostaining results showed ductal cells positive for CK7, CK14, CK19, CD117, and 34βE12, and negative for MYB, vimentin, ER, PR, and CEA. The myoepithelial cells were positive for p63, calponin and CK5/6. Metastatic salivary ACC was considered, and a sublingual gland mass was revealed by computed tomography. Histological evaluation confirmed primary sublingual gland ACC. Fluorescence in situ hybridization (FISH) did not find an MYB-NFIB fusion gene in specimens from either the primary or metastatic ACC tumors. The sublingual gland ACC relapsed in 20 mo. The recurrent lesion disappeared following local radiation therapy and computed tomography-guided radioactive seed implantation. The patient remains in good condition until now.
Metastatic sublingual gland ACC with initial clinical manifestation as a liver mass is very rare, and was pathologically confirmed in this patient by its histological appearance. Primary hepatic tumors and metastatic carcinomas should be included in the differential diagnosis. Immunohistochemical detection of MYB protein and MYB-NFIB fusion gene detection by FISH can be helpful, but occasional negative results confuse the diagnosis.
Core Tip: This is a report of a very rare case of liver metastasis as the initial clinical manifestation of sublingual gland adenoid cystic carcinoma (ACC). It has not been described in the English literature until now. We describe this case in detail, with a review of the clinical and pathological characteristics, differential diagnosis, and therapy of metastatic salivary gland ACC. Three previous reports and our case confirm that an isolated liver metastasis can be the primary presentation of salivary gland ACC. An MYB-NFIB fusion gene was not detected by fluorescence in situ hybridization in either the primary or metastatic ACC tumor.
- Citation: Li XH, Zhang YT, Feng H. Liver metastasis as the initial clinical manifestation of sublingual gland adenoid cystic carcinoma: A case report. World J Clin Cases 2021; 9(19): 5238-5244
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5238.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5238
Adenoid cystic carcinoma (ACC) is a common malignant tumor of the salivary gland and often involves the parotid, submaxillary, and minor salivary glands. ACC can develop at any age, but usually occurs in middle and old age, with no marked difference in sex distribution. ACC involves ductal cells and myoepithelial cells and includes tubiform, cribriform, and solid histological types. The involvement of nerves that results in pain is a predominant characteristic of ACC[1]. The most common sites of distant metastasis are the lung and liver, but salivary gland ACC metastasis has been reported in bone and the brain. We report a rare case that presented with liver metastasis as the initial clinical manifestation of sublingual gland ACC. We believe that this is only the fourth case report in the literature.
A 51-year-old Chinese woman presented in September 2016 with a painless 4.1 cm mass in the right lobe of liver during a routine radiological health examination.
A painless mass with diameter of 4.1 cm was found in the right lobe of the liver during a routine radiological health examination.
The patient had a clear medical history.
Physical examination revealed no positive signs.
Laboratory evaluation found normal blood serum levels of tumor biomarkers, including alpha fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen (CA)199.
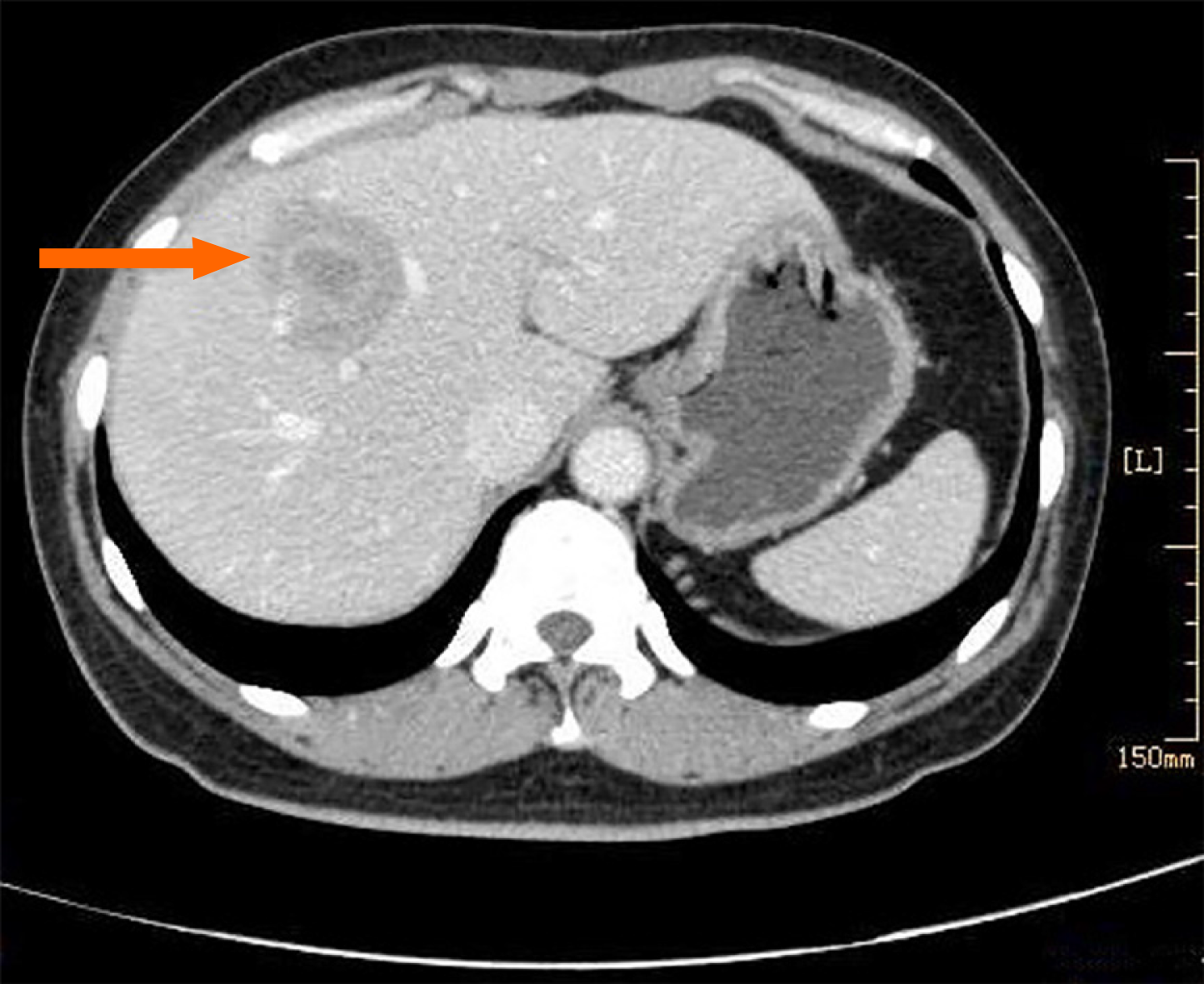
Ultrasound examination showed a 3.8 cm × 3.6 cm low echo-area with a distinct boundary in the right anterior lobe of liver. A bloodstream signal was found during color doppler flow imaging. Computed tomography with enhancement revealed a low-density mass shadow with a distinct boundary and a diameter of 4.1 cm in the anterior and superior segment of the right lobe of liver (Figure 1). The mass had heterogeneous enhancement with ring enhancement in the central area. The right hepatic artery passed through it. The right anterior branch of portal vein was adjacent to the mass, and the distal intrahepatic bile duct was expanded.
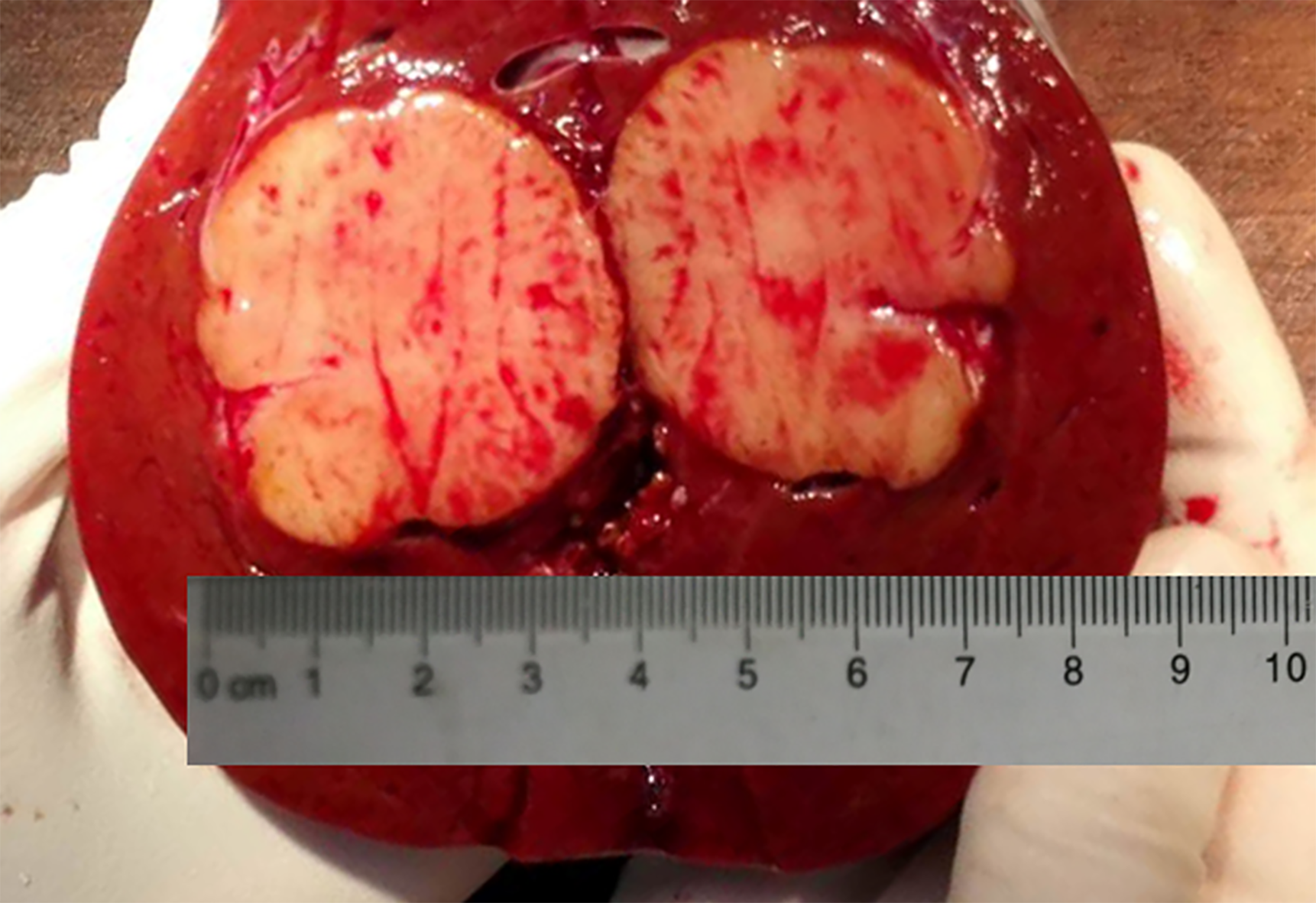
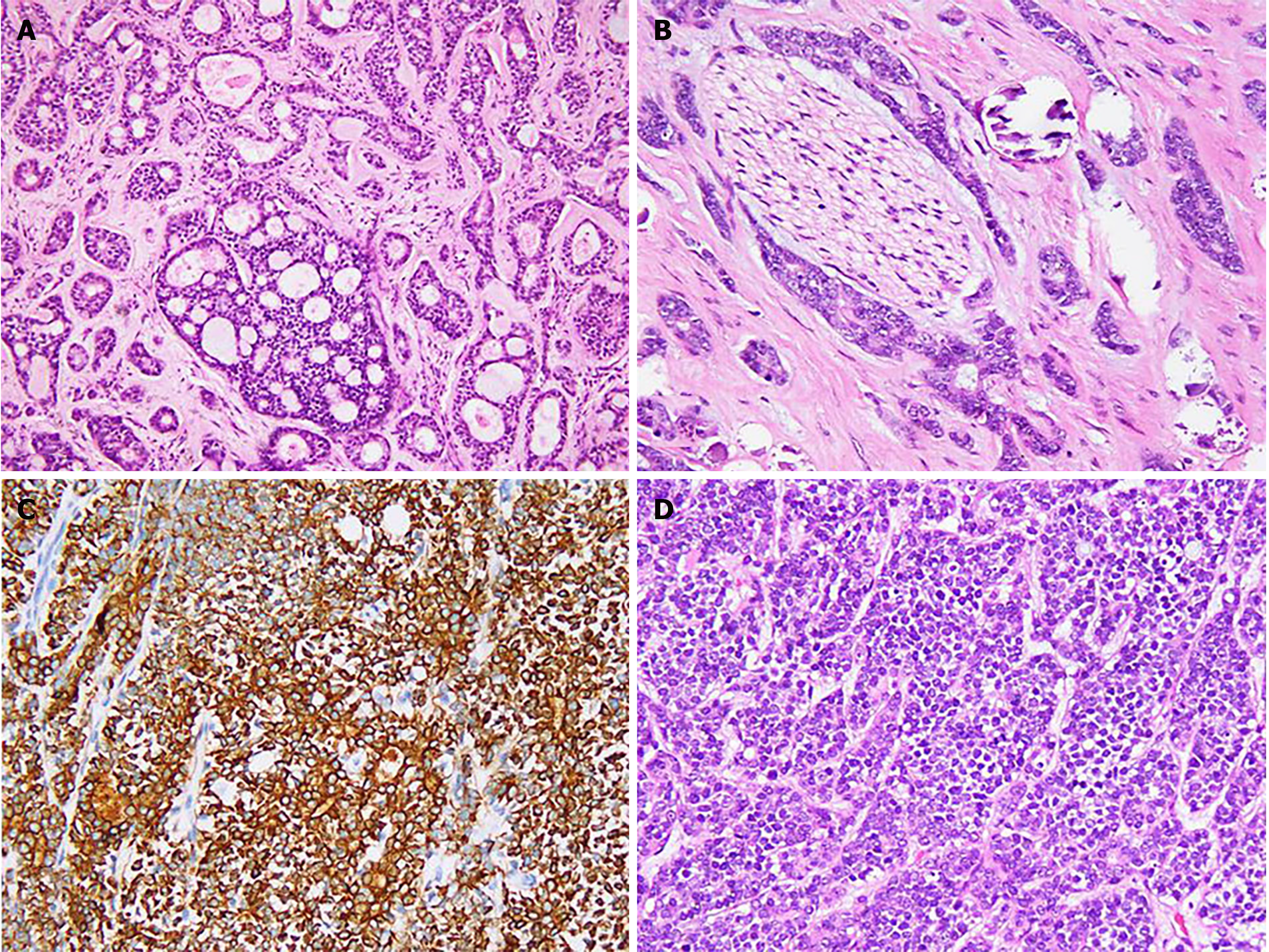
On frozen sections of tumor tissue, the diagnosis was adenocarcinoma of the biliary tract. The final diagnosis was ACC after evaluation of paraffin sections and immunostaining. Gross observation found a 3.8 cm × 3.5 cm × 3 cm grayish-white mass with a distinct boundary that was located 1 cm under the capsule of the liver (Figure 2). The cross section was grayish-white and the mass had a moderately solid quality. On histological evaluation, the tumor was composed of both ductal and myoepithelial cells and had both tubiform and cribriform histological structures, a solid component was rarely seen (Figure 3A). The tubular pattern included a true lumina. The cribri
A partial right hepatolobectomy was performed.
The pathologist advised the surgeon to explore the salivary glands for the primary lesion because primary ACC of the liver is very rare. A sublingual gland mass with a maximum diameter of 2.3 cm was revealed by computed tomography. The tumor was resected at West China Dental Hospital of Sichuan University, and a primary sublingual gland ACC was confirmed in paraffin sections. The sublingual gland ACC relapsed in May 2018. The patient refused reoperation. The recurrent lesion disappeared following local radiation therapy and computed tomography-guided radioactive seed implantation. The patient remains in good condition until now.
ACC was first described by Billroth in 1856 as a “cylindroma.” In 1954, Ewing[2] designated it as ACC for the first time and described its histology, including ductal and myoepithelial cells and having a cribriform structure. According to data from six dental hospitals of China, ACC accounts for about 11% of epithelial tumors and 27% of the malignant tumors of the salivary glands[3]. Three histologic types of ACC have been described, tubiform, cribriform, and solid. Combinations of the three types have been found in the same case. ACC grows slowly and always involves nerves and blood vessels.
Distant metastasis is common and often involves the lung, liver, bone, and brain. Metastasis may also occur to other sites, including the stomach, thyroid, kidney, spleen, and skin[4]. Distant metastasis of ACC may develop after diagnosis and therapy of the primary tumor, even several years afterward[5]. Metastatic ACC of the liver and lung that developed 5 years after surgery and chemotherapy has been reported[6]. However, there have been few reports of metastatic salivary ACC with initial clinical manifestation as a liver mass. Some investigators have independently reported liver metastasis as the initial clinical manifestation of ACC[7-9]. Until now, there have been only three case reports of liver metastasis as the initial clinical manifestation of submandibular ACC. This case is the first report of a primary sublingual gland ACC. The clinical information of the four cases is summarized in Table 1.
| Ref. | Gender | Age in yr | Primary site | Therapy | Follow-up |
| Deshpande et al[7] | Male | 68 | Submandibular gland | Chemotherapy | Not available |
| Garg et al[8] | Male | 75 | Submandibular gland | Refused treatment | Not available |
| Spolverato et al[9] | Female | 59 | Submandibular gland | Chemotherapy and radiation therapy | No recurrence after 5 mo |
| Our case | Female | 51 | Sublingual gland | Operation and radiation therapy after recurrence | Recurrence after 20 mo |
The initial clinical manifestation in this case was a liver mass, and ACC has three histological types resembling some primary or metastatic tumors of liver. Therefore, the tumors listed below should be considered in the differential diagnosis.
Intrahepatic cholangiocarcinoma: Early stage intrahepatic cholangicarcinoma has a relatively uniform tubiform architecture and a cord or papillary architecture with a rich fibrous stroma. The glandular cavity includes of pleiomorphic cubical and columnar epithelial cells with round nuclei and a small nucleolus. The tumor grows infiltratively along the bile duct wall or nerve and the tumor cells always secrete mucus. Atypical proliferation and carcinoma in situ of the adjacent bile duct progresses to primary intrahepatic cholangicarcinoma but not metastatic carcinoma. CD117, CK7, and MYB are positive in ACC but negative in intrahepatic cholangicarcinoma. Otherwise, myoepithelial markers can be helpful.
Poorly differentiated hepatocellular carcinoma: Poorly differentiated hepatocellular carcinoma always presents with solid architecture resembling solid type ACC. Fissure-like vessels can be seen in large nests of poorly differentiated hepatocellular carcinoma cells but without obvious blood sinus-like lacunae. The tumor cells have a high nucleus/cytoplasm ratio and obvious pleomorphism, even of paradoxical giant cells. Poorly differentiated hepatocellular carcinoma is positive for hepatocytes, alpha fetoprotein (AFP), and glypican-3 and ACC is always negative.
Epithelial-myoepithelial carcinoma: Epithelial-myoepithelial carcinoma is a rare low malignant tumor that usually occurs in the parotid gland. Epithelial-myoepithelial carcinoma accounts for fewer than 1% of all salivary gland tumors. Primary epithelial-myoepithelial carcinoma of the liver is very rare, with only two published case reports[10,11]. Like ACC, epithelial-myoepithelial carcinoma includes ductal and myoepithelial cells and involvement of blood vessels and nerves. It is difficult to distinguish epithelial-myoepithelial carcinoma from ACC by only histology and immunohistochemistry. However, ACC tends to have a cribriform architecture, MYB-NFIB gene fusion, and positive immunostaining for MYB protein. A recent study reported that MYB-NFIB gene fusion was not present in 15 cases of classical epithelial-myoepithelial carcinoma[12]. FISH might be a powerful “weapon” for differential diagnosis.
Metastatic breast ACC of the liver: A reported ACC case involved several nodular lesions in the right breast with a Breast Imaging-Reporting and Data System ultrasound result of grade Ш. The histology of the metastatic breast ACC resembled that of salivary gland ACC, but the differential diagnosis depended on immunohistochemical staining of breast cancer markers to identify the primary tumor. Breast ACC is not positive for estrogen receptor and progesterone receptor, but neither are all breast tumors. A recent advance in molecular pathology allows confirmation of ACC by a FISH assay that is positive for the characteristic translocation of t (6; 9) (q22-23; p23-24) and MYB-NFIB gene fusion. Metastatic breast and salivary gland ACC were distinguished by MYB protein immunostaining and a FISH assay positive for MYB-NFIB gene fusion.
Metastatic colorectal adenocarcinoma of liver: Advanced colorectal adenocarcinoma can metastasize to the liver through the hepatic portal system and moderately differentiated metastases can present with a cribriform architecture. For that reason, metastatic colorectal adenocarcinoma should be distinguished from metastatic salivary glands ACC. Colorectal adenocarcinoma is positive for villin, CDX-2, and CK20, but negative for CK7, CD117 and MYB. Moderately differentiated cribiform-type colorectal adenocarcinoma lacking peripheral myoepithelial cells are negative for p63, calponin and CK5/6.
A recent study found that the prognosis of ACC was closely correlated with clinical stage, histologic grade, complete excision of the tumor, and nerve involvement[13]. Surgical resection was performed because ACC is not sensitive to radiotherapy and chemotherapy. Postoperative radiotherapy reduced local recurrence, but did not completely avoid it. Chemotherapy was used to treat recurrent or metastatic cases and patients who were not candidates for surgery[14]. Recent studies that evaluated targeted therapy of ACC have reported successful treatment of cases that were positive for CD117 with imatinib[15,16]. In vitro, down regulation of p53 was found to promote epithelial-mesenchymal transition-like change and perineural invasive activity of human salivary ACC cells[17]. As ACC grows slowly, and the disease has a long survival time, novel immunotherapy might improve the prognosis.
Metastatic sublingual gland ACC with initial clinical manifestation as a liver mass is very rare. In this case, the histological appearance was the basis of a pathological diagnosis. Primary hepatic tumors and metastatic carcinomas should be included in the differential diagnosis. In addition to immunohistochemical detection of MYB protein, detection of an MYB-NFIB fusion gene by FISH can be helpful, but an occasional negative result can confuse the diagnosis.
We thank Prof. Geng N in the Department of Pathology, West China Dental Hospital of Sichuan University for diagnosis assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanase C S-Editor: Zhang H L-Editor: Filipodia P-Editor: Xing YX
| 1. | Binesh F, Akhavan A, Masumi O, Mirvakili A, Behniafard N. Clinicopathological review and survival characteristics of adenoid cystic carcinoma. Indian J Otolaryngol Head Neck Surg. 2015;67:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Ewing MR. Surgical treatment of oral cancer. Br J Plast Surg. 1954;7:108-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Yu SF. Oral Histopathology. Beijing: People’s Health Publishing House, 2013: 313-314.. |
| 4. | Schwentner I, Obrist P, Thumfart W, Sprinzl G. Distant metastasis of parotid gland tumors. Acta Otolaryngol. 2006;126:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Balducci G, Sagnotta A, Muroni M, Cacchi C, D'Amato A. An unusual case of exclusive liver metastases from adenoid cystic carcinoma of the submandibular gland: a role for surgery? Surg Today. 2011;41:596-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Sali PA, Yadav KS, Bushan K, Rajpurohit V, Varty PP, Sharma S. A rare case of lacrimal adenoid cystic carcinoma with large hepatic and multiple pulmonary metastases with successful surgical treatment. Int J Surg Case Rep. 2016;20:151-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Deshpande AH, Kelkar AA. Hepatic metastasis as an initial manifestation of salivary adenoid cystic carcinoma: Cytologic diagnosis. Diagn Cytopathol. 2009;37:45-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Garg N, Tomar R, Goyal S, Singh UR. Isolated liver metastases in an adenoid cystic carcinoma of the submandibular gland on fine needle aspiration cytology: an unusual presentation. Cytopathology. 2014;25:137-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Spolverato G, Fite J, Bishop J, Argani P, Pawlik TM. Liver metastasis as the initial presentation of adenoid cystic carcinoma. Dig Dis Sci. 2014;59:2004-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Tsuneyama K, Hoso M, Kono N, Kitagawa M, Masuda S, Matsuki N, Nakanuma Y. An unusual case of epithelial-myoepithelial carcinoma of the liver. Am J Surg Pathol. 1999;23:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Liu Y, Sang XT, Gao WS, Mao YL, Liu YW, Liu HF, Yang ZY, Yang SZ, Zhong SX, Huang JF. The first case of primary epithelial-myoepithelial carcinoma in the liver. Zhong hua Wai Ke Za Zhi. 2006;44:1477-1479. [PubMed] |
| 12. | Bishop JA, Westra WH. MYB Translocation Status in Salivary Gland Epithelial-Myoepithelial Carcinoma: Evaluation of Classic, Variant, and Hybrid Forms. Am J Surg Pathol. 2018;42:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Ozdemir C, Karacetin D, Tuna S, Karadeniz A. Treatment and clinicopathologic predictors for adenoid cystic carcinomas of the head and neck. J BUON. 2011;16:123-126. [PubMed] |
| 14. | Papaspyrou G, Hoch S, Rinaldo A, Rodrigo JP, Takes RP, van Herpen C, Werner JA, Ferlito A. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: a review. Head Neck. 2011;33:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Faivre S, Raymond E, Casiraghi O, Temam S, Berthaud P. Imatinib mesylate can induce objective response in progressing, highly expressing KIT adenoid cystic carcinoma of the salivary glands. J Clin Oncol. 2005;23:6271-3; author reply 6273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Alcedo JC, Fábrega JM, Arosemena JR, Urrutia A. Imatinib mesylate as treatment for adenoid cystic carcinoma of the salivary glands: report of two successfully treated cases. Head Neck. 2004;26:829-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Yang X, Jing D, Liu L, Shen Z, Ju J, Ma C, Sun M. Downregulation of p53 promotes in vitro perineural invasive activity of human salivary adenoid cystic carcinoma cells through epithelial-mesenchymal transition-like changes. Oncol Rep. 2015;33:1650-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |