Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5126
Peer-review started: April 13, 2021
First decision: April 23, 2021
Revised: May 6, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: July 6, 2021
Processing time: 71 Days and 21.9 Hours
Patients undergoing lumbar spine surgery usually suffer severe pain in the postoperative period. The erector spinae plane block (ESPB), first published in 2016, can anesthetize the ventral and dorsal rami of thoracic nerves and produce an extensive multi-dermatomal sensory block.
To assess whether bilateral ultrasound-guided ESPB at a lower thoracic level could improve pain control and quality of recovery in patients undergoing lumbar spine surgery.
A total of 60 patients aged 18-80 years scheduled to undergo lumbar spine surgery with general anesthesia were randomly assigned to two groups: ESPB group (preoperative bilateral ultrasound-guided ESPB at T10 vertebral level) and control group (no preoperative ESPB). Both groups received standard general anesthesia. The main indicator was the duration to the first patient controlled intravenous analgesia (PCIA) bolus.
In the ESPB group, the duration to the first PCIA bolus was significantly longer than that in the control group (h) [8.0 (4.5, 17.0) vs 1.0 (0.5, 6), P < 0.01], and resting and coughing numerical rating scale (NRS) scores at 48 h post operation were significantly lower than those in the control group (P < 0.05). There was no significant difference between the two groups regarding resting and coughing NRS scores at 24 h post operation. Sufentanil consumption during the operation was significantly lower in the ESPB group than in the control group (P < 0.01), while there was no significant difference between the two groups regarding morphine consumption at 24 or 48 h post operation. In the ESPB group, Modified Observer’s Assessment of Alertness/Sedation score within 20 min after extuba
In patients undergoing lumbar spine surgery, ultrasound-guided ESPB at a lower thoracic level improves the analgesic effect, reduces opioid consumption, and improves postoperative recovery.
Core Tip: The erector spinae plane block (ESPB) could anesthetize the dorsal rami of thoracic nerves and produce an extensive multi-dermatomal sensory block, which may improve the analgesic effect of patients undergoing lumber spine surgery. We designed this prospective randomized controlled trial and found that ESPB at a lower thoracic level could prolong the duration to the first patient controlled intravenous analgesia bolus, reduce the intraoperative opioid consumption, and improve postoperative recovery.
- Citation: Zhang JJ, Zhang TJ, Qu ZY, Qiu Y, Hua Z. Erector spinae plane block at lower thoracic level for analgesia in lumbar spine surgery: A randomized controlled trial. World J Clin Cases 2021; 9(19): 5126-5134
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5126.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5126
Patients undergoing major lumbar spine surgery usually suffer severe pain during the perioperative period. Conventional opioid-based analgesia techniques are associated with the side effects of nausea, vomiting, pruritus, and sedation. In recent years, with the introduction of ultrasonography into regional anesthesia practice, plane blocks have been frequently employed to improve postoperative analgesia and to reduce opioid consumption[1-3].
Anatomically, the posterior ramus of the thoracolumbar spinal nerves passes through the posterior surface of the transverse process of the corresponding lower vertebra and distributes to the back. The posterior ramus may innervate two to three segments caudal to its initial nerve root. The erector spinae plane block (ESPB), first described in 2016, is a paraspinal interfascial plane block targeting the ventral and dorsal rami of the spinal nerves. It has been reported that injection with 20 mL of fluid at the T5 vertebral level can produce 3-6 transverse processes cranially or caudally[4]. Therefore, ESPB performed at the T10 vertebral level can block the nerve innervating L1 to S1 vertebrae. Ropivacaine infusion related infection has also been reported[5]. Puncture site at the T10 vertebral level keeps a distance away from the surgical area, which may reduce the opportunity of infection. Therefore, we chose the puncture site at the T10 vertebral level and proposed that ESPB performed at the T10 vertebral level can provide effective analgesia for lumbar spine surgery.
Therefore, we designed this prospective randomized controlled study to observe the analgesic effect of ESPB in patients undergoing lumbar spine surgery.
This was a prospective randomized controlled study. The protocol was approved by the Beijing Hospital Medical Ethics Committee (2018BJYYEC-011-01) and registered with the Chinese Clinical Trial Registry at chictr.org.cn (ChiCTR1800015002). An informed consent form was signed by each patient or their legal guardian before enrollment. The study was conducted in the Department of Anesthesiology of Beijing Hospital.
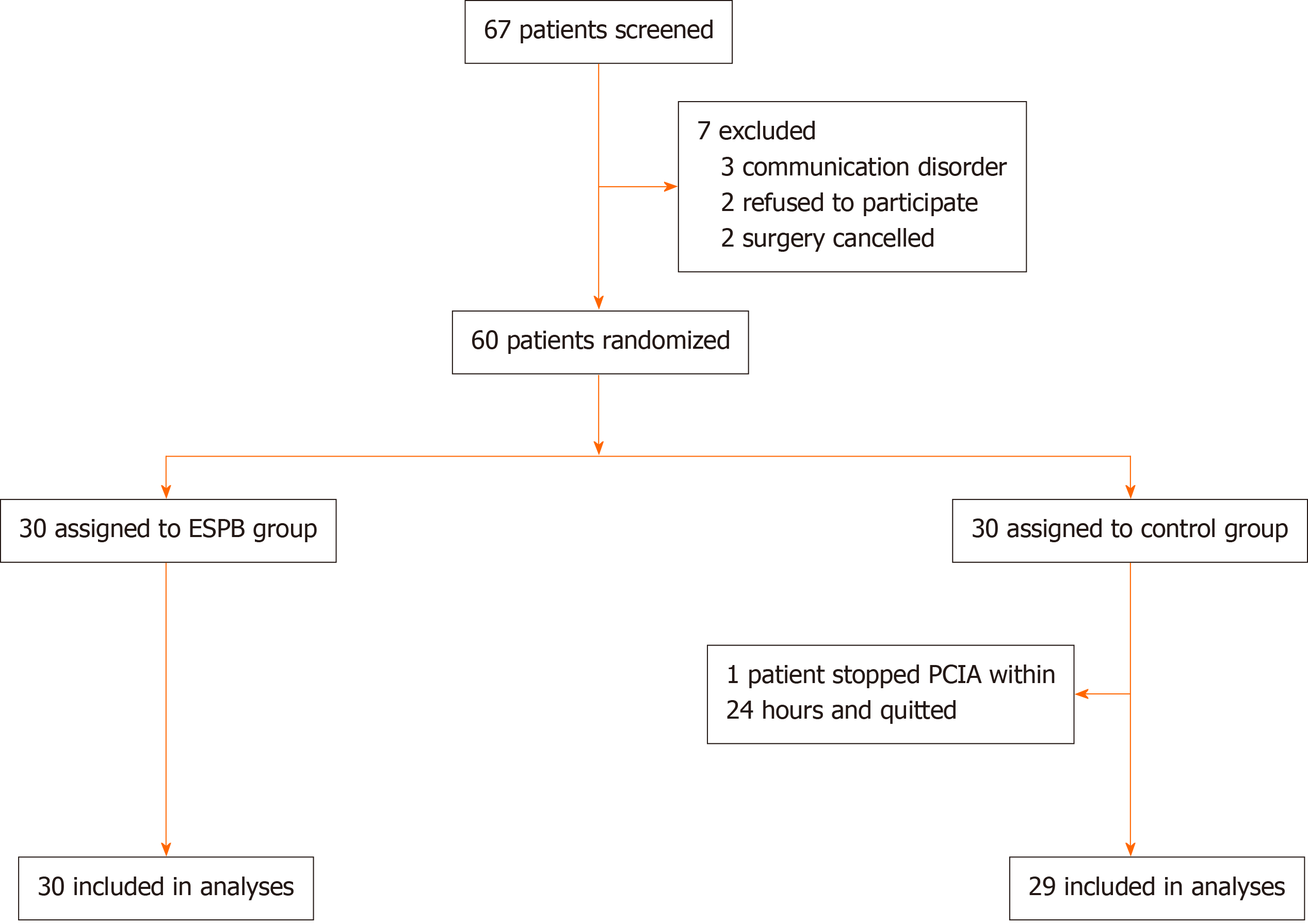
Patients were included in the study if they met the following criteria: (1) Age ≥ 18 years and ≤ 75 years; and (2) Scheduled to undergo lumbar spine surgery with general anesthesia. Patients were excluded if they met any of the following criteria: (1) Refusal to participate in the study; (2) American Society of Anesthesiologists physical classification of IV or higher; (3) Preoperative cognitive dysfunction or communication disorder; (4) Infection at the puncture site; (5) Allergy to amide-type local anesthetics; (6) Neuromuscular disease; (7) Hepatic or renal insufficiency; (8) Emergency surgery; (9) Chronic opioid use; or (10) Participation in another clinical study (Figure 1).
All patients who underwent preoperative assessment on the day before surgery were randomly assigned to either an ESPB group or a control group according to the random numbers sealed in the envelopes. The evaluating physician was blinded to the assignment. In the ESPB group, ultrasound-guided bilateral ESPB was performed before general anesthesia in the post-anesthesia care unit (PACU). The patients were placed in the right lateral decubitus position. The position of the T10 vertebra was marked on the skin. The regions scheduled for the procedure were sterilized in the ESPB group. A high-frequency linear US probe (Konica Minolta, Tokyo, Japan) in a sterile sheath was placed longitudinally 3 cm lateral to the T10 spinous process. The skin was then infiltrated with local anesthetic, and an echogenic 21-gauge block needle (Pajunk, Geisingen, Germany) was inserted using an out-of-plane approach. The tip was placed into the fascial plane on the deep aspect of the erector spinae muscle. The correct location of the needle tip was confirmed by visible fluid spread below the erector spinae muscle off the bony shadow of the transverse process. A total volume of 25 mL of 0.3% ropivacaine was injected through the needle. The procedure was then repeated on the opposite side. No intervention was performed in the control group.
Standard monitoring was established by electrocardiography, pulse oximetry, invasive arterial pressure, capnography, and the bispectral index (BIS). General anesthesia was induced with sufentanil at 0.2-0.3 µg/kg, propofol at 2.0-4.0 mg/kg, and cisatracurium at 0.2-0.3 mg/kg. After endotracheal intubation, mechanical ventilation was established with a tidal volume of 6-8 mL/kg and ventilation rate of 12-20/min to maintain PetCO2 at 35-45 mmHg. Anesthesia was maintained with sevoflurane in a mixture of 50% oxygen and 50% N2O and intermittent injection of sufentanil and cisatracurium when necessary. The target was to maintain the BIS between 45 and 60 and blood pressure and heart rate within 30% of the original preoperative values. Before skin incision, 50 mg of flurbiprofen axetil, dezocine at 0.1-0.2 mg/kg, and dexmedetomidine at 0.3 µg/kg were given intravenously for analgesia, and another 50 mg of flurbiprofen axetil was given before skin closure. Dexamethasone (5 mg) and palonosetron hydrochloride (0.5 mg) were given to prevent postoperative nausea and vomiting. At the end of the procedure, residual neuromuscular blockade was antagonized with 2 mg of neostigmine and 1 mg of atropine. After extubation, all patients were admitted to the PACU for at least 30 min. All patients received patient controlled intravenous analgesia (PCIA) with morphine at 0.5 mg/mL for 48 h, set at a continuous rate of 0.5 mL/h, bolus at 2 mL, and lock-out interval of 8 min.
The main indicator was the duration to the first PCIA bolus. The secondary indicators included morphine consumption and numerical rating scale (NRS) scores at 24 and 48 h post operation, intraoperative sufentanil and cisatracurium consumption, duration of stay in the PACU, and time to first ambulation after surgery.
Continuous variables were analyzed by the independent-sample t-test, the Mann-Whitney U test, or generalized linear mixed models. Categorical variables were analyzed by χ2 analysis or Fisher’s exact test. Statistical analyses were performed with SPSS 14.0 (SPSS Inc., Chicago, IL). All tests were two-sided, and P < 0.05 was considered statistically significant.
From June 2018 to May 2019, a total of 60 patients participated in the study, and one patient in the control group stopped PCIA because of omission within 24 h; therefore, 59 patients were included in the final analysis, including 30 in the ESPB group and 29 in the control group. The baseline patient demographics and characteristics were comparable between the two groups (Table 1).
| ESPB group (n = 30) | Control group (n = 29) | P value | |
| Sex (Male/Female) | 13/17 | 8/21 | 0.207 |
| Age, yr | 61 ± 12 | 64 ± 10 | 0.364 |
| Height, cm | 165 ± 9 | 163 ± 8 | 0.284 |
| Weight, kg | 70 ± 9 | 66 ± 9 | 0.146 |
| ASA (I/II/III) | 11/16/3 | 6/17/6 | 0.283 |
| Preoperative resting NRS | 4 (3-5) | 4 (3-5) | 0.871 |
| Preoperative moving NRS | 6 (5-7) | 5 (4-7) | 0.195 |
| Surgery segment | |||
| T12 | 1 (3.3) | 2 (6.9) | 0.557 |
| L1 | 2 (6.7) | 3 (10.3) | 0.643 |
| L2 | 5 (16.7) | 8 (27.6) | 0.351 |
| L3 | 17 (56.7) | 24 (82.8) | 0.054 |
| L4 | 27 (90.0) | 28 (96.6) | 0.643 |
| L5 | 28 (93.3) | 28 (96.6) | 1.000 |
| S1 | 14 (46.7) | 13 (44.8) | 0.797 |
Between the two groups, the durations of anesthesia were comparable. Sufentanil and cisatracurium consumption in the ESPB group was lower than that in the control group (P < 0.01). Compared with those in the control group, patients in the ESPB group showed better recovery, higher Modified Observer’s Assessment of Alertness/ Sedation score within 20 min after extubation, and shorter stay in the PACU (P < 0.01, Table 2).
| ESPB group (n = 30) | Control group (n = 29) | P value | |
| Duration of anesthesia, min | 193 ± 56 | 205 ± 54 | 0.242 |
| Dose of sufentanil, ug | 20 ± 6 | 25 ± 6 | 0.003 |
| Dose of cisatracurium, mg | 31 ± 12 | 41 ± 10 | 0.001 |
| MOAA/S at extubation | 4.1 ± 0.5 | 3.3 ± 0.6 | 0.000 |
| MOAA/S 10 min after extubation | 4.5 ± 0.5 | 4.0 ± 0.5 | 0.001 |
| MOAA/S 20 min after extubation | 4.9 ± 0.3 | 4.4 ± 0.6 | 0.000 |
| MOAA/S 30 min after extubation | 5.0 ± 0.0 | 4.9 ± 0.4 | 0.073 |
| Duration in the PACU, min | 35 ± 2 | 44 ± 15 | 0.001 |
The duration to the first PCIA bolus was significantly longer in the ESPB group than in the control group (h) [8.0 (4.5, 17.0) vs 1 (0.5, 6), P < 0.01]. Compared with those in the control group, the resting and coughing NRS scores at 48 h post operation in the ESPB group were lower (P < 0.05), while there was no significant difference between the two groups regarding resting and coughing NRS scores at 24 h post operation. Compared with that in the control group, the time to first ambulation in the ESPB group was significantly shorter, and the resting NRS score on postoperative 30th day was significantly lower (Table 3). The results show a better analgesia effect in the ESPB group. There were no adverse events in either group.
| ESPB group (n = 30) | Control group (n = 29) | P value | |
| Duration to the first PCIA bolus, h | 8.0 (4.5-17.0) | 1 (0.5-6) | 0.000 |
| Resting NRS 24 h post operation | 1 (1-2) | 2 (1-3) | 0.084 |
| Coughing NRS 24 h post operation | 3 (2-4) | 3 (2-5) | 0.075 |
| Resting NRS 48 h post operation | 1 (0-1) | 1 (1-2) | 0.012 |
| Coughing NRS 48 h post operation | 2 (1-2) | 3 (2-4) | 0.002 |
| Morphine consumption 24 h post operation, mg | 18 (14-24) | 18 (14-28) | 0.339 |
| Morphine consumption 48 h post operation, mg | 36 (28-45) | 36 (33-55) | 0.120 |
| Time to first ambulation, hour | 95 (72-96) | 96 (76-114) | 0.024 |
| Resting NRS on postoperative 30th d | 1 (0-1) | 2 (1-2) | 0.001 |
In the current study, compared with conventional opioid-based multimodal analgesia in lumbar surgery, the combination of ESPB provided superior pain relief in the perioperative period, reduced intraoperative opioid and relaxant consumption, shortened the duration in the PACU, and improved postoperative recovery.
Posterior lumbar surgery is one of the most painful surgical procedures, which delays early postoperative mobilization, increases the complications of thrombosis and infection, and prolongs the length of stay. Hence, postoperative analgesia is essential.
The erector spinae plane block was first described in 2016 as a regional block for thoracic neuropathic pain[4]. ESPB has emerged as a valuable regional anesthesia technique for a range of thoracic, abdominal, and other procedures[6-9]. Numerous cadaveric studies have confirmed successful staining of both ventral and dorsal rami of multiple spinal nerves above and below the site of injection when dye is injected deep into the erector spinae muscle[4,10]. However, a recent study showed that the ventral rami are not always blocked[11]. Most likely, ESPB cannot always provide effective analgesia for areas innervated by the ventral rami of the spinal nerve. Paraspinal muscles and bony elements are primarily innervated by the posterior rami of spinal muscles, and ESPB may provide effective analgesia for lumbar operation[12].
Based on anatomy and a previous study proposing that injection with 25 mL of fluid can spread more than five transverse processes caudally, we chose the T10 vertebral level as the local anesthetic injection site. Our study has shown that ESPB performed in patients undergoing lumbar operation decreases postoperative pain intensity, with a better NRS score and longer duration to first PCIA bolus. Recently, several reports showed successful use of ESPB in pain control after lumbar operation. In a case series, ESPB performed at the T10-T12 level was used for perioperative analgesia during lumbosacral spine surgery and produced effective perioperative opioid-sparing analgesia[13]. In a randomized controlled trial, preoperative bilateral ESPB performed at the T10 Level in lumbar spine surgery patients produced a significant reduction in postoperative opioid requirements and improved patient satisfaction compared with that in standard analgesia[14]. In our study, we also observed fewer intraoperative opioid requirements and no difference in postoperative opioid requirements. A previous cadaver study showed that injection with 20 mL of fluid at T7 spread more than five transverse processes caudally[6]. While the evidence to date indicates that the spread with 20 mL of injectate may extend 3-4 vertebral levels from the site of injection in a caudal direction[4,6,15], if injection with 20 mL of fluid at T10 produces a spread three transverse processes caudally, it cannot provide analgesia for operation at the L4, L5, and S1 vertebrae segments and causes opioid requirements similar to those observed in the control group. Patients in both groups received dexmedetomidine and nonsteroidal anti-inflammatory drugs for multimodal analgesia, and the elevated pain threshold reduced opioid requirements in both groups, which is another cause.
Sufentanil and relaxant consumption decreased in the ESPB group compared to the control group in our study and produced more rapid recovery in the PACU, which is important for reducing perioperative cardio-cerebrovascular complications, especially for elderly patients and patients with severe preoperative comorbidities. Earlier postoperative mobilization can reduce the risk of complications such as deep thrombosis, which is important for enhanced recovery after surgery.
We injected 25 mL of local anesthetic at the T10 erector spinae plane bilaterally and found no side effects in our study. While a similar analgesic effect might be obtained with bilateral paravertebral blocks, the peculiarity of ESPB is the simple identification of the ultrasound landmark and the likely safe procedure[16]. The transverse processes of the thoracic vertebrae and the erector spinae muscles are easily identified using a low-frequency curved-array transducer; the risk of pneumothorax is minimal because the transverse process acts as an anatomical barrier and avoids needle insertion to the pleura[7,17]. The relatively superficial location of the ESP block, distant from any major blood vessels and nervous structures, also minimizes concerns regarding anticoagulation and the development of a clinically significant hematoma[7]. The capacity for extensive cranial-caudal spread allows it to be performed at a distance from the surgical field. This factor is in contrast to another recently described regional analgesic technique for spine surgery, the thoracolumbar interfascial plane block, which requires injection at a vertebral level congruent with the surgical site[18,19].
There are several limitations in our study. First, the effective analgesic time of ropivacaine for peripheral nerve block is generally believed to be 5 to 8 h[20]. We observed NRS scores at 24 h and 48 h post operation, while we did not observe NRS scores at 2-12 h post operation at shorter time intervals. Second, sensory testing was not performed for each patient to map the block area, which might have revealed the exact limits of the analgesic effect of the block. Third, the sample size was small to observe the side effects of ESPB.
In patients undergoing lumbar spine surgery, our study showed that ESPB at the T10 level improved pain control and reduced opioid consumption, while a previous randomized controlled study showed ESPB at the T12 level reduced opioid consumption, and another randomized controlled study performing ESPB at the L3 level observed lower pain scores and less opioid consumption[21,22]. ESPB is a very promising technique in perioperative analgesia for patients undergoing lumbar spine surgery, while there is no conclusion regarding how to choose a correct injection site and which concentration and volume of local anesthetics are optimal. We need additional research to improve the role of ESPB in the future.
ESPB at the T10 vertebral level shows benefits over systemic multimodal analgesia in patients undergoing lumbar surgery. The analgesic benefits of ESPB can be attributed to the multimodal analgesia regimen, and we suggest the routine use of ESPB in lumbar surgery as a component of multimodal analgesia. Further clinical investigations, including prospective randomized controlled trials with a larger sample size, should be undertaken to clearly establish its efficacy in this setting.
Patients undergoing lumbar spine surgery usually suffer severe pain in the postoperative period.
The erector spinae plane block (ESPB) can anesthetize the ventral and dorsal rami of thoracic nerves and produce an extensive multi-dermatomal sensory block. We proposed that ESPB at a lower thoracic level could improve analgesia effect for patients undergoing lumbar spine surgery.
This study aimed to assess whether bilateral ultrasound-guided ESPB at a lower thoracic level could improve pain control and quality of recovery in patients undergoing lumbar spine surgery.
Patients scheduled to undergo lumbar spine surgery were randomly assigned to two groups: ESPB group (preoperative bilateral ultrasound-guided ESPB at T10 vertebral level) and control group (no preoperative ESPB). The main indicator was the duration to the first patient controlled intravenous analgesia (PCIA) bolus.
In the ESPB group, the duration to the first PCIA bolus was significantly longer, resting and coughing NRS scores at 48 h post operation and sufentanil consumption during the operation were significantly lower, Modified Observer’s Assessment of Alertness/Sedation score within 20 min after extubation was higher, and duration in the post-anesthesia care unit was shorter than those in the control group.
In patients undergoing lumbar spine surgery, ultrasound-guided ESPB at a lower thoracic level improves the analgesic effect, reduces opioid consumption, and improves postoperative recovery.
ESPB at the T10 vertebral level shows benefits over systemic multimodal analgesia in patients undergoing lumbar surgery. The analgesic benefits of ESPB can be attributed to the multimodal analgesia regimen.
The authors are grateful to their colleagues in the Department of Anesthesia for their help.
Manuscript source: Unsolicited manuscript
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nagata K S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Baeriswyl M, Kirkham KR, Kern C, Albrecht E. The Analgesic Efficacy of Ultrasound-Guided Transversus Abdominis Plane Block in Adult Patients: A Meta-Analysis. Anesth Analg. 2015;121:1640-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 2. | Ökmen K, Ökmen BM. The efficacy of serratus anterior plane block in analgesia for thoracotomy: a retrospective study. J Anesth. 2017;31:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 3. | Abdallah FW, MacLean D, Madjdpour C, Cil T, Bhatia A, Brull R. Pectoralis and Serratus Fascial Plane Blocks Each Provide Early Analgesic Benefits Following Ambulatory Breast Cancer Surgery: A Retrospective Propensity-Matched Cohort Study. Anesth Analg. 2017;125:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med. 2016;41:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1263] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 5. | Agarwal S, Nuttall GA, Johnson ME, Hanson AC, Oliver WC Jr. A prospective, randomized, blinded study of continuous ropivacaine infusion in the median sternotomy incision following cardiac surgery. Reg Anesth Pain Med. 2013;38:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Chin KJ, Adhikary S, Sarwani N, Forero M. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia. 2017;72:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 287] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 7. | Chin KJ, Malhas L, Perlas A. The Erector Spinae Plane Block Provides Visceral Abdominal Analgesia in Bariatric Surgery: A Report of 3 Cases. Reg Anesth Pain Med. 2017;42:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 8. | Hamilton DL, Manickam B. Erector spinae plane block for pain relief in rib fractures. Br J Anaesth. 2017;118:474-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 9. | Ueshima H, Otake H. Clinical experiences of ultrasound-guided erector spinae plane block for thoracic vertebra surgery. J Clin Anesth. 2017;38:137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Ivanusic J, Konishi Y, Barrington MJ. A Cadaveric Study Investigating the Mechanism of Action of Erector Spinae Blockade. Reg Anesth Pain Med. 2018;43:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 335] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 11. | Kose HC, Kose SG, Thomas DT. Lumbar versus thoracic erector spinae plane block: Similar nomenclature, different mechanism of action. J Clin Anesth. 2018;48:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Almeida CR, Oliveira AR, Cunha P. Continuous Bilateral Erector of Spine Plane Block at T8 for Extensive Lumbar Spine Fusion Surgery: Case Report. Pain Pract. 2019;19:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Melvin JP, Schrot RJ, Chu GM, Chin KJ. Low thoracic erector spinae plane block for perioperative analgesia in lumbosacral spine surgery: a case series. Can J Anaesth. 2018;65:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 14. | Singh S, Choudhary NK, Lalin D, Verma VK. Bilateral Ultrasound-guided Erector Spinae Plane Block for Postoperative Analgesia in Lumbar Spine Surgery: A Randomized Control Trial. J Neurosurg Anesthesiol. 2020;32:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 15. | Muñoz F, Cubillos J, Bonilla AJ, Chin KJ. Erector spinae plane block for postoperative analgesia in pediatric oncological thoracic surgery. Can J Anaesth. 2017;64:880-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | El-Boghdadly K, Pawa A. The erector spinae plane block: plane and simple. Anaesthesia. 2017;72:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Gürkan Y, Aksu C, Kuş A, Yörükoğlu UH, Kılıç CT. Ultrasound guided erector spinae plane block reduces postoperative opioid consumption following breast surgery: A randomized controlled study. J Clin Anesth. 2018;50:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 18. | Hand WR, Taylor JM, Harvey NR, Epperson TI, Gunselman RJ, Bolin ED, Whiteley J. Thoracolumbar interfascial plane (TLIP) block: a pilot study in volunteers. Can J Anaesth. 2015;62:1196-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Ueshima H, Ozawa T, Toyone T, Otake H. Efficacy of the Thoracolumbar Interfascial Plane Block for Lumbar Laminoplasty: A Retrospective Study. Asian Spine J. 2017;11:722-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia, 5th edition. Philadelphia: Lippincott Williams & Wilkins, 2006: 463. |
| 21. | Zhang TJ, Zhang JJ, Qu ZY, Zhang HY, Qiu Y, Hua Z. Bilateral Erector Spinae Plane Blocks for Open Posterior Lumbar Surgery. J Pain Res. 2020;13:709-717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Yayik AM, Cesur S, Ozturk F, Ahiskalioglu A, Ay AN, Celik EC, Karaavci NC. Postoperative Analgesic Efficacy of the Ultrasound-Guided Erector Spinae Plane Block in Patients Undergoing Lumbar Spinal Decompression Surgery: A Randomized Controlled Study. World Neurosurg. 2019;126:e779-e785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |