INTRODUCTION
Currently, gestational diabetes mellitus (GDM) is the most common medical complication accompanied by abnormal glucose tolerance during pregnancy, and the prevalence of undiagnosed hyperglycaemia is increasing, with this condition affecting approximately 14% of pregnancies[1]. Based on clinical reports, hyperglycaemia is associated with a well-documented range of adverse pregnancy outcomes for the mother and foetus, in terms of both short- and long-term consequences[2,3]. Maternal hyperglycaemia leads to foetal hyperglycaemia because glucose is easily transferred across the placenta; moreover, even lipids and amino acids may reach the foetus during pregnancy[3]. Deliveries of GDM pregnancies usually result in a range of adverse outcomes and health hazards for the mother and offspring, including increased risks of polyhydramnios, shoulder dystocia, operative delivery, and birth canal lacerations as short-term consequences, as well as increased risks of the subsequent development of obesity, type 2 diabetes (T2DM), and cardiovascular disease in the mother and the child as long-term consequences[1,4,5]. Currently, the use of continuous subcutaneous insulin injections is a potential alternative to the United States national recommendation[2,5]. However, insulin infusions and traditional hypoglycaemic drugs have been shown to induce insulin resistance and other side effects[1,3]; additionally, these treatments have resulted in long-term safety concerns for the mother and offspring. Additionally, increasing evidence suggests that insulin analogues (short- and long-acting) are safe alternatives to human insulin during pregnancy[6,7]. Therefore, it is critical to discover and develop new, nontoxic therapeutic agents, especially those agents of natural or plant origins.
Polysaccharides, which are natural macromolecular polymers, have diverse biological activities and pharmacological effects, such as the regulation of immunity[8-10], anticancer effects[11,12], and antioxidative effects[9,12]. As polysaccharides can be derived from existing natural sources, such as plants[13,14], animals, and microorganisms, they are easily available, nontoxic, inexpensive, biodegradable, and biocompatible; additionally, they are currently garnering interest as having valuable applications[9,11,12], especially polysaccharides that are isolated from P. ginseng C. A. Meyer[9,15]. Based on the current study, ginseng polysaccharide (GPS), the extract of polysaccharides from P. ginseng, is widely used in the fields of medicine and health foods, and it has broad market prospects[9,15]. To date, approximately 80 polysaccharides have been isolated from P. ginseng, and current reports demonstrate that GPS has promising biological activities and pharmacological effects, such as immunomodulation and antitumour, antiulcer, antiradiation, antioxidant, and hypoglycaemic activities[9,15]. P. ginseng has been used for the prevention and treatment of cardiovascular and cerebrovascular diseases for several hundred years[16-19]. Thus, it is highly possible that GPS can be developed as a novel candidate agent or treatment for cardiovascular and cerebrovascular diseases.
Based on current reports, it is hypothesised that GPS may be an ideal alternative or auxiliary hypoglycaemic agent for the treatment of GDM. However, to date, there have been no related research results to verify this possibility, and no systematic data analyses or summarized reviews have been conducted to assess the potential and feasibility of using GPS as a new therapeutic agent for treating GDM. Hence, we collect the available data from in vitro and in vivo experiments, organize and analyse the literature concerning the biological activities and pharmacological effects of GPS, provide related literature information, and analyse the potential use of GPS against GDM. The current study will provide additional rationales and a basis for the development of GPS in treating GDM.
BIOLOGICAL ACTIVITIES AND EFFECTS
Antioxidant activities and mitochondrial protection
It is known that excessive free radicals in the body can lead to different types of cell damage and can also cause several chronic human diseases. Based on related reports, polysaccharides from native P. ginseng (GPS) and its phosphorylated derivatives are clearly able to scavenge superoxide anion free radicals that are produced by the pyrogallol system[9,20]. In addition, these molecules are able to decrease 1,1-diphenyl-2-picrylhydrazyl and hydroxyl radical levels, notably inhibit the formation of malondialdehyde (MDA) in mouse brains in vivo[21], increase creatine kinase activities in mouse skeletal muscle under hypoxia-induced conditions[21], and improve the levels of cytosolic superoxide dismutase (cyt-SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and peroxiredoxin[22].
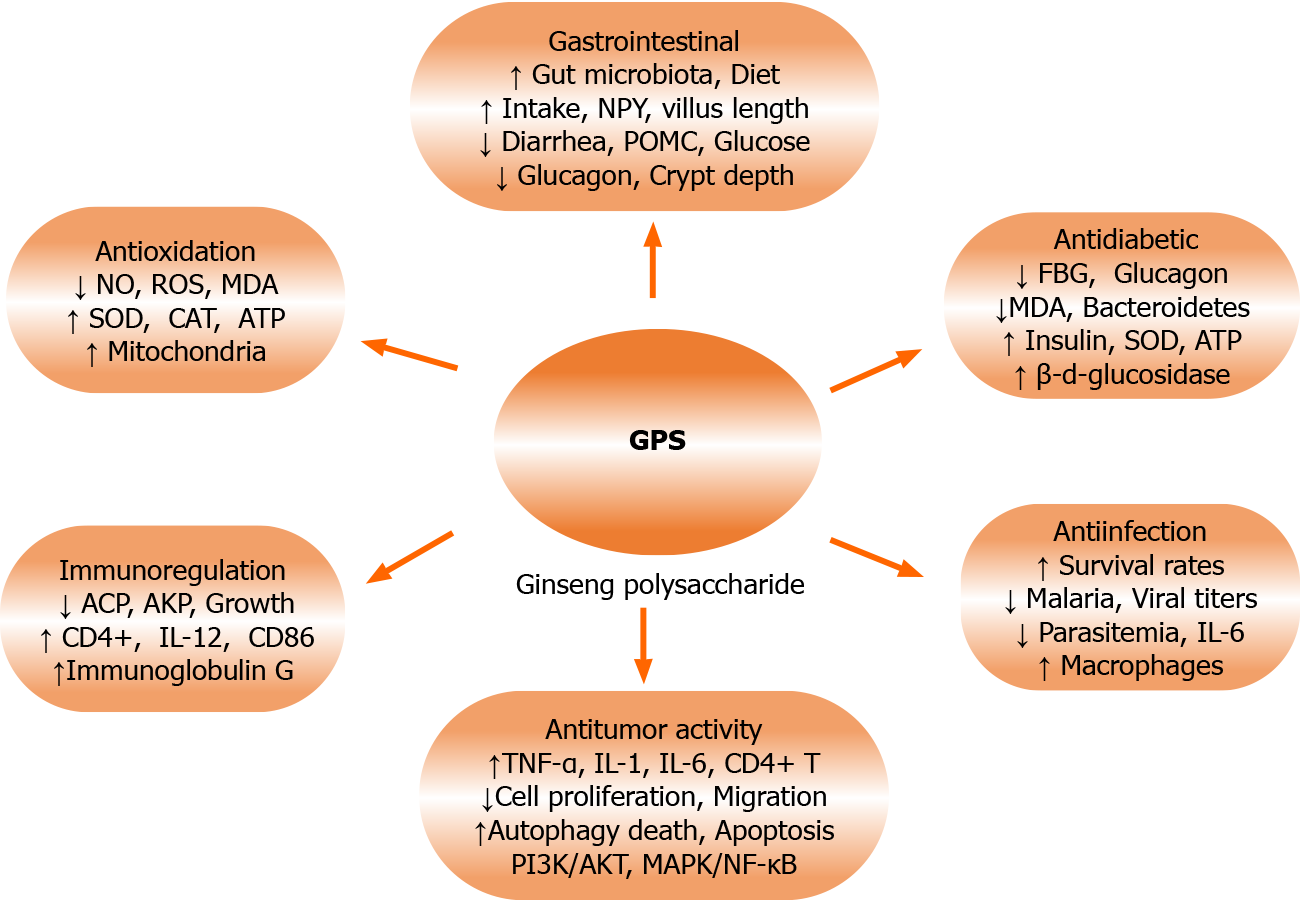
These results (Figure 1) suggest that GPS has antioxidative and antiaging effects (with a certain dose-effect relationship) and plays a vital role in mitochondrial function. Moreover, further research has indicated that GPS can inhibit mitochondrial swelling, increase the levels of adenosine diphosphate (ADP), adenosine triphosphate (ATP), and transversus abdominal plane (TAP) (as well as the ratio of ATP/ADP) in liver cells, and improve energy metabolism under hypoxic conditions[21]. Therefore, GPS may exert in vitro and in vivo pharmacological and protective effects associated with antihypoxia, antioxidation, and energetic metabolism.
Figure 1 Summary of the biological activities and effects of ginseng polysaccharide based on in vivo and in vitro experiments.
Ginseng polysaccharide exerts antioxidant, immunostimulatory, antitumor, anticancer, antimalarial, and anti-influenza activities and enhances mitochondrial protection, gastrointestinal protection, and probiotic balance. It can also improve bodily functions. ATP: Adenosine triphosphate; CAT: Catalase; DRD1: Dopamine D1 receptor; ERK: Extracellular signal-regulated kinase; FBG: Fasting blood glucose; GPS: Ginseng polysaccharide; IL: Interleukin; MDA: Malondialdehyde; MAPK: Mitogen-activated protein kinase; NF-κB: Nuclear factor-κB; NO: Nitric oxide; NPY: Hypothalamic neuropeptide Y; POMC: Proopiomelanocortins; ROS: Reactive oxygen species; SOD: Superoxide dismutase; TNF-α: Tumour necrosis factor α.
Immunostimulatory activities and bodily function
Humoral immunity plays an important role in disease resistance. Various polysaccharides may exert protective effects by promoting humoral immunity[9,12,23]. The current experimental results (Figure 1) have indicated that GPS can enhance the immunity of shrimp and Litopenaeus vannamei[22], as well as specifically regulate immune cell functions[24] and improve the immunity and growth of experimental sows and piglets[8,25].
In shrimp models, treatment with a GPS diet exerted immunoregulatory and antioxidant effects[22], significantly enhancing the activities of CAT, acid phosphatase (ACP), and alkaline phosphatase (AKP), increasing T-SOD and GSH-Px activities in the hepatopancreas, reducing MDA levels, and upregulating the levels of SOD, CAT, and GSH-Px mRNA[22]. Ginsan, a P. ginseng polysaccharide, has shown marked immunomodulatory effects on dendritic cells (DCs), which are critical antigen-presenting cells that participate in immune responses[24]. Ginsan treatment has also been shown to increase the levels of interleukin (IL)-12 and tumour necrosis factor (TNF)-α in DCs, as well as enhancing the expression of maturation markers (CD86) and significantly stimulating the proliferation of allogeneic CD4+ T lymphocytes; however, this treatment only marginally improved the expression of major histocompatibility complex class II molecules[24]. Moreover, GPS supplementation can improve the bodily functions of pregnant and lactating sows and enhance the immunity of sows and the growth of their piglets[25,26], with no adverse effects on reproduction[25]. GPS dietary supplementation has been demonstrated to significantly improve weaning weight and increase the total immunoglobulin G and M levels and glutathione peroxidase activity in the milk and serum of sows[25,26]. Furthermore, this supplementation has been shown to increase the concentrations of IL-2 and TNF-α in piglet serum[25], while also significantly reducing MDA levels[25].
These results (Figure 1) indicate that GPS can improve immunity-related biomolecule levels during late pregnancy and lactation, which may be beneficial to health and growth via biological transmission effects.
Gastrointestinal protection and probiotics
At present, the anti-inflammatory and immunomodulatory effects of plant polysaccharides have been recognized. Related reports (Figure 1) have shown that GPS might improve gastrointestinal function, promote food intake[27], regulate the composition and diversity of the gut microbiota in animals, restore the gut microbiota, balance metabolic processes[21], and promote the recovery of the mucosa[26,28]. Further results have indicated that GPS can significantly increase the average daily food intake[27] and feed conversion rate[26], and promote hedonic eating behaviour in mice[27] and piglets[26], in addition to decreasing serum glucose and glucagon levels[27]. The effects of GPS might be related to the upregulation of hypothalamic neuropeptide Y and decreases in dopamine D1 receptor and proopiomelanocortin levels in the midbrain[27]. GPS has also been observed to markedly increase body weight and serum immunoglobulin M levels[26], reduce the rate of diarrhoea and aspartate aminotransferase levels[26], decrease jejunal crypt depth, increase jejunal villus length[26,28], and enhance the levels of butyrate, isobutyric acid, and acetic acid in the colon[26], thus indicating that GPS might contribute to improving gastrointestinal functions.
Interestingly, the current data (Figure 1) have suggested that GPS can mediate noticeable improvements in probiotics and profitable strain balance[26,28,29], which are closely associated with gastrointestinal function. GPS has also been shown to significantly improve bacterial quantity and diversity in the colon, increase species richness and balance in piglets, and induce a higher relative abundance of Lachnospiraceae and Anaerostipes[26]. Furthermore, GPS can beneficially improve lactic acid bacteria (LAB) stability[29], significantly decrease Mycoplasma gallinarum and Asteroleplasma anaerobium abundances in chickens, regulate crypt depth in the jejunum in Xuefeng backbone chickens, increase Bacteroides vulgatus and Eubacterium tortuosum abundances[28], and significantly inhibit LAB cell death[29].
These findings (Figure 1) suggest that GPS may be used as an effective alternative for improving intestinal and gastric functions, morphology, and microbial compositions. Therefore, GPS plays a critical role in promoting sustainable gastrointestinal functions and probiotic protection.
Antitumour and anticancer activities
Tumours are involved in the interactions of diverse and complex cells and unregulated processes of cell growth and metabolism, but these processes are only partially understood[11,30-32]. Hence, cancer is seen as one of the most important problems that threaten human health worldwide[30,31]. Currently, increasing evidence (Figure 1) shows that various polysaccharides from natural product extracts have obvious antitumour properties and effects through cytotoxic or immunomodulatory mechanisms[10-12,15].
In human gastric cancer cells, two types of polysaccharides isolated from P. ginseng has been shown to potently inhibit the growth, proliferation, migration, and invasiveness of HGC-27 cells in a dose-dependent manner[33,34]. Specifically, GPS has been shown to markedly induce a typical apoptotic morphology, increase the G2/M phase population of cells, and induce HGC-27 cell apoptosis[33,34]. Western blot analysis indicated that GPS can dramatically decrease the expression levels of Twist, AKR1C2, and vimentin, but it can upregulate the expression levels of NF1 and E-cadherin in HGC-27 cells[33,34]. Moreover, GPS can dramatically inhibit HT-29 cell proliferation, induce cell cycle arrest in the G2/M phase[35], increase early apoptosis and autophagy in MG-63 cells[36], and further cause caspase-3 activation[35]. All of these findings demonstrate that GPS mediates gastrointestinal protective actions and antitumour activities.
For lung cancer cells, GPS treatment has been shown to significantly inhibit A549 cell growth[37], cell migration and invasion in lung epithelial BEAS-2B cells[37,38], and the growth of transplantable Lewis lung carcinoma tumors[39,40] in model mice. Additionally, GPS treatment can block A549 cell cycle arrest[37], induce cell apoptosis in vitro and vitro[37,39,40], and markedly increase the ratio of peripheral blood CD4+/CD8+ T lymphocytes, splenocyte proliferation, and the weights of spleen and thymus tissues in model mice[40]. Further research results have demonstrated that these effects might be associated with the induction of serum IL-2 and interferon-γ levels and NK cytolytic activity. In addition, these effects may be associated with[40] improving p-PTEN expression[38], mediating cell cycle arrest[37], and subsequently inhibiting the PI3K/AKT signalling pathway[38]. Hence, GPS may have great potential value for lung cancer treatment via immunostimulatory regulation.
In human leukaemia cells, GPS has been observed to significantly inhibit HL-60 cell growth[41] and K562 cell proliferation[42,43], induce cell apoptosis[41-43] in vitro, and mediate K562 cell cycle arrest[43]. Furthermore, GPS treatment has been shown to activate caspase-9, caspase-3, and poly-ADP ribose polymerase (PARP) cleavage[41], increase proapoptotic Bax expression, and regulate the loss of mitochondrial membrane potential in HL-60 cells[41]. Moreover, GPS has been shown to significantly augment P38 and c-Jun NH2-terminal kinase (JNK) mRNA levels, reduce extracellular signal-regulated kinase (ERK) mRNA levels, markedly suppress the expression of phosphorylated (p)-ERK, nuclear factor (NF)-κB p65, and cyclin D1, and increase the synthesis of p-P38 and p-JNK protein in K562 cells[42,43]. In conclusion, the potential molecular mechanisms of GPS against lung cancer may be associated with the caspase-9/caspase-3-mediated cleavage of PARP, cytochrome c, and the mitogen-activated protein kinase (MAPK)/NF-κB/cyclin D1 signalling pathways.
Furthermore, results from other studies have revealed that GPS has no direct effect in killing K562, HL-60, and KG1 (tumor) cells, but it can significantly enhance phagocytic activity and cell apoptosis by mediating mouse peritoneal macrophage (PM) activity[42,44,45]. GPS treatment has been observed to increase the expression levels of CD68, ACP, and ANE in mouse PMs, increase the expression levels of cytokines, and enhance the nitric oxide levels, including TNF-α, IL-1, and IL-6, which suggests that GPS might exert anticancer effects by stimulating macrophages and immunoregulation[42,44,45]. GPS has also been shown to have synergistic effects against tumours in MG-63 cells with Gy irradiation treatment via autophagy death and apoptosis, decrease the phosphorylation of p38 and AKT, increase the ratio of LC3II/LC3I, and induce MG-63 cell autophagy[36].
Taken together (Figure 1), these findings suggest that GPS can regulate tumour cell cycle arrest, induce cancer cell apoptosis via the autophagy death and apoptosis pathways, and regulate the PI3K/AKT-PTEN/signalling pathway, the MAPK/NF-κB/cyclin D1 signalling pathway, the caspase-9/caspase-3-mediated cleavage of the PARP pathway, and immune-stimulating actions, indicating that GPS may have the potential to be developed as a novel antitumor agent.
Antimalarial and anti-influenza activities
For the prevention and treatment of malaria and influenza virus infections, it is vital to explore medicinal plants with antiparasitic activities[8,11,12,15]. Based on related reports (Figure 1), GPS has been demonstrated to have significant prophylactic activities against malaria and infection with influenza viruses by stimulating the immune system[46,47].
Treatment with GPS for 6 d has been shown to reduce 64.73% of parasitaemia cases[46], inhibit residual malaria infections during early infection phases[46], activate macrophages, and restore phagocytosis activity in malaria-bearing mice[46], indicating that GPS may exert antimalarial actions via the initiation and regulation of immune responses. GPS pre-treatments have been observed to significantly promote the survival benefits of model mice that were infected with H1N1 (A/PR/8/34) and H3N2 (A/Philippines/82) influenza viruses[47], moderately enhance survival rates and lower the levels of lung viral titres and IL-6 levels in model mice that were infected by the 2009 H1N1 virus[47], and improve their survival against infections and heterosubtypic lethal challenges in vaccinated mice[47]. These results demonstrate that GPS has great potential for treating influenza viral infections, which is closely related to the immunomodulatory effects that result from GPS treatment.
POTENTIAL FOR TREATING GDM
Anti-diabetic activity
Diabetes is a global social disease that affects several million people; at present, diabetic patients are commonly treated with oral antidiabetic drugs, such as glyburide[48,49]. However, because blood sugar changes over a short period of time, hypoglycaemic drugs often produce side effects[48,50], such as hypoglycaemia, diarrhoea, and lactic acidosis, which may be seen as hazardous factors for certain patients[1,5]. Safer and more economical drugs from natural plant sources for the control of blood sugar are urgently needed.
First, neutral polysaccharide (WGPN) and acidic polysaccharide (WGPA) fractions were separated from GPS[51]. In streptozotocin-induced diabetic mice, WGPN treatment has been shown to significantly reduce the fasting blood glucose (FBG) of mice[51] and to decrease serum glucose and glucagon levels[27]. Additionally, WGPA treatment has been shown to decrease serum MDA levels, scavenge reactive oxygen species and related free radicals, improve SOD activities, increase hepatic glycogen levels, and increase serum insulin levels[51], indicating that the impacts of GPS on FBG may be involved in antioxidant effects and in increasing insulin secretion to promote glycogen synthesis.
In diabetic model rats, GPS has been demonstrated to significantly increase the relative abundance of Firmicutes, as well as reverse the dysbiosis of intestinal flora at the phylum level in diabetic rats, upregulate the relative abundance of Bacteroidetes, restore the entire gut microbiota from perturbation, improve faecal β-d-glucosidase activity, and potentiate the hypoglycaemic effects of ginsenosides[52,53]. Metabolomics research has shown that water-soluble GPS (WGP) treatment can significantly increase the levels of inosine, serotonin, phenylpropionylglycine, and dodecanedioic acid in rat urine samples, indicating that WGP can improve the metabolism levels of purine, tryptophan, and fatty acids[52,54]. Additionally, WGP has been shown to significantly decrease the levels of 1-methyladenine, 4-deoxyerythronic acid, 5-hydroxyhexanoic acid, and tetrahydrocortisol, indicating that WGP can regulate DNA, organic acid, and steroid hormone metabolisms[52,54]. All of these results suggest that GPS may affect glucose metabolism, nucleic acid metabolism, and lipid metabolism and could be used as an antidiabetic candidate drug.
In addition, GPS can protect mitochondria, improve energy metabolism, and increase the levels of ATP, ADP, and TAP by inhibiting mitochondrial damage[21]. Taken together (Figure 1), these findings suggest that GPS can mediate distinct hypoglycaemic and antidiabetic effects against diabetes. Hence, GPS has great potential and clinical application prospects as an antidiabetic candidate drug or remedy.
Potentiality analysis
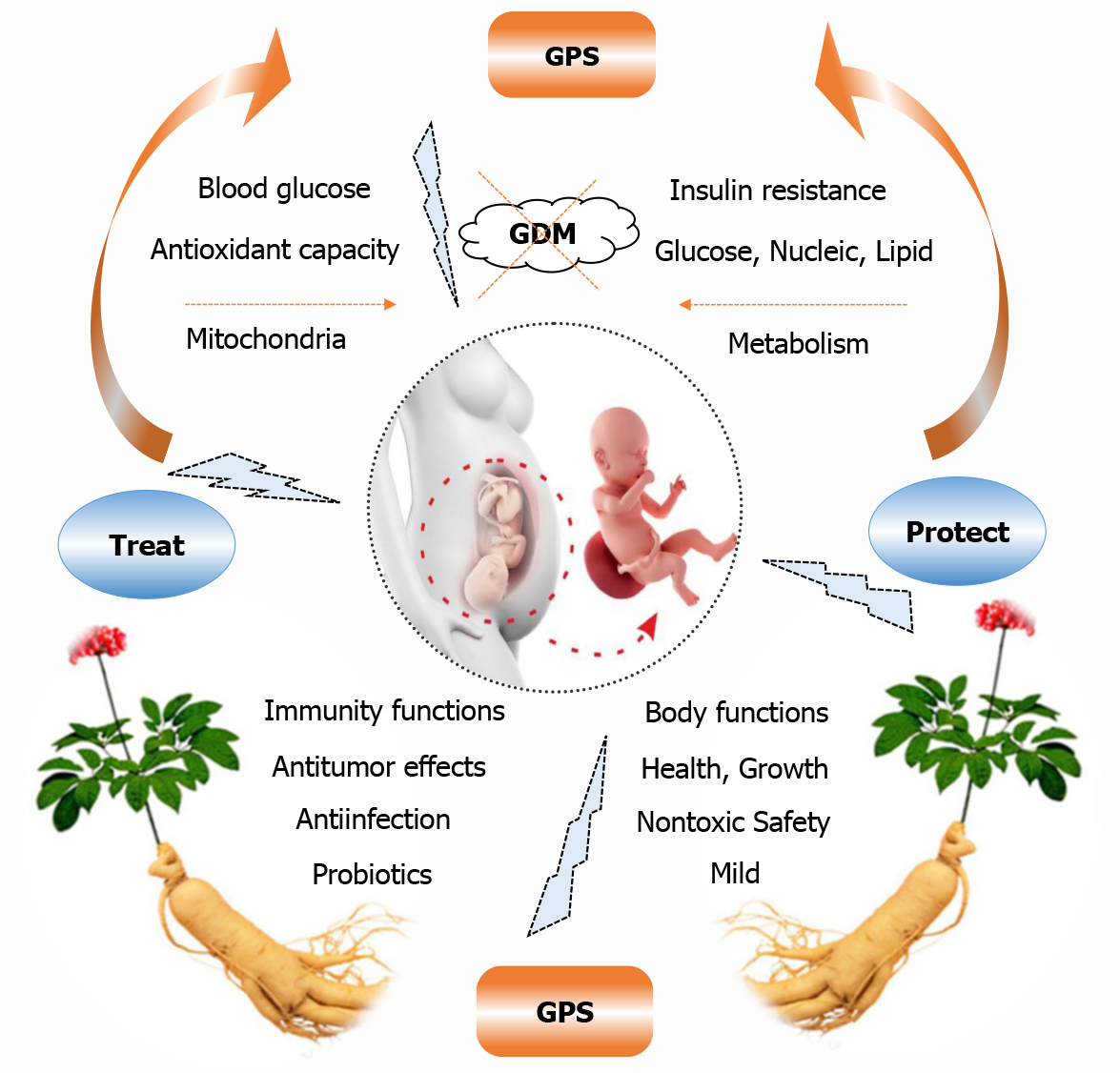
GDM is a common pregnancy complication that is characterized by abnormal glucose tolerance during pregnancy, leading to potential adverse outcomes for the mothers and offspring[3], and can often cause short- and long-lasting health hazards. These hazards can include increased risks for polyhydramnios, shoulder dystocia, operative delivery, birth canal lacerations, obesity, T2DM, and cardiovascular disease in the mother and child[1,4,5]. However, traditional hypoglycaemic drugs may lead to safety concerns for mothers and children, in terms of their long-term effects[1,3]. Therefore, it is essential to develop new, nontoxic therapeutic agents from natural plants. Based on previously summarized reports (Figure 2), we have concluded that GPS, with its mild hypoglycaemic effects, lack of toxicity, high biodegradability, and high biocompatibility, has great potential as an ideal antidiabetic drug and remedy to prevent and treat GDM.
Figure 2 Potential analysis and exploration of ginseng polysaccharide as an ideal antidiabetic drug and remedy for use in preventing and treating gestational diabetes mellitus.
GPS: Ginseng polysaccharide; GDM: Gestational diabetes mellitus. Copyright permission: The picture of pregnant woman was cited from the Rocketparent.com. The picture of ginseng was cited from the http://616pic.com/sucai/1pkid6dwz.html. All of these were complied with the Creative Commons Attribution-NonCommercial (CC BY-NC 4.0) license.
First, GPS can mildly downregulate blood glycogen levels[27,51], enhance antioxidant capacity, protect mitochondria, improve energy metabolism, regulate the metabolisms of glucose, nucleic acids, and lipids, exert synergistic hypoglycaemic effects with other anti-diabetic drugs, and inhibit insulin resistance. In addition, GPS has no effect on normal FBG functions[27,51]. Hence, GPS is appropriate for mildly lowering blood glucose and may be used as a novel agent and therapy for GDM, wherein it does not result in risks of short-term hypoglycaemia following oral administration of hypoglycaemic drugs[21,27].
Second, GPS can regulate immune cell functions, enhance immune functions, exert immunoregulatory and antioxidant effects, and improve ATP production. All of these factors contribute to improvements in the physical functions of the mother and offspring, thus protecting them from external infection and damage and improving the health and growth of the offspring, as has been verified in sows and their piglets [8,25]. Therefore, GPS plays crucial roles in the health and growth of mothers and offspring during pregnancy and lactation.
Third, GPS can increase daily food intake, improve gastrointestinal functions, and maintain probiotics and a beneficial strain balance. Probiotics are beneficial to glucose metabolism during and after pregnancy; thus, they have been suggested and recommended as an intervention for preventing and treating GDM[3]. Hence, GPS may improve bodily functions, regulate the gastrointestinal tract, and lower blood sugar in the mother and offspring via probiotics.
In addition, GPS regulates cell cycle arrest, induces cancer cell apoptosis via the autophagy death and apoptosis pathways, regulates immune-stimulating responses in a variety of tumor cells, and exhibits antiradiation effects. GPS also has significant prophylactic activity against malaria and influenza viruses. Furthermore, GPS has no adverse effects on the mother and progeny, with excellent safety and no toxic side effects[26,39,40]. These biological activities and effects of GPS are beneficial to the bodily health and growth of the mother and offspring (Figure 2).