Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5064
Peer-review started: January 14, 2021
First decision: April 4, 2021
Revised: April 13, 2021
Accepted: May 6, 2021
Article in press: May 6, 2021
Published online: July 6, 2021
Processing time: 161 Days and 2.9 Hours
Hepatectomy is the first choice for treating neuroendocrine tumor liver metastases. However, most patients with neuroendocrine tumor liver metastases are not suitable for hepatectomy. Ablation combined with hepatectomy can be an alternative to liver resection.
To explore the clinical effect of microwave ablation combined with hepatectomy for the treatment of neuroendocrine tumor liver metastases.
In this study, the data of patients who underwent microwave ablation combined with hepatectomy for the treatment of neuroendocrine tumor liver metastases from June 2015 to January 2018 were reviewed. Before the operation, the patients did not receive any treatment for liver neuroendocrine tumors. After a multidisciplinary expert group discussion, all patients were deemed unsuitable for liver resection. All patients were diagnosed with neuroendocrine tumors by pathology. The overall survival time and progression-free survival time were followed by telephone calls and outpatient visits after surgery.
Eleven patients with neuroendocrine tumor liver metastases were treated by microwave ablation combined with hepatectomy between June 2015 and January 2018. The median number of liver metastatic nodules was 4 (range, 2 to 43). The median number of lesions resected was 1 (range, 1 to 18), and the median number of lesions ablated was 3 (range, 1 to 38). The mean operation time was 405.6 (± 39.4) min. The median intraoperative blood loss was 600 mL (range, 50 to 3000). Ten patients had a fever after surgery. The median duration of fever was 3 d (range, 0 to 21). Elevated bilirubin levels occurred in all patients after surgery. The median bilirubin on the first day after surgery was 28.5 (range, 10.7 to 98.9) µmol/L. One patient developed respiratory failure, renal insufficiency, and pneumonia after the operation. No patient died postoperatively during hospitalization. The mean overall survival time after surgery was 34.1 (± 3.7) mo, and the median progression-free survival time was 8 (range, 2 to 51) mo. One year after surgery, ten patients survived and five patients survived without progression. Three year after surgery, eight patients survived and two patients survived without progression.
Microwave ablation combined with hepatectomy not only makes the patients obtain a survival rate similar to that of patients undergoing hepatectomy, but also has a low incidence of postoperative complications.
Core Tip: Liver resection is the first choice for treating neuroendocrine tumor liver metastases. Due to the strict indications for hepatectomy, most patients with neuroendocrine tumor liver metastases are not suitable for hepatectomy. Ablation combined with hepatectomy is an alternative treatment option. Here, this study analyzed the clinical effect of microwave ablation combined with hepatectomy in the treatment of neuroendocrine tumor liver metastases. The results show that microwave ablation combined with hepatectomy not only makes the patients obtain a survival rate similar to that of patients undergoing hepatectomy, but also has a low incidence of posto
- Citation: Zhang JZ, Li S, Zhu WH, Zhang DF. Microwave ablation combined with hepatectomy for treatment of neuroendocrine tumor liver metastases. World J Clin Cases 2021; 9(19): 5064-5072
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5064.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5064
Neuroendocrine tumors originate from neuroendocrine cells and grow relatively slowly. Neuroendocrine tumors occurs mostly in the gastrointestinal tract and pancreas[1,2]. Patients with neuroendocrine tumors often progress to liver metastases. Liver metastases can be found in more than half of patients with neuroendocrine tumors[1]. A previous study has shown that neuroendocrine tumors with liver metastases often have a worse prognosis[3]. Liver resection is the first choice for treating neuroendocrine tumor liver metastases. Due to the strict indications for hepatectomy, most patients with neuroendocrine tumor liver metastases are not suitable for hepatectomy. Ablation combined with hepatectomy has attracted the attention of clinicians in the treatment of neuroendocrine tumor liver metastases.
Ablation techniques include radiofrequency ablation, microwave ablation, cryoablation, laser ablation, and other means. Radiofrequency ablation combined with hepatectomy for treating neuroendocrine tumor liver metastases has been reported by Taner et al[4] and Elias et al[5]. Cryoablation combined with hepatectomy for treating neuroendocrine tumor liver metastases has been reported by Saxena et al[6]. Due to the frequent postoperative complications, long operation times, and more complicated operations, compared with radiofrequency ablation and microwave ablation, the use of cryoablation is rare[7,8]. Compared with radiofrequency ablation, microwave ablation produces higher local temperature and ablation range. And microwave ablation heat transfer is not affected by tissue carbonization, which makes the heat transfer more rapid and the ablation more complete. The stronger heat generation efficiency of microwave ablation often makes the operation time of microwave ablation shorter[9]. In addition, microwave ablation has greater advantages in treating nodules larger than 3 cm and nodules close to large vessels[10,11]. Therefore, our institution has attempted to use microwave ablation combined with hepatectomy for the treatment of neuroendocrine tumor liver metastases. This is the first report about microwave ablation combined hepatectomy for treating neuroendocrine tumor liver metastasis.
This study reviewed patients who underwent microwave ablation combined with hepatectomy for the treatment of neuroendocrine tumor liver metastases.
All patients were treated by microwave ablation (FORSEA MTC-3 Microwave Ablation System, Nanjing, Jiangsu, China) combined with hepatectomy in our institution. All patients were confirmed to have neuroendocrine tumors by pathology. After a multidisciplinary expert group discussion, all patients were deemed unsuitable for liver resection. None of the patients received any treatment for neuroendocrine tumors before surgery, such as liver resection, ablation, radiotherapy, or chemo
All patients underwent computed tomography (CT) or magnetic resonance imaging (MRI) examination before the operation to clarify the number and location of the tumors. Intraoperative ultrasound exploration further clarified the number and size of the nodules. For nodules larger than 3 cm, a large number of nodules smaller than 3 cm concentrated on a liver segment, or tumors located on the liver surface, surgical resection was usually selected. For nodules less than 3 cm, microwave ablation was usually selected. When tumors are located deep in the liver, hepatectomy will lead to the severe loss of normal liver tissue and liver function. Therefore, microwave ablation was usually selected. For intrahepatic nodules, all hepatectomy margins were greater than 0.5 cm from the tumor. All pathologies after hepatectomy confirmed R0 resection. All nodules found by intraoperative ultrasound were removed by liver resection or microwave ablation. The microwave ablation power was 70 W. For tumors less than 1 cm, the ablation time was 5 min. For tumors with a diameter of 1-2 cm, the ablation time was 10 min. For tumors with a diameter of 2-3 cm, the ablation time was 20 min. For tumors larger than 3 cm, the ablation was repeated multiple times.
All patients returned to the ward or intensive care unit (ICU) for close observation after surgery. All patients underwent CT or MRI examination 1 mo after surgery to determine whether the lesions were ablated completely. After that, all patients underwent CT/MRI examination every 3 mo. All patients were followed by telephone calls and outpatient visits.
Continuous variables were tested by the Shapiro-Wilk test to determine whether they followed a normal distribution. Continuous variables that were proved to have a normal distribution are reported as the mean and standard deviation. Otherwise, continuous variables are reported as medians. Categorical variables are reported as frequencies or percentages. P values less than 0.05 were considered significant. Data were analyzed with Statistical Package for the Social Sciences version 21.0 (SPSS 21.0).
Except for operation time and postoperative overall survival time, other continuous variables were not normally distributed (Shapiro-Wilk test for all continuous variables; Table 1).
| Continuous variable | P value |
| Age | 0.036a |
| Number of lesions | 0.000a |
| Number of hepatectomy | 0.000a |
| Number of microwave ablation | 0.001a |
| Blood loss | 0.042a |
| Operative time | 0.121 |
| Postoperative hospital stay | 0.019a |
| Bilirubin on the first day after surgery | 0.000a |
| Duration of the fever | 0.003a |
| Progression-free survival time | 0.003a |
| Postoperative survival time | 0.832 |
Eleven patients with neuroendocrine tumor liver metastases underwent microwave ablation combined with hepatectomy from June 2015 to January 2018. There were three males and eight females with a median age of 62 (range, 36 to 69) years. The pathological grades of the 11 patients included G3 in six cases, G2 in four, and G1 in one. The primary site of neuroendocrine tumors included four cases in the pancreas, one in the rectum, one in the colon, and five in unclear sites. All patients had multiple liver tumors. Patients had a median liver nodule of 4 (range, 2 to 43). Seven patients had bilobar tumors, and four patients had tumors in only one lobe. Because the single lobe tumors of the four patients were too deep, a large amount of normal liver tissue would be lost if only hepatectomy was performed. Due to the high recurrence rate of neuroendocrine tumors after liver metastasis, normal liver tissue should be preserved as much as possible for the second operation. Therefore, only hepatectomy was not considered as a surgical option for these four patients with tumors in one lobe. In addition, deep liver tumors are often close to large blood vessels, and patients undergoing hepatectomy will have a higher surgical risk. Carcinoid syndromes such as blushing, diarrhea, heart valve injury, bronchial stenosis, and asthma were not found in any patients. Of the 11 patients, four were associated with metabolic-related diseases such as hypertension, diabetes, and hyperlipidemia, and one was associated with systemic lupus erythematosus and asthma. The details of the information of the patients are summarized in Table 2.
| Patient No. | Sex | Age | Origin | Pathological grade | Bilobar or not | Number of lesions |
| 1 | Female | 62 | Colon | G2 | Yes | 23 |
| 2 | Male | 62 | Unknown | G3 | Yes | 7 |
| 3 | Male | 53 | Pancreatic | G2 | Yes | 41 |
| 4 | Male | 53 | Unknown | G3 | No | 2 |
| 5 | Female | 69 | Unknown | G3 | Yes | 43 |
| 6 | Female | 36 | Unknown | G1 | Yes | 4 |
| 7 | Female | 63 | Rectum | G3 | No | 2 |
| 8 | Female | 65 | Pancreatic | G2 | Yes | 8 |
| 9 | Female | 37 | Pancreatic | G3 | No | 4 |
| 10 | Female | 57 | Pancreatic | G3 | No | 3 |
| 11 | Female | 63 | Unknown | G2 | Yes | 2 |
Of the six patients with liver metastases with a clear primary site, all of them had their primary tumors removed at the same time. All patients underwent ultrasound examinations to confirm the number and distribution of liver metastases. Intraoperative ultrasound examination of all patients revealed all the nodules found by preoperative MRI, among which three patients had additional nodules found by intraoperative ultrasound. The mean operation time was 405.6 (± 39.4) min. The patient's median intraoperative blood loss was 600 (range, 50 to 3000) mL. The median nodules removed by hepatectomy was 1 (range, 1 to 18). The median nodules removed by microwave ablation was 3 (range, 1 to 38). The total number of liver metastasis nodules was 139, and the total number of microwave ablation nodules was 104. Among 11 patients with microwave ablation combined with hepatectomy, the total number of microwave ablation nodules accounted for 74.8% of the total nodules found in the patients by intraoperative ultrasound. There were 43 liver metastases in No. 5 patient. Fourteen nodules were concentrated in segment V of the liver, and four were located on the liver surface. The other 25 nodules were diffusely distributed in the liver. The surgeon removed the nodules in segment V and on the liver surface through hepatectomy. The remaining diffusely distributed nodules were removed by microwave ablation. The details of the surgical information are summarized in Table 3.
| Patient No. | Number of hepatectomy | Number of microwave ablations | Blood loss (mL) | Operative time (min) |
| 1 | 2 | 21 | 50 | 360 |
| 2 | 1 | 6 | 1500 | 427 |
| 3 | 3 | 38 | 3000 | 690 |
| 4 | 1 | 1 | 1500 | 480 |
| 5 | 18 | 25 | 2400 | 510 |
| 6 | 1 | 3 | 200 | 320 |
| 7 | 1 | 1 | 300 | 270 |
| 8 | 4 | 4 | 700 | 310 |
| 9 | 2 | 2 | 500 | 510 |
| 10 | 1 | 2 | 600 | 305 |
| 11 | 1 | 1 | 200 | 280 |
Of the 11 patients, four entered the ICU after surgery. The median postoperative hospital stay for all patients was 14 (range, 7 to 37) d. Ten patients had a fever after surgery. The median duration of fever was 3 (range, 0 to 21) d. Elevated bilirubin levels occurred in all patients after surgery. The median bilirubin on the first day after surgery was 28.5 (range, 10.7 to 98.9) µmol/L. No patient died during postoperative hospitalization. One patient (No. 3 patient) developed respiratory failure, renal insufficiency, and pneumonia after surgery. The remaining patients had no complications. The details of the postoperative information are summarized in Table 4.
| Patient No. | Postoperative hospital stay (d) | Bilirubin on first day after surgery (μmol/L) | Duration of the fever (d) |
| 1 | 12 | 27.2 | 4 |
| 2 | 22 | 34.2 | 2 |
| 3 | 22 | 98.9 | 21 |
| 4 | 37 | 30.5 | 4 |
| 5 | 15 | 28.7 | 2 |
| 6 | 8 | 15.9 | 0 |
| 7 | 12 | 10.7 | 3 |
| 8 | 12 | 23.4 | 2 |
| 9 | 14 | 28.5 | 8 |
| 10 | 14 | 14.3 | 12 |
| 11 | 7 | 34.8 | 1 |
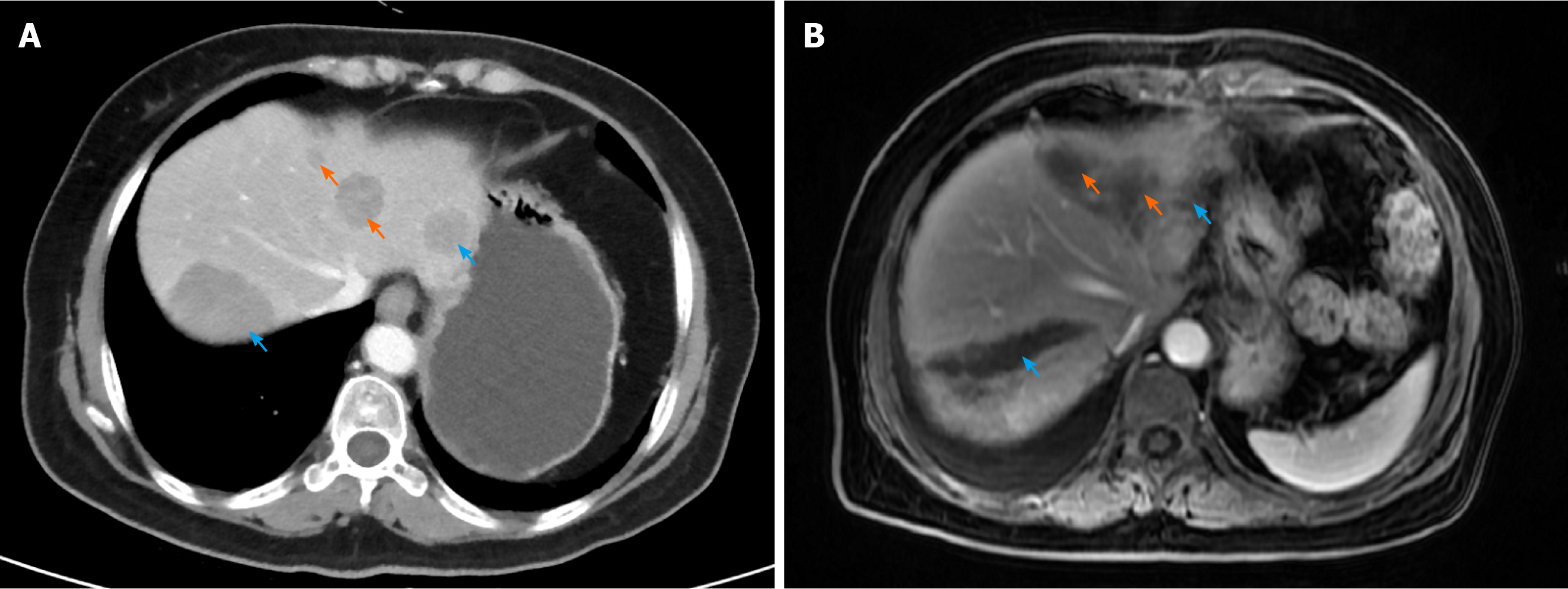
Eleven patients underwent regular re-examination after the operation, and all the patients were re-examined by CT or MRI 1 mo after the operation, which indicated complete ablation. Thereafter, CT or MRI of the abdomen was performed every 3 mo. The preoperative and postoperative imaging examinations of No. 7 patient are shown in Figure 1. All patients were treated with octreotide after the operation. The patient's mean overall survival time after surgery was 34.1 (± 3.7) mo, and the median postoperative progression-free survival time was 8 (range, 2 to 51) mo. One year after surgery, ten patients survived and five patients survived without progression. Three year after surgery, eight patients survived and two patients survived without progression. The details of the follow-up information are summarized in Table 5.
| Patient No. | Progression-free survival time (mo) | Relapse before follow-up | Postoperative survival time (mo) | Died before follow-up |
| 1 | 26 | No | 26 | No |
| 2 | 2.5 | Yes | 48 | Yes |
| 3 | 13 | Yes | 38 | No |
| 4 | 8 | Yes | 24 | Yes |
| 5 | 20 | Yes | 23 | Yes |
| 6 | 51 | No | 51 | No |
| 7 | 7 | Yes | 45 | Yes |
| 8 | 2 | Yes | 34 | No |
| 9 | 2 | Yes | 11 | Yes |
| 10 | 8 | Yes | 41 | No |
| 11 | 6 | Yes | 35 | No |
Hepatectomy is the first choice for the treatment of neuroendocrine tumor liver metastases. Surgical resection can not only improve the patient's ectopic endocrine-related symptoms but also greatly improve the survival time[12]. Due to the widespread nature of tumors, most patients with neuroendocrine tumor liver metastases do not meet the indications for liver resection. Only 10% of patients with neuroendocrine tumor liver metastasis meet the indications for hepatectomy[13]. In addition, patients with neuroendocrine tumor liver metastases after hepatectomy still have a higher recurrence rate. Zhang et al[14] reported that the recurrence-free rate in patients with neuroendocrine tumor liver metastasis after liver resection is approximately 60% after 3 years. Most patients cannot stand secondary hepatectomy. The emergence of ablation technology provides new treatment options for patients with neuroendocrine tumor liver metastases who cannot undergo hepatectomy. The application of ablation technology is flexible. It can be used alone to ablate lesions to reduce the burden of patients' tumors, or it can be combined with hepatectomy for patients who do not have indications for hepatectomy. Some retrospective clinical studies have confirmed the clinical efficacy of ablation in the treatment of liver neuroendocrine tumors. However, reports about surgical resection combined with ablation for neuroendocrine tumor liver metastases are still limited.
Patients with unresectable liver neuroendocrine tumors had a 5-year survival rate of only 22%[15]. According to previous studies and data from our center, after undergoing ablation combined with liver resection, the 1, 3, and 5-year survival rates of these patients were more than 87.5%, 73%, and 61%, respectively[4-6]. This result was similar to the survival rate of patients who only underwent hepatectomy[14]. Therefore, hepatectomy combined with ablation not only expands the surgical indications of patients who do not meet the indications for hepatectomy but also results in an increased survival rate similar to that of hepatectomy. For patients with neuroendocrine tumor liver metastases who are not suitable for hepatectomy, ablation combined with hepatectomy can be used as a new treatment option. However, previous studies have shown that the 1, 3, and 5-year progression-free survival rates of patients undergoing ablation combined with hepatectomy were approximately 60%, 28%, and 17%, respectively, which were much lower than those of patients undergoing hepatectomy[5,14]. Therefore, microwave ablation combined with hepatectomy may lead to a higher recurrence rate than hepatectomy.
Among the 11 patients with microwave ablation combined with hepatectomy, the number of nodules removed by microwave ablation accounted for 74.8% of the total number of nodules found during intraoperative ultrasound. No patient died during postoperative hospitalization. One case had complications including respiratory failure, renal insufficiency, and pneumonia. All patients only had a slight increase in bilirubin level after surgery. Of the 11 patients, ten developed a fever. Among them, eight patients had a fever that lasted less than 10 d, and two patients had a fever that lasted more than 10 d. Because ablation produces a large number of necrotic cells, the absorption of heat is a very common phenomenon in ablation therapy. The previous literature also reported that the ablation of abdominal tumors was accompanied by a large amount of heat absorption. The absorbed heat generated after ablation can be relieved within 10 d after surgery. For fever that lasts longer, it is necessary to consider whether a postoperative infection has occurred[16]. The incidence of complications was low in patients receiving microwave ablation combined with hepatectomy.
Microwave ablation combined with hepatectomy expands the indications for patients with neuroendocrine tumor liver metastasis that are not suitable for hepatectomy. The patients not only obtain a survival rate similar to that of patients undergoing hepatectomy, but also have improved quality of life. However, microwave ablation combined with hepatectomy has a higher recurrence rate than hepatectomy.
Hepatectomy is the first choice for treating neuroendocrine tumor liver metastases. However, most patients with neuroendocrine tumor liver metastases are not suitable for hepatectomy. Ablation combined with hepatectomy can be an alternative to liver resection.
This study evaluated the clinical efficacy of microwave ablation combined with hepatectomy for the treatment of neuroendocrine tumors with liver metastases. It provides new treatment options for patients who are not suitable for only hepat
This study aimed to explore the clinical effect of microwave ablation combined with hepatectomy for the treatment of neuroendocrine tumor liver metastases.
In this study, the data of patients who underwent microwave ablation combined with hepatectomy for the treatment of neuroendocrine tumor liver metastases from June 2015 to January 2018 were reviewed.
Eleven patients with neuroendocrine tumor liver metastases were treated by microwave ablation combined with hepatectomy between June 2015 and January 2018. One patient developed respiratory failure, renal insufficiency, and pneumonia after the operation. No patient died postoperatively during hospitalization. The mean overall survival time after surgery was 34.1 (± 3.7) mo, and the median progression-free survival time was 8 (range, 2 to 51) mo.
Microwave ablation combined with hepatectomy not only makes the patients obtain a survival rate similar to that of patients undergoing hepatectomy, but also has a low incidence of postoperative complications. For patients with neuroendocrine tumor liver metastases who are not suitable for hepatectomy, ablation combined with hepatectomy can be used as a new treatment option.
Due to the low incidence of neuroendocrine tumors, there are currently no prospective studies on neuroendocrine tumor liver metastases. Limited research suggests that ablation combined with hepatectomy for neuroendocrine tumor liver metastasis can prolong the survival rate of patients and improve the quality of life of patients. Prospective or retrospective studies of large cases will be needed in the future.
We thank Liu HX of Peking University People's Hospital for reviewing statistical methods.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kordzaia D S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3246] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 2. | Kose E, Kahramangil B, Aydin H, Donmez M, Takahashi H, Aucejo F, Siperstein A, Berber E. Outcomes of laparoscopic tumor ablation for neuroendocrine liver metastases: a 20-year experience. Surg Endosc. 2020;34:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Pape UF, Berndt U, Müller-Nordhorn J, Böhmig M, Roll S, Koch M, Willich SN, Wiedenmann B. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 4. | Taner T, Atwell TD, Zhang L, Oberg TN, Harmsen WS, Slettedahl SW, Kendrick ML, Nagorney DM, Que FG. Adjunctive radiofrequency ablation of metastatic neuroendocrine cancer to the liver complements surgical resection. HPB (Oxford). 2013;15:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Elias D, Goéré D, Leroux G, Dromain C, Leboulleux S, de Baere T, Ducreux M, Baudin E. Combined liver surgery and RFA for patients with gastroenteropancreatic endocrine tumors presenting with more than 15 metastases to the liver. Eur J Surg Oncol. 2009;35:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Saxena A, Chua TC, Chu F, Al-Zahrani A, Morris DL. Optimizing the surgical effort in patients with advanced neuroendocrine neoplasm hepatic metastases: a critical analysis of 40 patients treated by hepatic resection and cryoablation. Am J Clin Oncol. 2012;35:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Tait IS, Yong SM, Cuschieri SA. Laparoscopic in situ ablation of liver cancer with cryotherapy and radiofrequency ablation. Br J Surg. 2002;89:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Ryan MJ, Willatt J, Majdalany BS, Kielar AZ, Chong S, Ruma JA, Pandya A. Ablation techniques for primary and metastatic liver tumors. World J Hepatol. 2016;8:191-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 235] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 360] [Article Influence: 60.0] [Reference Citation Analysis (1)] |
| 11. | Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation vs radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Knox CD, Anderson CD, Lamps LW, Adkins RB, Pinson CW. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol. 2003;10:1171-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Maithel SK, Fong Y. Hepatic ablation for neuroendocrine tumor metastases. J Surg Oncol. 2009;100:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Zhang XF, Beal EW, Weiss M, Aldrighetti L, Poultsides GA, Bauer TW, Fields RC, Maithel SK, Marques HP, Pawlik TM. Timing of disease occurrence and hepatic resection on long-term outcome of patients with neuroendocrine liver metastasis. J Surg Oncol. 2018;117:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79:813-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 16. | Carrafiello G, Laganà D, Ianniello A, Dionigi G, Novario R, Recaldini C, Mangini M, Cuffari S, Fugazzola C. Post-radiofrequency ablation syndrome after percutaneous radiofrequency of abdominal tumours: one centre experience and review of published works. Australas Radiol. 2007;51:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |