Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5037
Peer-review started: December 15, 2020
First decision: January 17, 2021
Revised: January 28, 2021
Accepted: March 10, 2021
Article in press: March 10, 2021
Published online: July 6, 2021
Processing time: 190 Days and 21.6 Hours
Endometrial lesions include endometrial cancer and inferior fibroids. Among them, endometrial cancer as a malignant tumor seriously endangers the life and health of patients. Ultrasonography is an important means of diagnosing female reproductive system diseases, and it is of critical value for the early diagnosis of endometrial cancer. However, different ultrasound inspection programs have achieved different results. It is of great significance to choose a suitable inspection program.
To explore the diagnostic efficacy of different ultrasonic examination methods in clinical endometrial lesions.
The 140 patients with endometrial lesions who were treated in our hospital from April 2018 to October 2019 were used as the research subjects. All patients underwent transvaginal color ultrasound and transabdominal color ultrasound. We compared the diagnostic coincidence and image display effects of the two different examination methods, and the endometrial thickness, blood flow, uterine effusion and resistance index of different diseases were observed by transvaginal color ultrasound.
The diagnostic coincidence rate of all types of diseases of transvaginal color ultrasound was significantly higher than that of transabdominal color ultrasound (P = 0.001, 0.005, 0.001 and 0.001). In addition, the excellent and good rate of image display of transvaginal color ultrasound was higher than that of transabdominal color ultrasound (P = 0.001). There were significant differences in endometrial thickness in patients with different types of endometrial lesions through the transvaginal color examination (P = 0.001). The incidence rate of uterine effusion in patients with endometrial carcinoma was significantly higher than that in patients with other types of endometrial lesions (P = 0.001), and the rate of the blood flow was the highest (P = 0.001). The comparison of blood flow resistance index indicated that the blood flow resistance index in endometrial cancer patients was the lowest, which shows that the difference was statistically significant (P = 0.001).
The overall diagnostic efficacy of transvaginal color ultrasound in the clinical diagnosis of endometrial lesions is better than that of transabdominal color ultrasound, which held higher diagnostic coincidence rate and image display effect. There were significant differences in the thickness of the endometrium and the blood flow in different types of lesions.
Core Tip: Ultrasonography is an important means of diagnosing female reproductive system diseases, and it is of critical value for the early diagnosis of endometrial cancer. We explored the diagnostic efficacy of different ultrasonic examination methods in clinical endometrial lesions. The display effect of the patients with clinical endometrial lesions who received transvaginal color ultrasound examination was better than that of patients who received transabdominal color ultrasound, and the diagnostic coincidence rate was higher.
- Citation: Lin XL, Zhang DS, Ju ZY, Li XM, Zhang YZ. Diagnostic value of different color ultrasound diagnostic method in endometrial lesions. World J Clin Cases 2021; 9(19): 5037-5045
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5037.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5037
Endometrial lesions are a common gynecological disease in women that ranges in severity and types, including endometrial carcinoma and uterine submucous myoma. The relative research on endometrial lesions suggests that there is high sensitivity of the endometrium to estrogen and progesterone. Endometrial lesions may occur in women when the endocrine system is chronically disturbed[1].
Epidemiological analysis suggests that endometrial carcinoma is a common malignant tumor in women. Increasing work, stress and changing living habits have led to universalization of endocrine disorders, and the incidence rate of this disease increased significantly[2]. The development of early treatment to detect the endometrial lesions and identify them as benign or malignant is imperative[3].
As one of the commonly used methods of clinical examination, the ultrasound has shown good diagnostic efficacy in many female reproductive system diseases. It has the advantages of being noninvasive, simple and easy to review[4]. However, the selection of different ultrasonic examination schemes in clinical endometrial lesions has an effect on the diagnosis. Two commonly used methods of examination include transvaginal color ultrasound and transabdominal color ultrasound. In order to facilitate the selection of the color ultrasound examination scheme, this paper used comparative study to further clarify the diagnostic efficacy of these two color ultrasound examination schemes.
A total of 140 patients with endometrial lesions treated in our hospital from April 2018 to October 2019 were selected for this research approved by the Ethics Committee. Inclusion criteria: (1) Patients with confirmed endometrial lesions; (2) Patients who were informed of the direction and objective of this study and signed the agreement voluntarily; and (3) Patients with nonemergency procedures. Exclusion criteria: (1) Patients with combined severe infection; (2) Patients with combined liver, heart, lung and other organ lesions; (3) Patients with cognitive impairment or inability to follow up; and (4) Patients with hematologic diseases. These 140 patients, aged between 33 and 78, with an average age of 56.52 (± 3.87), were collected under the observation of education level, including 18 cases of junior high school, 55 cases of high school, 38 cases of junior college and 29 cases of undergraduate degree and above.
The color ultrasound examination was performed 3-7 d after their menstruation with a Voluson E8 color ultrasound diagnostic instrument (GE, United States) with the probe frequency set to 5.0-9.0 MHz.
Transabdominal color ultrasound examination: One hour before the examination, the patient was advised to drink water properly until filling her bladder to clearly display her uterus. During the examination, the patient was supine, and the ultrasound probe was placed over the pubic symphysis to complete the multiangle plain scan. Then, the patient was told to change the position, and uterine, pelvic and uterine rectum effusion of the patient were observed.
Transvaginal color ultrasound examination: The patient was asked to empty the bladder taking the lithotomy for examination, sterilizing the condom and applying the coupling agent to the ultrasonic probe. The probe was inserted slowly into the vaginal fornix of the patient, and the uterine shape, size and position of the endometrium were observed.
The diagnosis conformity situation of various endometrial lesions (endometrial carcinoma, endometrial polyp, submucous myoma, endometrial hyperplasia) between the two groups was compared according to the pathological results of surgery.
The endometrial thickness, intrauterine effusion, blood flow display and blood flow resistance index of patients with different types of transvaginal ultrasound were compared.
The images of excellent and good conditions displayed by the two detection methods were compared and divided into excellent, good and poor. Excellent: clear image; good: relatively blurred image; poor: unable to identify. The image display excellent rate = (the number of excellent examples + the number of good examples)/ the total cases × 100%.
All the data in this observation were analyzed by SPSS20.0 statistical software. mean ± SD indicated measurement information. The four groups were tested by F test and χ2 test, with the counting data represented with the rate. P < 0.05 was defined as statistically significant.
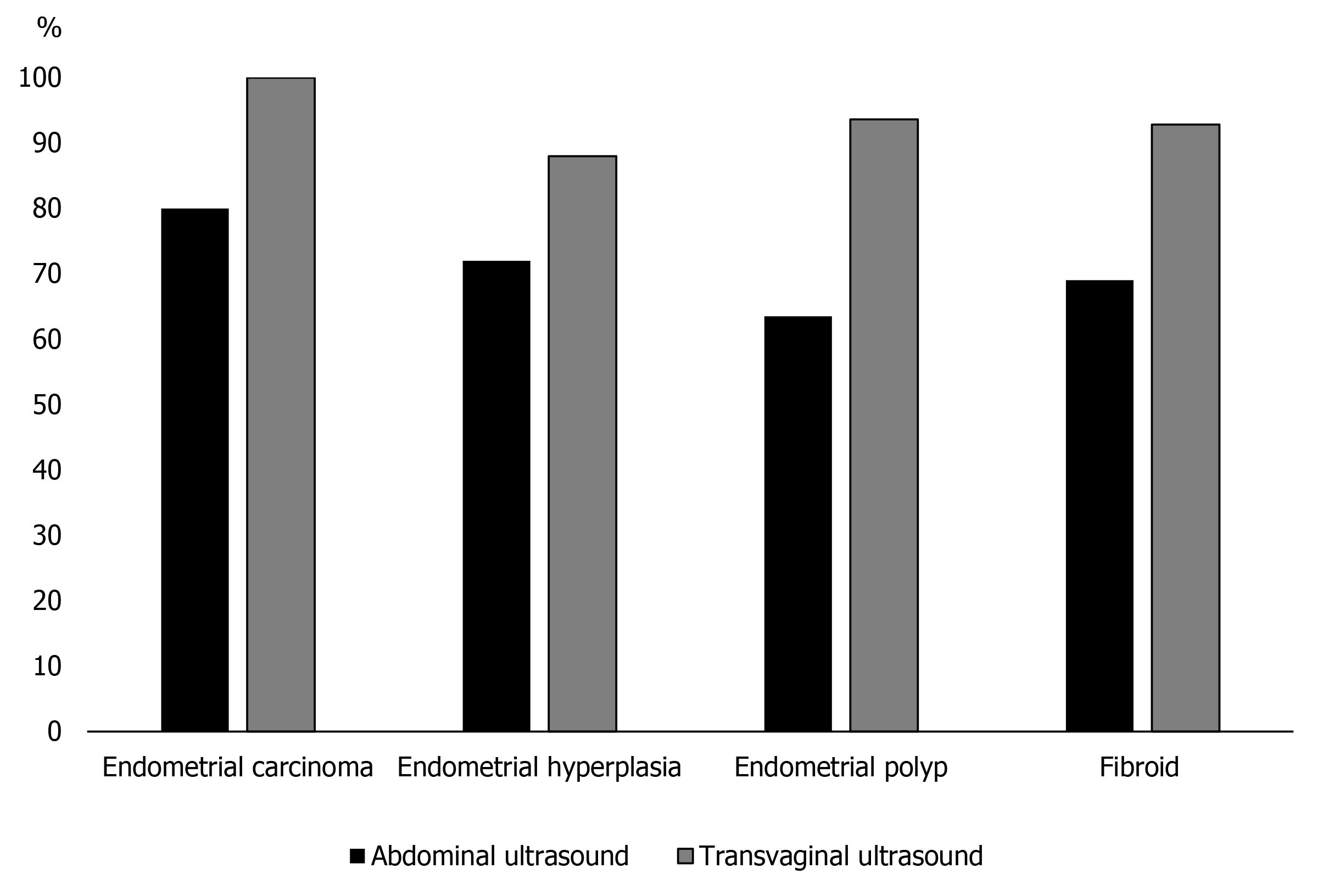
As can be seen from Table 1 and Figure 1, the diagnostic coincidence rate of the transvaginal color Doppler ultrasound for endometrial carcinoma, endometrial polyp, endometrial hyperplasia and submucosal fibroids of the uterus were significantly higher, and the final surgical examination was taken as the reference standard (P = 0.001, 0.005, 0.001 and 0.001, respectively).
| Type | Cases, n | Transabdominal color ultrasound, n (%) | Transvaginal color ultrasound, n (%) | χ2 | P value |
| Endometrial carcinoma | 10 | 8 (80.00) | 10 (100.00) | 22.222 | 0.001 |
| Endometrial hyperplasia | 25 | 18 (72.00) | 22 (88.00) | 8.000 | 0.005 |
| Endometrial polyp | 63 | 40 (63.49) | 59 (93.65) | 27.012 | 0.001 |
| Submucous myoma of uterus | 42 | 29 (69.05) | 39 (92.86) | 18.385 | 0.001 |
As can be seen from Table 2, the excellent and good rate of transvaginal color Doppler ultrasound was higher than that of transabdominal color Doppler ultrasound (P = 0.001).
| Display effect | Transabdominal color ultrasound, n of 140 (%) | Transvaginal color ultrasound, n of 140 (%) | χ2 | P value |
| Excellent | 79 (56.43) | 106 (75.71) | 8.291 | 0.004 |
| Good | 22 (15.71) | 21 (15.00) | 0.019 | 0.889 |
| Poor | 39 (27.86) | 13 (9.29) | 11.400 | 0.001 |
| Showing excellent rate | 101 (72.14) | 127 (90.71) | 11.400 | 0.001 |
As can be seen from Table 3, endometrial thickness of patients with endometrial carcinoma and endometrial hyperplasia was greater than 10 mm, with significant differences in endometrial thickness among different lesion types (P = 0.001).
| Endometrial thickness | Endometrial carcinoma, n of 10 (%) | Endometrial hyperplasia, n of 25 (%) | Endometrial polyp, n of 63 (%) | Submucous myoma of uterus, n of 42 (%) | χ2 | P value |
| < 5 mm | 0 (0.00) | 0 (0.00) | 12 (19.05) | 3 (7.14) | 36.214 | 0.001 |
| 5-10 mm | 0 (0.00) | 0 (0.00) | 20 (31.75) | 24 (57.14) | ||
| > 10 mm | 10 (100.00) | 25 (100.00) | 31 (49.21) | 15 (35.71) |
It can be seen from the data in Table 4 that the incidence rates of the uterine cavity effusion and blood flow display were the highest in endometrial carcinoma through the transvaginal color ultrasound, which indicated that the difference was statistically significant (P = 0.0001 and 0.001, respectively), and the comparison of blood flow resistance index indicated that the blood flow resistance index in endometrial carcinoma patients was the lowest (P = 0.001).
| Project | Endometrial carcinoma, n of 10 (%) | Endometrial hyperplasia, n of 25 (%) | Endometrial polyp, n of 63 (%) | Submucous myoma of uterus, n of 42 (%) | χ2/F | P value |
| Uterine cavity effusion | 10 (100.00) | 6 (24.00) | 10 (15.87) | 10 (23.81) | 25.631 | 0.001 |
| Blood flow display situation | 10 (100.00) | 6 (24.00) | 9 (14.29) | 9 (21.43) | 23.414 | 0.001 |
| Resistance index | 0.34 (0.05) | 0.72 (0.11) | 0.70 (0.09) | 0.53 (0.07) | 79.014 | 0.001 |
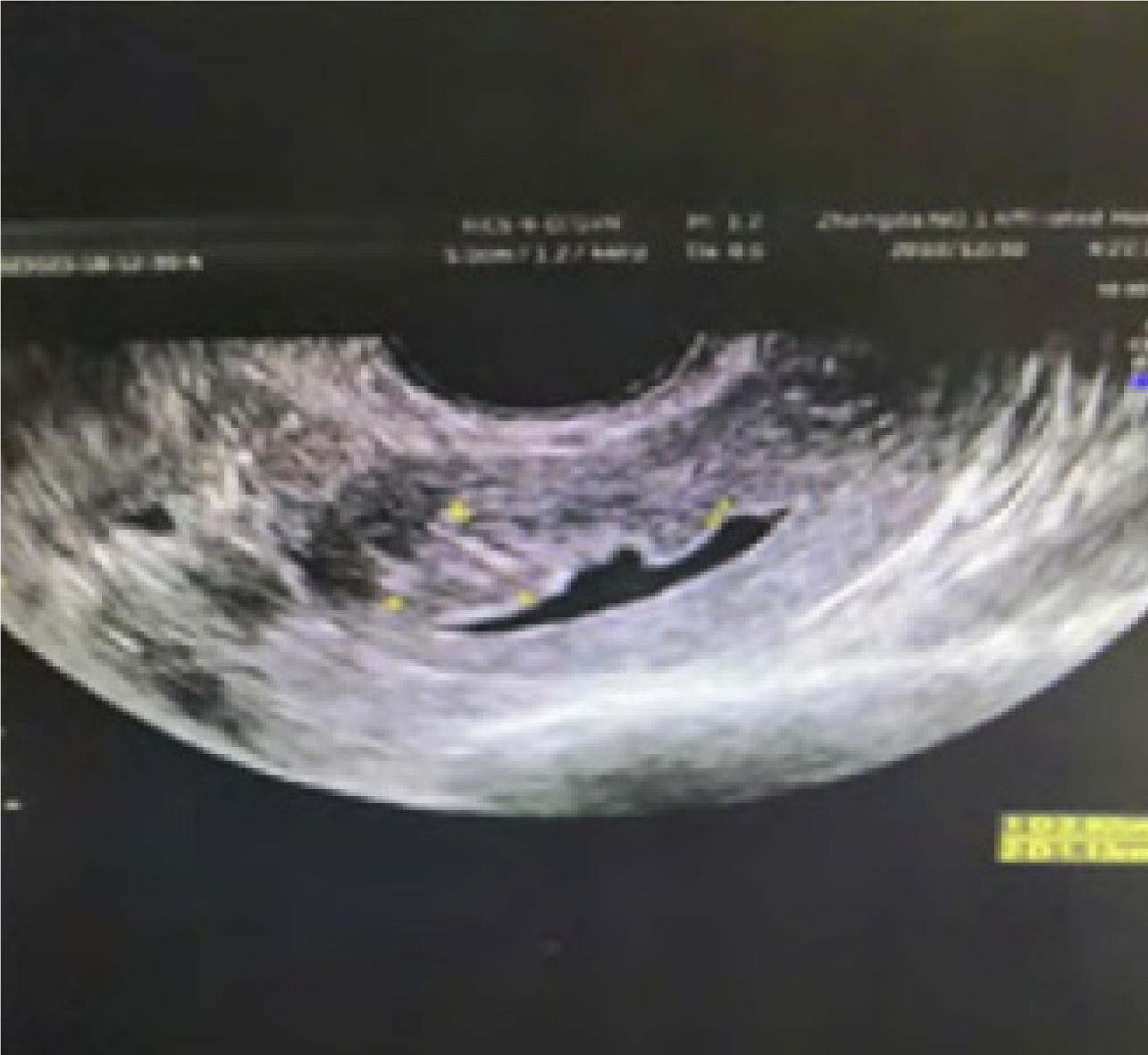
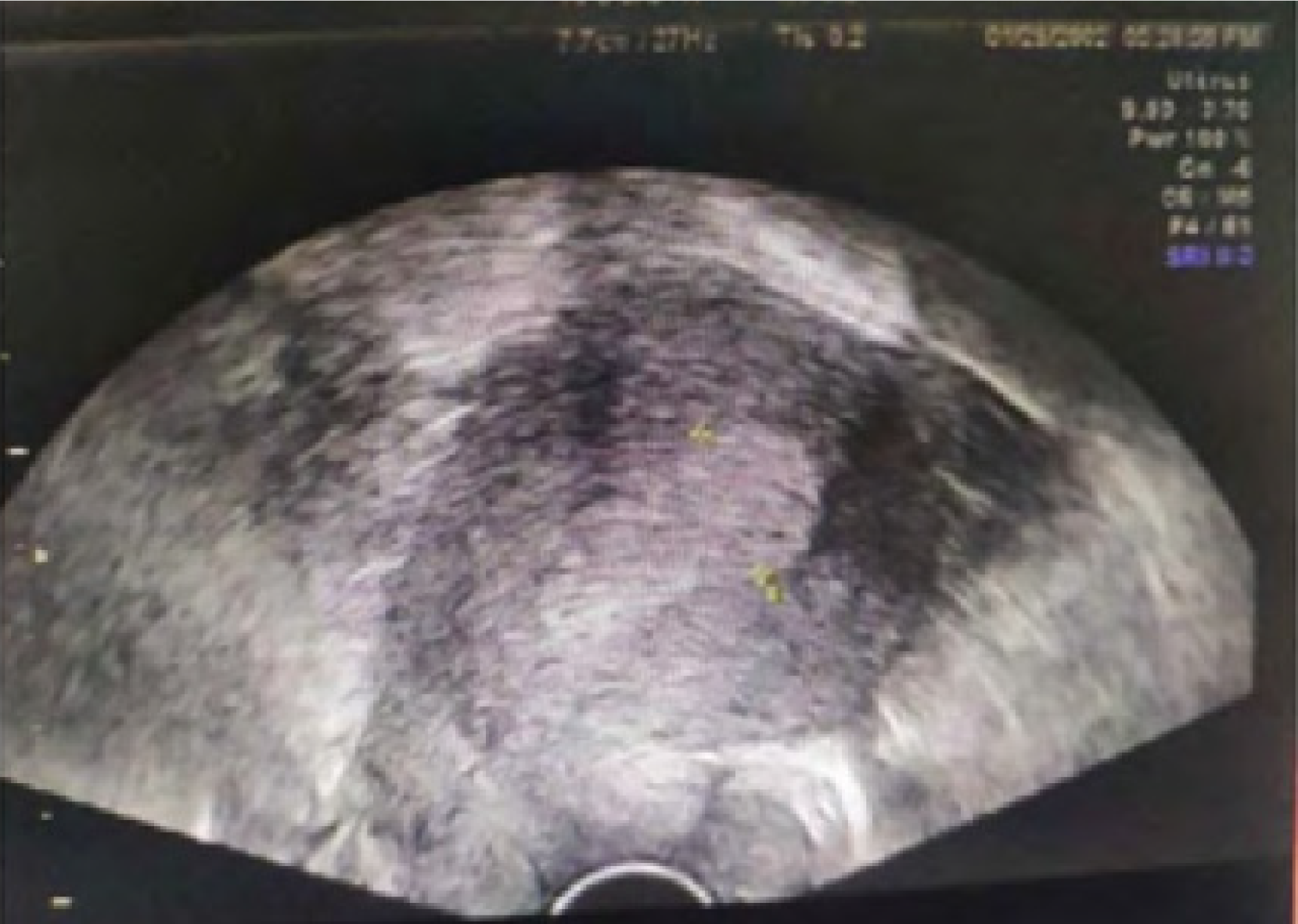
Figure 2 is a transvaginal color ultrasound image of patients with endometrial carcinoma. There is usually an enhanced/weakened echo region in patients with endometrial carcinoma, whose uterine morphology is irregular, occurring endometrial thickening typically, with blurred myometrium boundary and abundant and messy blood flow signals in the lesion. Figure 3 is the image of a transvaginal color ultrasound in patients with endometrial hyperplasia, with irregular thickening endometrium and uneven echo as well as cystic echo and relatively full uterine in shape.
There are many types of endometrial lesions, and they account for 20%-30% of malignant tumors of the female genital tract. It is difficult to distinguish many kinds of lesions directly from clinical symptoms. Thus, the type of lesion needs to be identified by imaging and pathological biopsy[5,6]. If patients can obtain an effective diagnosis in time and have a timely intervention, progression of the disease can be fully avoided, and deterioration of some benign lesions can be prevented. Diagnostic curettage is often used to obtain intrauterine tissue from patients for pathological examination. However, the uterine curettage examination itself is traumatic, has a small focus for some malignant lesions, has a high missed diagnosis rate of no targeted uterine curettage examination and can result in infertility[7,8].
Imaging examination is one of the most commonly used examination methods in the clinic, as it can obtain information on almost all visceral, skeletal and vascular diseases of the human body and has an important reference basis in clinical diagnosis[9,10]. Common imaging methods of endometrial lesions include color ultrasound, computed tomography (CT), X-ray, magnetic resonance imaging (MRI), catheter and angiography, among others. However, there are radiation hazards from X-ray imaging and CT[11]. In contrast to X-ray imaging and CT, MRI has no radiation hazard and high soft tissue resolution. It has high diagnostic efficiency for endometrial lesions[12]. However, MRI is expensive and takes a long time to examine. Therefore, MRI is not applied in the clinical as often as ultrasound, CT, X-ray or color ultrasound.
Color ultrasound is based on the basic principle of different reflected waves when ultrasonic waves travel in different human tissues and encounter different media. By transmitting a special set of ultrasonic waves to the subject, the echo delay time and strength information are obtained. The image information of the tested part is formed by computer system processing[13]. In the diagnosis of endometrial lesions, color ultrasound can clearly show the boundary between the lesion and the surrounding tissue and the situation of hemodynamic changes, which provides a sufficient diagnostic basis for clinical doctors. However, during routine transabdominal color ultrasound examination, the imaging effect is easily disturbed by the frequency of the probe, the depth of the probe, the scope of the patient’s focus, the thickness of the fat layer, whether the bladder is filled and other factors that lead to the deterioration of the quality of endometrial imaging, which can result in the occurrence of a missed diagnosis and misdiagnosis[14].
Compared with transabdominal color ultrasound, transvaginal ultrasound is less dependent on bladder filling and is not disturbed by body weight, intestinal gas or other factors. The closer the position of the vaginal fornix probe is to the focus, the better the image display effect will be. Thus, the endometrial thickness can be measured accurately, and the scope, size and nature of the lesion can be located and quantitatively diagnosed[15,16]. Some clinical studies also believe that transvaginal color ultrasound on the basis of two-dimensional ultrasound combined with three-dimensional ultrasound technology can be used to observe the uterine conditions of patients from different angles. Multiple sections and multiple perspectives were used to conduct the observations. This is more helpful to observe the relationship between the lesion and the endometrial wall so that the doctor can judge the depth of lesion infiltration and to improve the diagnostic accuracy[17-19].
Ultrasound performance varies among patients with different types of lesions when transvaginal color ultrasound is used. In uterine endometrial carcinoma patients, there is usually an enhanced/weakened echo region. Its uterine morphology is irregular, and endometrial thickening typically occurs. The boundary of the myometrium is blurred, and there are abundant and messy blood flow signals in the lesion. In patients with endometrial hyperplasia, the thickening of the endometrium is irregular, and the echo is uneven. There is a cystic echo, and the uterine shape is relatively full. There is no obvious blood flow signal, and the boundary between the endometrium and myometrium is clear. In patients with submucous myoma of the uterus, the performance of the line of the uterine cavity is biased. The boundary between the endometrium and edge is clear and presented as oval or round nodules. The echo is relatively smooth, the blood flow signal in the tumor is punctate, and the periphery is annular. Endometrial polyps are generally round and hyperechoic. The patient’s intimal base layer can be seen as a point-like blood flow signal, and the boundary is relatively clear.
The results of this study show that the diagnostic coincidence rate of transvaginal color ultrasound was higher than that of transabdominal color. The overall image shows that the excellent and good rate of transvaginal color Doppler ultrasound is higher than that of transabdominal color ultrasound. There were significant differences in endometrial thickness, uterine effusion, blood flow, resistance index and other parameters in different types of patients undergoing transvaginal ultrasound examination. In the research by Leonardi et al[20], the value of transvaginal color ultrasound and transabdominal color ultrasound in the examination of endometrial lesions was compared. The result showed that the diagnostic coincidence rate of transvaginal color ultrasound, the total excellent and good rates of image clarity and the score of image definition were higher than those of transabdominal color ultrasound, which is similar to the findings of this study, and that transvaginal color ultrasound can be confirmed to have higher application value than transabdominal color ultrasound in the diagnosis of endometrial lesions.
In summary, the display effect of the patients with clinical endometrial lesions who receive transvaginal color ultrasound examination is better than that of patients who receive transabdominal color ultrasound, and the diagnostic coincidence rate is higher. Thus, it can be preferred.
Endometrial lesions include endometrial cancer and inferior fibroids. Ultrasonography is an important means of diagnosing female reproductive system diseases, and it is of critical value for the early diagnosis of endometrial cancer.
In clinical application, the choice of different ultrasound examination schemes has a certain impact on the diagnosis of endometrial lesions. In order to facilitate the choice of clinical color ultrasound examination schemes, two commonly used examination methods including vaginal color ultrasound and abdominal color ultrasound were compared.
To explore the diagnostic effects of different ultrasound examination methods in clinical endometrial lesions and provide guidance for clinical diagnosis in choosing appropriate examination schemes.
All 140 patients underwent transvaginal color ultrasound and transabdominal color ultrasound. We compared the diagnostic coincidence and image display effects of two examination methods, and the endometrial thickness, blood flow, uterine effusion and resistance index of different diseases were observed by transvaginal color ultrasound.
Compared with transabdominal color ultrasound, transvaginal ultrasound is less dependent on bladder filling and is not disturbed by body weight, intestinal gas or other factors. The closer the position of the vaginal fornix probe is to the focus, the better the image display effect will be. Thus, the endometrial thickness can be measured accurately, and the scope, size and nature of the lesion can be located and quantitatively diagnosed.
The diagnostic coincidence rate of patients with clinical endometrial lesions examined by transvaginal color ultrasound is relatively high, which is worthy of promotion.
This research innovatively compares the diagnostic value of different ultrasound examination schemes for endometrial lesions and provides a more reasonable scheme.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hatta W, Park SJ S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH
| 1. | Xu J, Qiao L, Xiong K, Cheng S, Luo H, Wang Y, He J, Chen X, Pan M. Diagnostic Value of Quantitative Analysis by Contrast-Enhanced Ultrasound of Endometrial Lesions. J Ultrasound Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Méndez Fernández R, Barrera Ortega J. Magnetic resonance imaging of pelvic endometriosis. Radiologia. 2017;59:286-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Gupta A, Bhatnagar A, Seth BN, Dang A, Gupta V. Bladder Endometriosis Mimicking TCC - A Case Report. J Clin Diagn Res. 2016;10:PD12-PD13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Soljačić Vraneš H, Djaković I, Kraljević Z, Nakić Radoš S, Leniček T, Kuna K. Clinical value of transvaginal ultrasonography in comparison to hysteroscopy with histopathologic examination in diagnosing endometrial abnormalities. Acta Clin Croat. 2019;58:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Desplats V, Vitte RL, du Cheyron J, Roseau G, Fauconnier A, Moryoussef F. Preoperative rectosigmoid endoscopic ultrasonography predicts the need for bowel resection in endometriosis. World J Gastroenterol. 2019;25:696-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Loh SH, Lew BL, Sim WY. Primary Cutaneous Endometriosis of Umbilicus. Ann Dermatol. 2017;29:621-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Sudderuddin S, Helbren E, Telesca M, Williamson R, Rockall A. MRI appearances of benign uterine disease. Clin Radiol. 2014;69:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Gerges B, Lu C, Reid S, Chou D, Chang T, Condous G. Sonographic evaluation of immobility of normal and endometriotic ovary in detection of deep endometriosis. Ultrasound Obstet Gynecol. 2017;49:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Jafarey YS, Hanley CS, Berlinski RA, Warner C, Armstrong A. Medical management of leiomyomata and suspected endometriosis in an Allen's swamp monkey (allenopithecus nigroviridus). J Zoo Wildl Med. 2015;46:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Agostinho L, Cruz R, Osório F, Alves J, Setúbal A, Guerra A. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017;8:549-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Liu X. Clinicopathological features of endometriosis in abdominal wall--clinical analysis of 151 cases. Clin Exp Obstet Gynecol. 2016;43:379-383. [PubMed] |
| 12. | Zhang Y, Xiao X, Xu F, Lin Q, Xu J, Du B. Evaluation of Uterosacral Ligament Involvement in Deep Endometriosis by Transvaginal Ultrasonography. Front Pharmacol. 2019;10:374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Seo JW, Lee DY, Yoon BK, Choi D. The Efficacy of Postoperative Cyclic Oral Contraceptives after Gonadotropin-Releasing Hormone Agonist Therapy to Prevent Endometrioma Recurrence in Adolescents. J Pediatr Adolesc Gynecol. 2017;30:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Shi H, Chen X, Lv B, Zhang X. Concurrent tamoxifen-related Müllerian adenofibromas in uterus and ovary. Int J Clin Exp Pathol. 2015;8:15381-15385. [PubMed] |
| 15. | DE Oliveira R, Adami F, Mafra FA, Bianco B, Vilarino FL, Barbosa CP. Causes of endometriosis and prevalent infertility in patients undergoing laparoscopy without achieving pregnancy. Minerva Ginecol. 2016;68:250-258. [PubMed] |
| 16. | Green RW, Epstein E. Dynamic contrast-enhanced ultrasound improves diagnostic performance in endometrial cancer staging. Ultrasound Obstet Gynecol. 2020;56:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Alborzi S, Rasekhi A, Shomali Z, Madadi G, Alborzi M, Kazemi M, Hosseini Nohandani A. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine (Baltimore). 2018;97:e9536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Novelli AA, Puppo A, Ceccaroni M, Olearo E, Monterossi G, Mantovani G, Pelligra S, Olearo PL, Fanfani F, Scambia G. Diagnostic accuracy and economic impact of three work-up strategies identifying risk groups in endometrial cancer, fully incorporating sentinel lymph node algorithm. Facts Views Vis Obgyn. 2020;12:169-177. [PubMed] |
| 19. | Capozzi VA, Merisio C, Rolla M, Pugliese M, Morganelli G, Cianciolo A, Gambino G, Armano G, Sozzi G, Riccò M, Berretta R. Confounding factors of transvaginal ultrasound accuracy in endometrial cancer. J Obstet Gynaecol. 2020;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Leonardi M, Robledo KP, Espada M, Vanza K, Condous G. SonoPODography: A new diagnostic technique for visualizing superficial endometriosis. Eur J Obstet Gynecol Reprod Biol. 2020;254:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |