Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5019
Peer-review started: March 4, 2021
First decision: April 6, 2021
Revised: April 8, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: July 6, 2021
Processing time: 112 Days and 3.7 Hours
The poor prognosis and rising incidence of esophageal cancer highlight the need for improved therapeutics that are essential prior to treatment. LCL161 is an SMAC (second mitochondrial activator of caspases) mimic and inhibitor of apoptosis protein (IAP) antagonist which exhibits anti-tumor effects and improves the chemical sensitivity of many cancers.
To ascertain the effects and mechanisms of the SMAC analog LCL161 on esophageal cancer cells.
MTT assay and TUNEL assay were used to detect cell proliferation and apoptosis, respectively. Western blot analysis was used to study the molecular mechanisms of LCL161-induced death of ECA109 cells.
LCL161 decreased ECA109 cell proliferation in dose- and time-dependent manner and induced apoptosis of ECA109 cells in a dose-dependent manner. Also, LCL161 induced a significant decrease in the expression of the XIAP and significant increase in the expression of Caspase-3. In addition, Bax increased significantly with increasing concentrations of LCL161, and the relative expression of Bax was significantly different between groups.
These findings support the hypothesis that LCL161 can inhibit proliferation and induce apoptosis in esophageal cancer cells by regulating the expression of IAP family members, suggesting that it has potential to be an effective treatment for esophageal squamous cell carcinoma.
Core Tip: The poor prognosis and rising incidence of esophageal cancer highlight the need for improved therapeutics that are essential prior to treatment. The aim of this study was to explore the mechanisms by which SMAC (second mitochondrial activator of caspases) mimic inhibits proliferation and induce apoptosis in ECA109 esophageal cancer cells. The findings support the hypothesis that LCL161 can inhibit proliferation and induce apoptosis in esophageal cancer cells by regulating the expression of inhibitor of apoptosis proteins family members, suggesting that it has potential to be an effective treatment for esophageal squamous cell carcinoma.
- Citation: Jiang N, Zhang WQ, Dong H, Hao YT, Zhang LM, Shan L, Yang XD, Peng CL. SMAC exhibits anti-tumor effects in ECA109 cells by regulating expression of inhibitor of apoptosis protein family. World J Clin Cases 2021; 9(19): 5019-5027
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5019.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5019
Esophageal cancer is one of the most lethal forms of malignancy worldwide, and there has been a dramatic increase in the incidence of esophageal cancer over the past few decades[1]. Esophageal cancer has two main subtypes: Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma. More than 90% of esophageal cancer cases worldwide are ESCC, which is currently predominant in China[2]. Despite improvements in the management and treatment of esophageal cancer patients, the diagnosis of ESCC at advanced or late stages means that most ESCC patients have no alternative but to receive chemotherapy, radiotherapy, and adjuvant treatments. The general outcome remains very poor in terms of the overall 5-year survival rate (approximately 10%)[3]. Considering the strong potential for invasion and metastasis, the poor prognosis of esophageal cancer highlights the need to determine the factors that affect the prognosis and recurrence or metastasis.
At present, tumor cell apoptosis processes have received widespread attention. SMAC (second mitochondrial activator of caspases) is a mitochondrial protein that interacts with inhibitor of apoptosis proteins (IAPs) and, upon apoptosis initiation, is released into the cytoplasm to inhibit the caspase-binding activity of IAPs, including XIAP, IAP1, IAP2, and survivin[4,5]. Previous reports have indicated that the SMAC mimic LCL161 significantly inhibits the proliferation and induces the apoptosis of lung cancer, liver cancer, leukemia, breast cancer, and other tumor cells[6-8]. Furthermore, SMAC has been demonstrated to be a predictive factor in many cancers treated with chemotherapy[4,9-12]. These results suggest that the expression of SMAC in tumor cells may predict a good response to anticancer processes. However, few studies have focused on the effects of SMAC mimics on cell proliferation and apoptosis in esoph
Therefore, in this study, we used MTT assay, TUNEL assay, and Western blot analysis of the expression of XIAP, Caspase-3, and Bax to explore the mechanisms by which SMAC mimic inhibits proliferation and induce apoptosis in ECA109 esophageal cancer cells.
Human ESCC cell line ECA109, which was purchased from Shanghai Cell Institute of Chinese Academy of Sciences, was cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, United States) supplemented with 10% heat-inactivated fetal bovine serum (Gibco) and 100 μg/mL penicillin-streptomycin. The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2. LCL161 (cat. No. 16169) was purchased from MedChemExpress (MedChemExpress, Monmouth Junction, NJ, United States). The TUNEL kit, thiazolyl blue tetrazolium bromide (MTT), and DMSO were purchased from Sigma (St. Louis, MO, United States). Antibodies against XIAP, Bax, and Caspase-3 were purchased from Cell Signaling Technology.
Cell proliferation was detected by MTT assay according to the manufacturer's protocol. Cells in logarithmic growth phase were used for experiments. ECA109 cells were seeded into 96-well plates and treated with different concentrations of LCL161 (5, 10, and 20 mmol/L) for 24, 48, and 72 h, respectively. Subsequently, 20 μL of MTT (5 mg/mL) reagent was added into each well, and the plates were incubated for 4 h at the incubator chamber. Then, the supernatants were gently aspirated and 200 μL of DMSO was added. Absorbance was measured at 490 nm with a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, United States). Cells in each group were plated in triplicate; three independent experiments were performed.
Cell apoptosis was detected by TUNEL assay according to the manufacturer's protocol. ECA109 cells were seeded on coverslips and incubated with serum-free medium for 48 h. Cells were treated with different concentrations of LCL161 (5, 10, and 20 mmol/L) or DMSO for 24 h. After dewaxing and rehydrating with xylene and ethanol, ECA109 cells were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 1 h at 25 °C, blocked with 3% H2O2 for 10 min, and permeabilized with 0.1% Triton X-100 sodium citrate solution for 3 min. Apoptotic cells were labelled by TUNEL assay, and cell nuclei were labelled with DAPI. Images (magnification × 40) were obtained using a BX43 fluorescence microscope (Olympus Corporation, Tokyo, Japan), and apoptotic cells were analyzed with ImageJ software, each with five randomly selected fields.
Cells treated with different concentrations of LCL161 (5 and 10 mmol/L) for 48 h were lysed using RIPA buffer (Thermo Fisher Scientific, Inc.). Total protein was quantified with a BCA kit (Beyotime Institute of Biotechnology, Haimen China). Subsequently, 50 µg of denatured protein per lane was subjected to SDS-PAGE on 12% polyacrylamide gels and then transferred to polyvinylidene difluoride membranes. Following blocking with 5% skim milk in TBS containing 0.05% Tween-20 for 1 h at room temperature, the membranes were incubated with primary antibodies against XIAP, Bax, Caspase-3, and β-actin at 4 °C overnight. After washing with TBST, the corresponding fluorescently labelled secondary antibodies were incubated with the membranes at room temperature for 1.5 h. The proteins were detected using the Luminata Forte Western HRP substrate (EMD Millipore) according to the manufacturer's protocol. Band intensity was analyzed using ImageJ software.
SPSS version 19.0 software (IBM Corp., Armonk, NY, United States) was used for statistical analyses. Significance was determined using one-way ANOVA with Tukey’s multiple comparisons. All values are expressed as the mean ± SD (n ≥ 3). P < 0.05 was considered statistically significant.
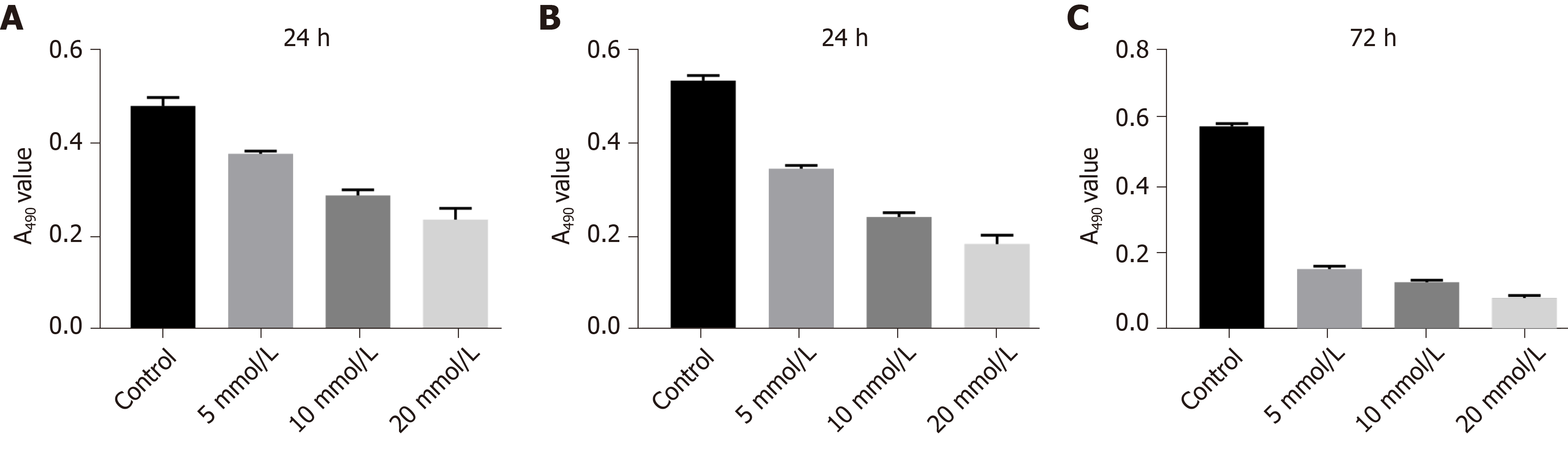
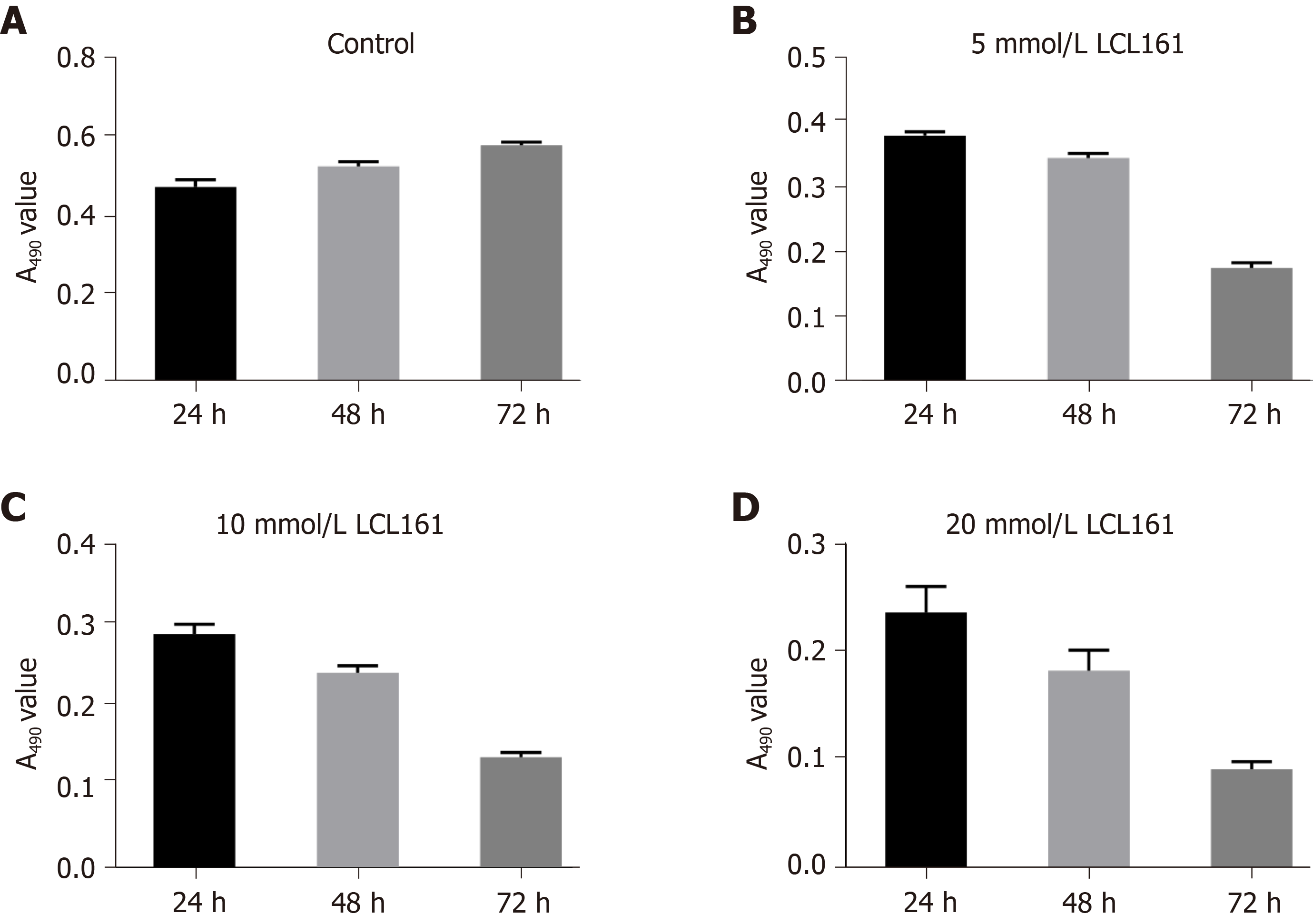
The cytotoxicity of LCL161 against ECA109 cells was evaluated by MTT assay. As shown in Figures 1 and 2, the exposure of ECA109 cells to various concentrations of LCL161 (5, 10, and 20 mmol/L) for 24, 48, and 72 h were detected by MTT assay. After LCL161 treatment, the proliferation rates of the three groups changed obviously. At each time point, the proliferation rate of ECA109 cells in the LCL161 groups was significantly decreased compared with the control group (P < 0.05; Figure 1). Additionally, the proliferation of ECA109 cells in the LCL161 groups decreased with time (P < 0.05; Figure 2). These results showed that LCL161 decreased ECA109 cell proliferation in dose- and time-dependent manner.
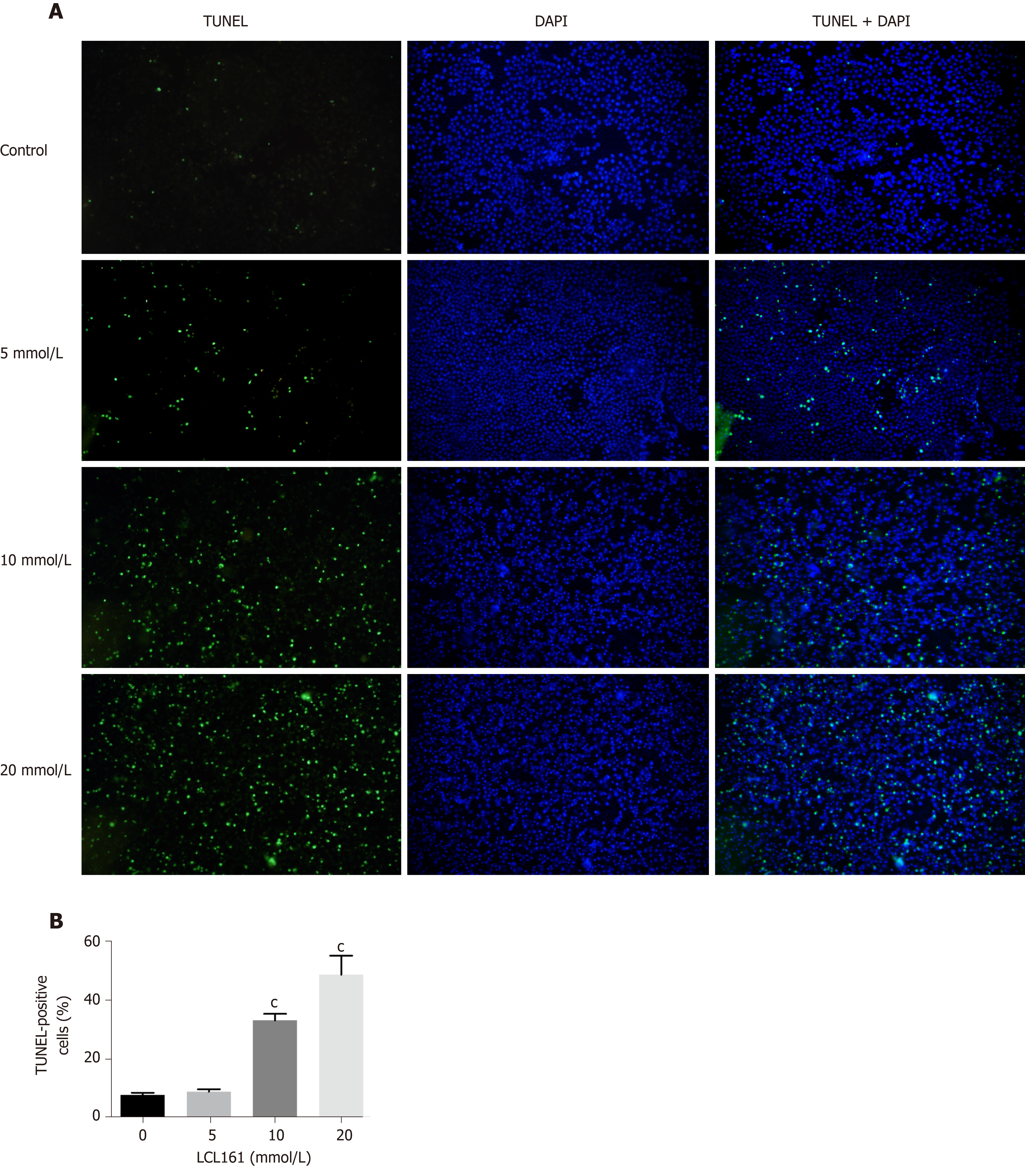
We next investigated whether LCL161 inhibits the proliferation of ECA109 cells, and whether LCL161 could promote ECA109 cell apoptosis by using TUNEL staining. As shown in Figure 3A, the apoptotic rate of ECA109 cells in the control group, 5 mmol/L, 10 mmol/L, and 20 mmol/L LCL161 groups were 7.73 + 0.78, 8.88 + 0.83, 33.13 + 2.26, and 48.67 + 6.50, respectively. The apoptotic cells in the LCL161 groups were significantly increased compared with those in the control group (P < 0.05). Additionally, the apoptosis rate of ECA109 cells was significantly higher in the 20 mmol/L group than in the control, 5 mmol/L, and 10 mmol/L groups (P < 0.001). However, the apoptotic cells significantly increased with the increase of drug concentration. Together, these TUNEL staining results indicated that LCL161 induced apoptosis of ECA109 cells in a dose-dependent manner.
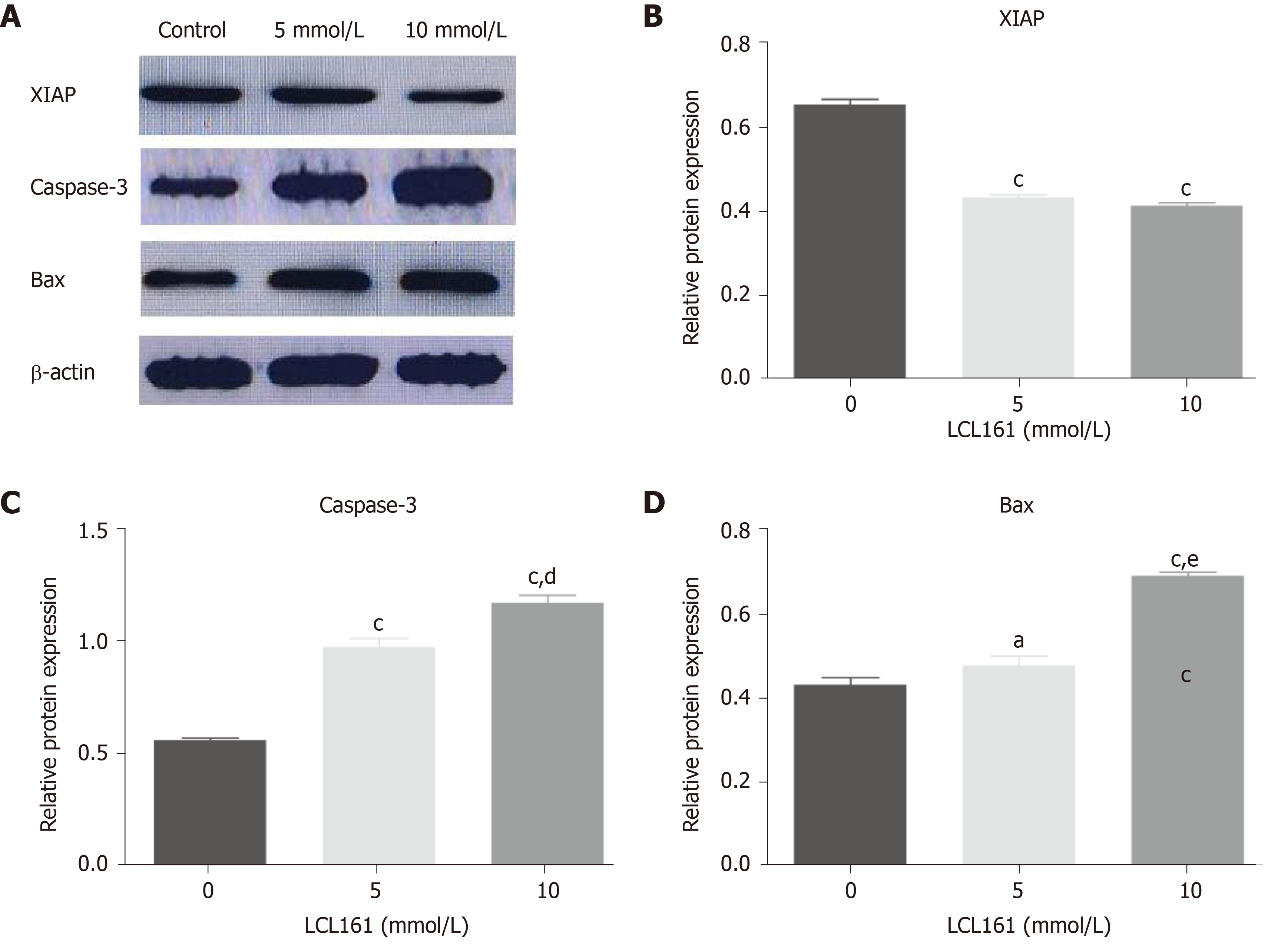
Abnormal regulation of cell apoptosis is related to the occurrence and progression of tumor. To further find the underlying mechanism of apoptosis-promoting effect of LCL161, the expression of XIAP, Caspase-3, and Bax was observed by Western blot. In this part, 20 mmol/L LCL161 was not included in the study due to the large apoptosis of cells. As shown in Figure 4, LCL161 induced a significant decrease in the expression of XIAP and significant increase in the expression of Caspase-3, which is necessary for their apoptosis-promoting effects. In addition, Bax increased significantly with increasing concentrations of LCL161, and the relative expression of Bax was signifi
Esophageal cancer ranks eighth among the most common cancers and sixth in terms of cancer-related mortality worldwide[1]. Unfortunately, the current therapeutic options for ESCC are far from satisfactory due to its strong potential for invasion and metastasis and its poor prognosis. Therefore, effective therapeutic agents urgently need to be developed. Previous reports have suggested that the SMAC mimic LCL161 inhibits the proliferation and induces the apoptosis of liver cancer, leukemia, breast cancer, and other tumor cells[6,7]. Previously, our team demonstrated that SMAC overexpression significantly inhibited A549 cell invasion and promoted apoptosis, providing a potential approach for the biological therapy of lung cancer[8]. Further
SMAC contains factors that reduce various aspects of tumorigenicity. In the current study, we confirmed that the SMAC mimic LCL161 decreased ECA109 cell proliferation in dose- and time-dependent manner and induced ECA109 cell apoptosis in a dose-dependent manner, consistent with previous reports[11,13]. SMAC is an endogenous IAP antagonist that promotes apoptosis by removing the inhibition of Caspase by IAP. LCL161 is a small molecule analog of SMAC that can be taken orally and is an IAP inhibitor. LCL161 has a high affinity for IAPs to hydrolyze them, activate Caspases, and then induce apoptosis[13].
Apoptosis plays a key role in maintaining the homeostasis of cell proliferation and cell death, and disorder of its regulatory mechanism is generally considered an important marker of tumors[14]. Many factors are involved in the regulation of cell apoptosis, among which IAPs, a key negative regulator of apoptotic pathways, play an important role in tumor development[15]. Members of the IAP family are endogenous inhibitors of the Caspase family that can inhibit apoptosis by binding to Caspases via a highly conserved baculovirus IAP sequence[16]. Among the IAP family, XIAP has the strongest inhibitory effect on apoptosis, and its binding to Caspase-3 can cause it to lose its original activity and prevent apoptosis[17]. The SMAC protein activates Caspase-3 and other Caspases, granule B, calpain, and the tissue albumin lysis protein Bid through the death receptor apoptosis pathway. Afterwards, Bid is transferred to the mitochondria, which enhances the expression of the apoptotic protein Bax and increases the permeability of the mitochondrial outer membrane, thereby promoting the release of SMAC[18-21]. SMAC can specifically bind the apoptotic inhibitor XIAP, relieve the inhibitory effect of XIAP on Caspase-3, and release activated Caspase-3, thus promoting the occurrence of apoptosis[17]. The results of our study showed that the expression levels of the XIAP protein in the control group and in the 5 mmol/L and 10 mmol/L LCL161 groups decreased successively, while the expression levels of the Bax and Caspase-3 proteins increased successively, suggesting that LCL161 could upregulate the expression of Bax and Caspase-3 and inhibit the expression of XIAP, thereby inducing apoptosis of ECA109 cells.
There are few experiments on cell function verification. In this study, cell proliferation and apoptosis were confirmed only by MTT and TUNEL assays. Transwell and scratch experiments also confirmed the effect of LCL161 on the migration and infiltration of ECA109 cells. Flow cytometry can also be added to verify apoptosis. The mechanism studies were relatively superficial, and only the key proteins of IAPS family were verified by Western blot. Genomics can be used to find the key molecules involved in the mechanism of action.
In conclusion, our results confirmed that the SMAC mimic LCL161 decreased ECA109 cell proliferation in a dose- and time-dependent manner and induced ECA109 cell apoptosis in a dose-dependent manner. Additionally, changes in the apoptotic signals of XIAP, Caspase-3, and Bax provide a theoretical basis for the treatment of ESCC with LCL161.
The poor prognosis and rising incidence of esophageal cancer highlight the need for improved therapeutics that are essential prior to treatment. LCL161 is an SMAC (second mitochondrial activator of caspases) mimic and inhibitor of apoptosis protein (IAP) antagonist which exhibits anti-tumor effects and improves the chemical sensitivity of many cancers.
In order to improve the chemical sensitivity of esophageal cancer, we studied the effects and mechanisms of the SMAC on esophageal cancer. Our study will provide new ideas for the treatment of esophageal cancer.
The aim of this study was to ascertain the effects and mechanisms of the SMAC analog LCL161 on esophageal cancer cells.
MTT assay and TUNEL assay were used to detect cell proliferation and apoptosis, respectively. Western blot analysis was used to study the molecular mechanisms of LCL161-induced death of ECA109 cells.
LCL161 decreased ECA109 cell proliferation in a dose- and time-dependent manner and induced apoptosis of ECA109 cells in a dose-dependent manner. Also, LCL161 induced a significant decrease in the expression of the XIAP and significant increase in the expression of Caspase-3. In addition, Bax increased significantly with increasing concentrations of LCL161, and the relative expression of Bax was significantly different between groups.
These findings support the hypothesis that LCL161 can inhibit proliferation and induce apoptosis in esophageal cancer cells by regulating the expression of IAP family, suggesting that it has potential to be an effective treatment for esophageal squamous cell carcinoma (ESCC).
Our results confirmed that the Smac mimic LCL161 decreased ECA109 cell proliferation in a dose- and time-dependent manner and induced ECA109 cell apoptosis in a dose-dependent manner. Additionally, changes in the apoptotic signals of XIAP, Caspase-3, and Bax provide a theoretical basis for the treatment of ESCC with LCL161. The underlying molecular mechanisms need to be further investigated.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carey I, Chon HY S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Lordick F, Hölscher AH, Haustermans K, Wittekind C. Multimodal treatment of esophageal cancer. Langenbecks Arch Surg. 2013;398:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598-5606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 736] [Cited by in RCA: 747] [Article Influence: 62.3] [Reference Citation Analysis (8)] |
| 3. | Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg. 2018;41:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 542] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 4. | Ye T, Yao H, Xu Y, Zhao X, Lu H, Zhang R. Role of Smac, survivin, XIAP, and Omi/HtrA2 proteins in determining the chemotherapeutic response of patients with cervical cancer treated with neoadjuvant chemotherapy. Cancer Biomark. 2019;26:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Rathore R, McCallum JE, Varghese E, Florea AM, Büsselberg D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis. 2017;22:898-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 6. | Chen Z, Chen J, Liu H, Dong W, Huang X, Yang D, Hou J, Zhang X. The SMAC Mimetic APG-1387 Sensitizes Immune-Mediated Cell Apoptosis in Hepatocellular Carcinoma. Front Pharmacol. 2018;9:1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Nikkhoo A, Rostami N, Hojjat-Farsangi M, Azizi G, Yousefi B, Ghalamfarsa G, Jadidi-Niaragh F. Smac mimetics as novel promising modulators of apoptosis in the treatment of breast cancer. J Cell Biochem. 2019;120:9300-9314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Yang C, Wang H, Zhang B, Chen Y, Zhang Y, Sun X, Xiao G, Nan K, Ren H, Qin S. LCL161 increases paclitaxel-induced apoptosis by degrading cIAP1 and cIAP2 in NSCLC. J Exp Clin Cancer Res. 2016;35:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Zhao YC, Wang Y, Ni XJ, Li Y, Wang XM, Zhu YY, Luo CY. Clinical significance of Smac and survivin expression in breast cancer patients treated with anthracyclinebased neoadjuvant chemotherapy. Mol Med Rep. 2014;9:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Xu Y, Zhou L, Huang J, Liu F, Yu J, Zhan Q, Zhang L, Zhao X. Role of Smac in determining the chemotherapeutic response of esophageal squamous cell carcinoma. Clin Cancer Res. 2011;17:5412-5422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Hao Y, Sun Q, Peng C. Role of Smac in apoptosis of lung cancer cells A549 induced by Taxol. Clin Lab. 2015;61:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Hou L, Chen K, Hao Y, Zhao Y, Sun Q, Zhao X, Peng C. Effect of Smac in combination with cisplatin on esophageal cancer cell line ECA109. Int J Clin Exp Med. 2015;8:18506-18511. [PubMed] |
| 13. | Tian A, Wilson GS, Lie S, Wu G, Hu Z, Hebbard L, Duan W, George J, Qiao L. Synergistic effects of IAP inhibitor LCL161 and paclitaxel on hepatocellular carcinoma cells. Cancer Lett. 2014;351:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Liu Q, Wang HG. Anti-cancer drug discovery and development: Bcl-2 family small molecule inhibitors. Commun Integr Biol. 2012;5:557-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Luan H, Ye F, Wu L, Zhou Y, Jiang J. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg. 2014;14:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Aslam R, Speck ER, Kim M, Freedman J, Semple JW. Transfusion-related immunomodulation by platelets is dependent on their expression of MHC Class I molecules and is independent of white cells. Transfusion. 2008;48:1778-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Federici AB, Vanelli C, Arrigoni L. Transfusion issues in cancer patients. Thromb Res. 2012;129 Suppl 1:S60-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Billen LP, Shamas-Din A, Andrews DW. Bid: a Bax-like BH3 protein. Oncogene. 2008;27 Suppl 1:S93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Deraco M, Baratti D, Cabras AD, Zaffaroni N, Perrone F, Villa R, Jocollè J, Balestra MR, Kusamura S, Laterza B, Pilotti S. Experience with peritoneal mesothelioma at the Milan National Cancer Institute. World J Gastrointest Oncol. 2010;2:76-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Jensen S, Seidelin JB, LaCasse EC, Nielsen OH. SMAC mimetics and RIPK inhibitors as therapeutics for chronic inflammatory diseases. Sci Signal. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Abhari BA, McCarthy N, Le Berre M, Kilcoyne M, Joshi L, Agostinis P, Fulda S. Smac mimetic suppresses tunicamycin-induced apoptosis via resolution of ER stress. Cell Death Dis. 2019;10:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |