Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.4939
Peer-review started: February 24, 2021
First decision: April 18, 2021
Revised: April 26, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: July 6, 2021
Processing time: 120 Days and 2.5 Hours
Coronavirus disease 2019 (COVID-19) distresses the pulmonary system causing acute respiratory distress syndrome, which might lead to death. There is no cure for COVID-19 infection. COVID-19 is a self-limited infection, and the methods that can enhance immunity are strongly required. Enhancing oxygenation is one safe and effective intervention to enhance immunity and pulmonary functions. This review deliberates the probable influences of enhancing oxygenation on immunity and other health-connected conditions in patients with COVID-19. An extensive search was conducted through Web of Science, Scopus, Medline databases, and EBSCO for the influence of enhancing oxygenation on immunity, pulmonary functions, psycho-immune hormones, and COVID-19 risk factors. This search included clinical trials and literature and systematic reviews. This search revealed that enhancing oxygenation has a strong effect on improving immunity and pulmonary functions and psycho-immune hormones. Also, enhancing oxygenation has a self-protective role counter to COVID-19 risk factors. Lastly, this search revealed the recommended safe and effective exercise protocol to enhance oxygenation in patients with COVID-19. Enhancing oxygenation should be involved in managing patients with COVID-19 because of its significant effects on immunity, pulmonary functions, and COVID-19 risk factors. A mild to moderate cycling or walking with 60%-80% Vo2max for 20-60 min performed 2-3 times per week could be a safe and effective aerobic exercise program in patients with COVID-19 to enhance their immunity and pulmonary functions.
Core Tip: Coronavirus disease 2019 is a self-limited infection, and interventions that can increase immunity are strongly recommended. Thus, enhancing oxygenation is one of the safe and effective interventions in enhancing immunity and pulmonary functions. This review discusses the possible effects of enhancing oxygenation of patients with coronavirus disease 2019 on immunity and other health-related condi
- Citation: Mohamed A, Alawna M. Enhancing oxygenation of patients with coronavirus disease 2019: Effects on immunity and other health-related conditions . World J Clin Cases 2021; 9(19): 4939-4958
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/4939.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.4939
The coronavirus or coronavirus disease 2019 (COVID-19) is defined by the World Health Organization as a world disaster. The World Health Organization has announced that through February 11, 2021 that there were 106797721 confirmed cases, among them 2341145 deaths[1]. COVID-19 is recognized as a surrounded RNA beta-coronavirus commonly identified as the severe acute respiratory syndrome coronavirus 2[2]. Communal signs of COVID-19 include cough and fever[3]. Fever presents in 43.8% of patients with COVID-19 on early admission; though, it rises to 88.7% throughout hospitalization. The next communal symptom is cough, which happens in approximately 67.8%[2]. Other symptoms contain fatigue, dyspnea, and myalgia.
COVID-19 has been demonstrated to be self-limited where the host’s immune strength plays a key role in decreasing its associated disorders and death rates[4]. Thus, interventions that increase immune functions are strongly needed to either people in lockdown or people with COVID-19. Enhancing oxygenation has been demonstrated as a safe and effective intervention to increase immune functions. The advantage of enhancing oxygenation over other interventions is that it causes an autonomic modulation; this autonomic modulation increases pulmonary functions and controls pyscho-immune changes that occur due to high stressors (such as COVID-19). Thus, this review highlights the influence of enhancing oxygenation on immunity, pulmonary functions, psycho-immune hormones, and obesity. Also, this review highlights the defensive role of enhancing oxygenation on COVID-19 risk factors.
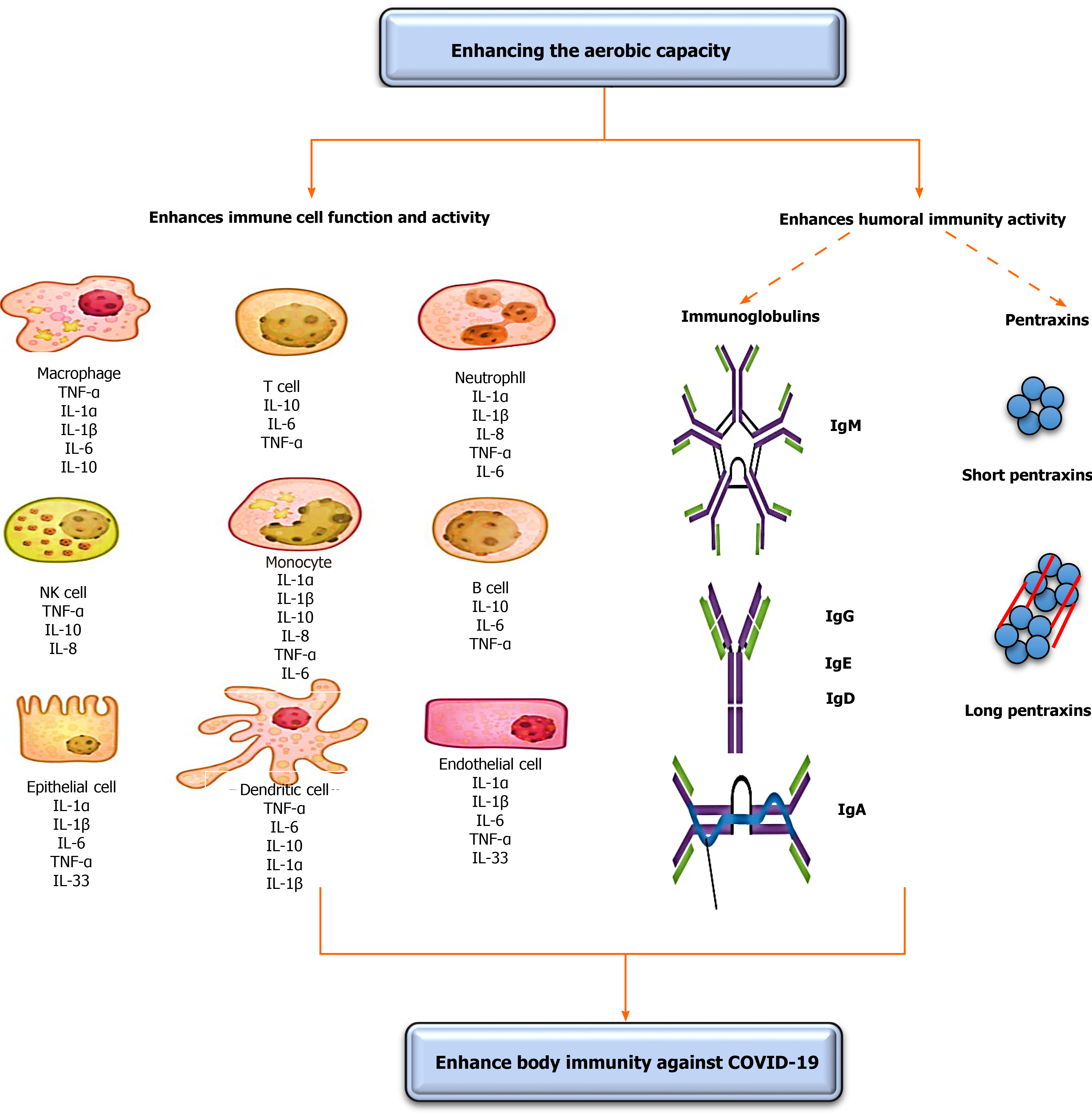
The strength of immunity plays a fundamental role in the treatment of any infection. COVID-19 is a self-limited infection; thus, strengthening immunity might have a significant role in lessening its associated disorders and death rates[5]. However, the existence of some vaccines, the development of specific drugs to treat patients with COVID-19 could continue for about 1 year. Thus, the need for a fast and safe intervention for COVID-19 is a necessity to decreasing its associated disorders and death rates. Enhancing oxygenation can afford a safe and immediate enhancement in immunity strength[5]. This subtopic discusses the vital role of enhancing oxygenation on enhancing COVID-19 specified immunity components vital for decreasing associated disorders and death rates. Immunity mainly divides into two components, including immune cells and humoral immunity. The effects of increasing the oxygenation on immunity are illustrated in Figure 1.
The first immunity component that fights COVID-19 infection is immune cells. COVID-19, severe acute respiratory syndrome coronavirus, and Middle East respir
Pascal et al[11] stated that the response of T-lymphocytes is substantial to minimize the number of Middle East respiratory syndrome coronaviruses[11]. Chen et al[12] reported that the reduction of serum T-lymphocytes causes a decline in the respiratory T-lymphocyte cytokine production and neutralizing antibody, which can result in an extensive immune-intermediated interstitial pneumonia and delayed washing out of severe acute respiratory syndrome coronaviruses from infected lungs[12]. Also, T-helper cells, the most significant cells in adaptive immunity, activate the production of proinflammatory cytokines via triggering the nuclear factor kappa B pathways[13]; these proinflammatory cytokines activate the secretion and relocation of neutrophils and monocytes to the region of infection to start other chemokine and cytokine cascades, such as tumor necrosis factor β, interleukin (IL)-1, IL-10, IL-6, IL-12, IL-8, and monocyte chemotactic protein-1[14,15].
Enhancing oxygenation causes a short-term enhancement in the function and number of T-lymphocytes. Gonçalves et al[16] conducted a recent systematic review to investigate the influence of enhancing oxygenation on the function and number of immune markers. They established that enhancing oxygenation causes fast and short-term enhancements in the function and number of T-lymphocytes, B-lymphocytes, immunoglobulins, leukocytes, and interleukins. Previous studies showed that only one session of aerobic exercise causes a significant increase in almost all immune markers, including T-lymphocytes, immunoglobulins, and leukocytes in humans[17-20]. Lippi et al[17,18] demonstrated that mild aerobic exercise (running for 21.1 km) significantly increases the number of monocytes, leukocytes, and neutrophils among amateur runners. Lira et al[20] showed that a single session of moderate aerobic exercise (5 km running) significantly increases the number of cytokines, IL-10 and IL-6, for 60 min. Li et al[19] showed that one session of extended aerobic exercise increases the number of serum neutrophils, monocytes, and leukocytes for 9 h. Gonçalves et al[21] investigated the defensive role of enhancing oxygenation on artificially-produced acute lung inflammations in rats. They detected that 5 wk of low aerobic exercise significantly increased the number of neutrophils in bronchoalveolar lavage fluid, pulmonary resistance and pliability, protein leakage, tumor necrosis factor-α, serum IL-10, IL-6, IL-1beta, and KC (murine homolog to IL-8) levels.
The second immunity component essential against COVID-19 infection is humoral immunity. Humoral immunity consists mainly of B-cell subdivisions; these subdivisions present with phenotype features of modest and un-isotype interchanged method. They consist of antibody-secreting and memory cells, which rise during coronavirus infection[7,22]. The antigen stimulation of coronavirus is detected via employing a particular 9-mer peptide “CYSSLILDY” that lies in the zone from 437-445 of the S-glycoprotein region[7,22]. This construction has the supreme B-cell antigenicity outline to form high connections to major histocompatibility complex class I alleles into a computerized model[23]. Several reports mentioned the important role of humoral immunity in controlling the exitance period of coronavirus infection[24-26].
The normal humoral immunity involves numerous elements, such as serum complements (C3-C9), pentraxins [C-reactive proteins (CRPs)], immunoglobulins [IgM, IgE, IgG, and IgA and contact cascades (FXIIa)][27]. The serum complements primarily act in the antiviral defense, and they are strongly regulated by specific proteins produced into the bloodstream. Viruses regularly include encrypted proteins that aid them to evade their discover by serum complement[7,28]. Thus, increasing the function of the serum complement system might aid in recognizing these encrypted proteins.
To the best of our knowledge, infrequent human articles examined the influence of enhancing oxygenation on role of complements in respiratory infections. These infrequent studies concentrated more on athletes than normal subjects to examine the influence of overtraining syndrome in weakening immunity among athletes. In these studies, there were conflicts among their results[29-32]. These high conflicts might have occurred because of athletes’ highly strenuous and repeated activities, which can cause negative effects on immunity (overtraining syndrome)[33].
The influence of enhancing oxygenation on the function and level of serum immunoglobins has been extensively documented in the literature. Immunoglobulins play a chief role in fighting COVID-19 infection and decreasing the severity of its associated disorders. Immunoglobulins chiefly are IgA, IgG, IgE, and IgM. The IgA and IgG are the predominant Ig in the mucosal fluid, and they have a vibrant role in inhibiting upper and lower respiratory tract infection[34-36]. The influence of enhancing oxygenation on increasing the function and activity of immunoglobulins has been documented in the literature. Karacabey et al[30] showed that the performance of continual moderate aerobic exercise significantly increased the secretion of immunoglobulins IgM, IgA, and IgG. Mohamed et al[37] examined the long influence of aerobic vs anaerobic exercise on serum immunoglobulins amid obese females. They observed that unlike anaerobic exercise, aerobic exercise significantly increased serum Ig, predominantly IgG and IgM.
The second subdivision of humoral immunity is CRPs. CRPs in the body principally act in inflammatory mechanisms and responses to viral infection by encouraging the production of serum complements, nitric oxide secretion, phagocytosis, apoptosis, and cytokines[38]. Increasing CRPs levels is a natural body defense mechanism to counter viral infection. Conversely, persistent high serum CRPs can cause considerably fast lung destruction because high serum CRPs lead to a resultant decrease in lung function[39]. The serum CRPs are considered a chief laboratory test to detect COVID-19 infection. Patients who suffer from COVID-19 have elevated serum CRPs[40,41]. Enhancing oxygenation plays an exciting role in controlling serum CRPs by creating a temporary slight rise in serum CRPs[42-44] to counter lung infection and a lasting reduction in serum CRPs[45,46] to inhibit the decline in lung functions.
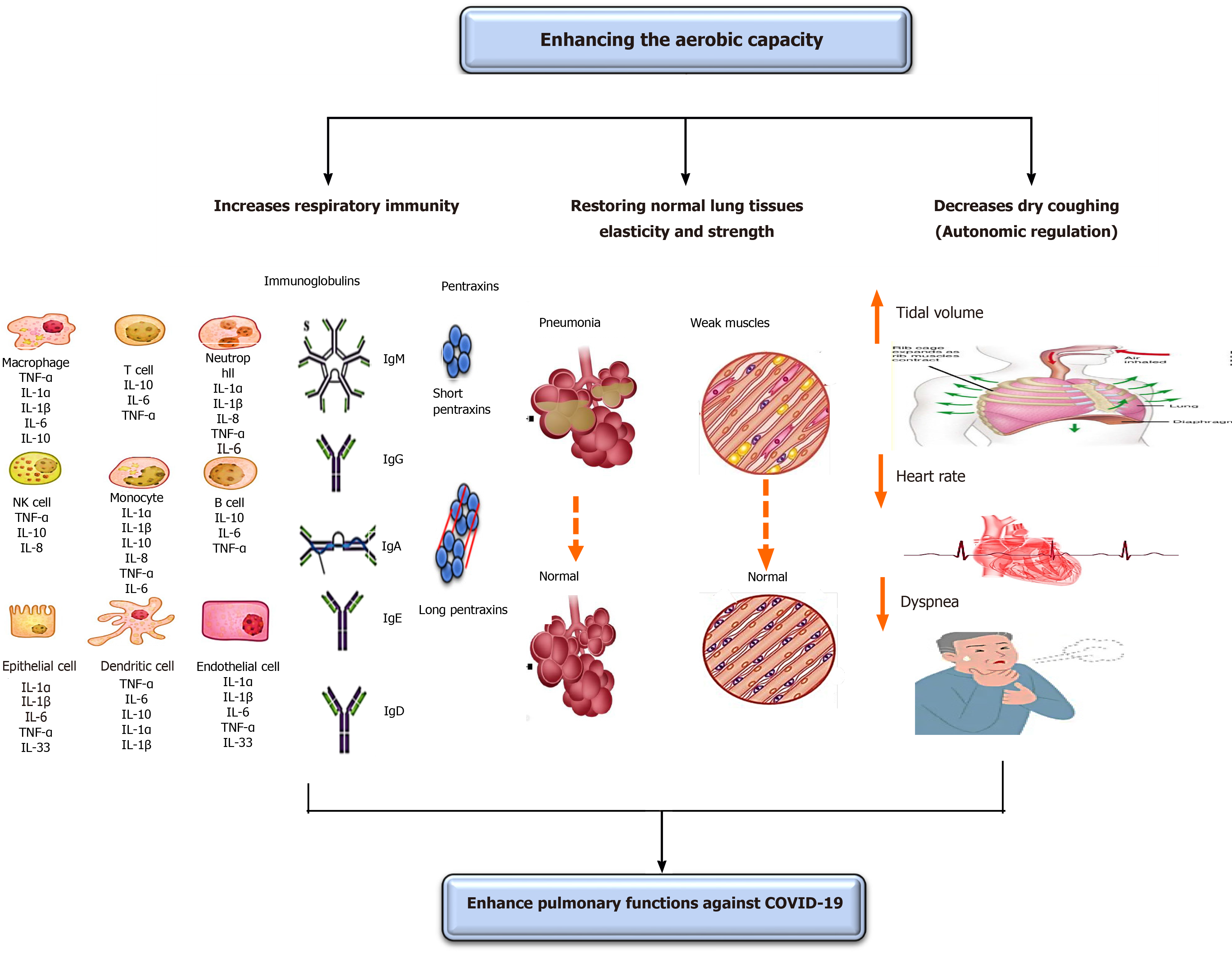
COVID-19 negatively disturbs respiratory functions leading to respiratory disorders, particularly pneumonia and acute respiratory distress syndrome (ARDS)[47-51]. The respiratory symptoms that commonly arise amid patients with COVID-19 are fever and cough. The subtopic discusses in-depth the important role of enhancing oxygenation in improving COVID-19-associated respiratory disorders and symptoms. The effects of enhancing oxygenation on pulmonary functions are shown in Figure 2.
Enhancing oxygenation can inhibit pneumonia or reduce its development from moderate to severe. Baumann et al[52] stated that the performance of aerobic exercise for a short time can inhibit the incidence of fever and pneumonia in patients with malignancy. Williams[53] has shown that mild aerobic exercise, like walking and running, significantly declines the peril of pneumonia and aspiration pneumonia death. Stravinskas Durigon et al[54] showed that the performance of aerobic exercise for 8 wk averts Pseudomonas aeruginosa prompted bacterial settlement and lung inflammation among older mice. Neuman et al[55] demonstrated that enhancing oxygenation plays a key role in reducing the incidence of pneumonia in United States women. Olivo et al[56] reported that the performance of mild aerobic exercise has an important anti-inflammatory influence in patients with Streptococcus pneumonia, which aids in offsetting pulmonary inflammations.
Also, enhancing oxygenation can limit the development of ARDS or reduce its advance from moderate to severe. Rigonato-Oliveira et al[57] showed that enhancing oxygenation prevents acute lung inflammation by diminishing oxidative stress signs and inflammatory cytokines in rats and people. Vieira et al[58] stated that enhancing oxygenation significantly rises serum IL-10, which has an indispensable role in immunity strength to decline recent lung injuries. A very fresh study conducted by Shi et al[59] has revealed that the performance of mild aerobic exercise for 5 wk prevents the occurrence of acute lung injuries in rats via forming neutrophil extracellular traps (NETs), which play a key role in limiting recent lung inflammation. Neutrophil extracellular traps are structures that have an extracellular web-like shape and particularly are comprised of neutrophil elastase, DNA, histones, and myeloperoxidase. These formed neutrophil extracellular traps can proficiently trap attacking pathogens by using high local amounts of antimicrobial peptides to destruct virulence elements. Interestingly, enhancing oxygenation has a superior role in COVID-19 respiratory-related symptoms than breathing exercise[5]. The influence of enhancing oxygenation on COVID-19 respiratory-related symptoms can be summarized in four mechanisms.
Enhancing oxygenation acts as antimycotic or antibiotic prophylaxis. It was previously mentioned in the section of “the influence of enhancing oxygenation on immunity” that enhancing oxygenation improves respiratory and bodily immunity through: (1) Enhancing the level and function of immune cells, such as T-lymphocytes, macrophages, monocytes, and neutrophils, which are necessary cells in the body’s resistance to infection; (2) Enhancing serum immunoglobulins “IgA, IgM, IgG,” predominantly IgA as a result of its chief function to counter lung infection; and (3) Adjusting serum CRP levels, by producing a transient rise in them to counter lung infection and a latent reduction to prevent falling of lung functions.
Enhancing oxygenation can significantly enhance the pliability of lung tissues and increase the strength and stamina of respiratory muscles. A short-term increase in oxygenation can increase lung tissue elasticity and recoil properties. Guimarães et al[60] studied the influence of aerobic exercise on the incidence of an artificially created emphysema in rats. They observed that the performance of mild aerobic exercise for 4 wk significantly increased lung biomechanics and flow acceleration ratio by decreasing lung hyperinflation and increasing elasticity and strength of lung tissues. Park and Han[61] examined the effect of performing regular aerobic exercise on peak expiratory lung capacity in elderly females. They observed that the performance of mild aerobic exercise for 20 min and lasting for 12 wk significantly increased alveoli functions and lung pliability. Taskin et al[62] examined the influence of aerobic exercise on respiratory muscle strength in people with ankylosing spondylitis. They observed that the performance of mild aerobic exercise for 40 min/d and lasting for 12 wk significantly augmented the end maximal exercise capacity, respiratory muscle strength, and inspiratory muscle performance and lessened dyspnea perception.
Enhancing oxygenation has an antioxidant role to diminish free radicals and oxidative stress in the blood. Free radicals, like reactive oxygen species, are formed during ordinary cellular functions as one of the normal physiological mechanisms in all living creatures. Free radicals play both useful and poisonous effects. Once serum-free radicals extremely raise and can no longer be handled, they lead to an oxidative stress[63]. Oxidative stress can significantly help in the development of numerous diseases, amid them lung infection and diseases[63,64]. Mild aerobic exercise can aid in the removal of these increased serum free radicals and decrease the incidence of lung infection and disorders such as pneumonia and ARDS.
Also, enhancing oxygenation increases the body’s resistance to consequential oxidative challenges by enhancing the mitochondrial function and permitting enhanced oxygenation of body and lung tissues[63,65]. Toledo et al[66] studied the influence of aerobic exercise on the incidence of pulmonary disorders in rats. They assessed the reactive oxygen species as a biomarker of the beginning of lung disorders. They observed that mild aerobic exercise implemented for 24 wk significantly decreased serum reactive oxygen species in the bronchoalveolar lavage fluid in rats. They concluded that increasing oxygenation is vital to inhibit or reduce the worsening of ARDS and pneumonia. Da Cunha et al[67] studied the consequence of performing aerobic exercise on induced oxidative stress by an artificially occurring lung injury in rats. They observed that the performance of mild aerobic exercise for 20 min significantly inhibited the accumulation of nuclear factor kappa B/p65, reactive species, and nitrite amounts. They proposed that mild aerobic exercise may have a major function as a protector to counter the development of recent lung inflammation.
Enhancing oxygenation can decrease coughing in patients with COVID-19; this mainly occurs through modulating the autonomic nervous system over mucociliary clearance[68,69]. Borghi-Silva et al[69] examined the consequence of routine aerobic exercise on the function of lungs in patients with chronic obstructive pulmonary disease. They observed that performing mild aerobic exercise for 6 wk significantly reduced respiratory rate and elevated the tidal volume during exercise; these changes mainly occurred due to autonomic modulations, which caused a decrease in the heart rate. Recently, Leite et al[68] explored the influence of aerobic exercise on the cough and function of autonomic nervous system in patients with chronic obstructive pulmonary disease. They observed that performing 12 wk of mild aerobic exercise significantly reduced the cough and heart rate. The influence of enhancing oxygenation on reducing heart rate might be valuable in lessening dyspnea observed in patients with COVID-19[69]. Thus, performing mild aerobic exercise might significantly reduce both cough and dyspnea, which often arise in patients with COVID-19 over breathing exercise as they significantly control the parasympathetic nervous system leading to a major drop in these two signs.
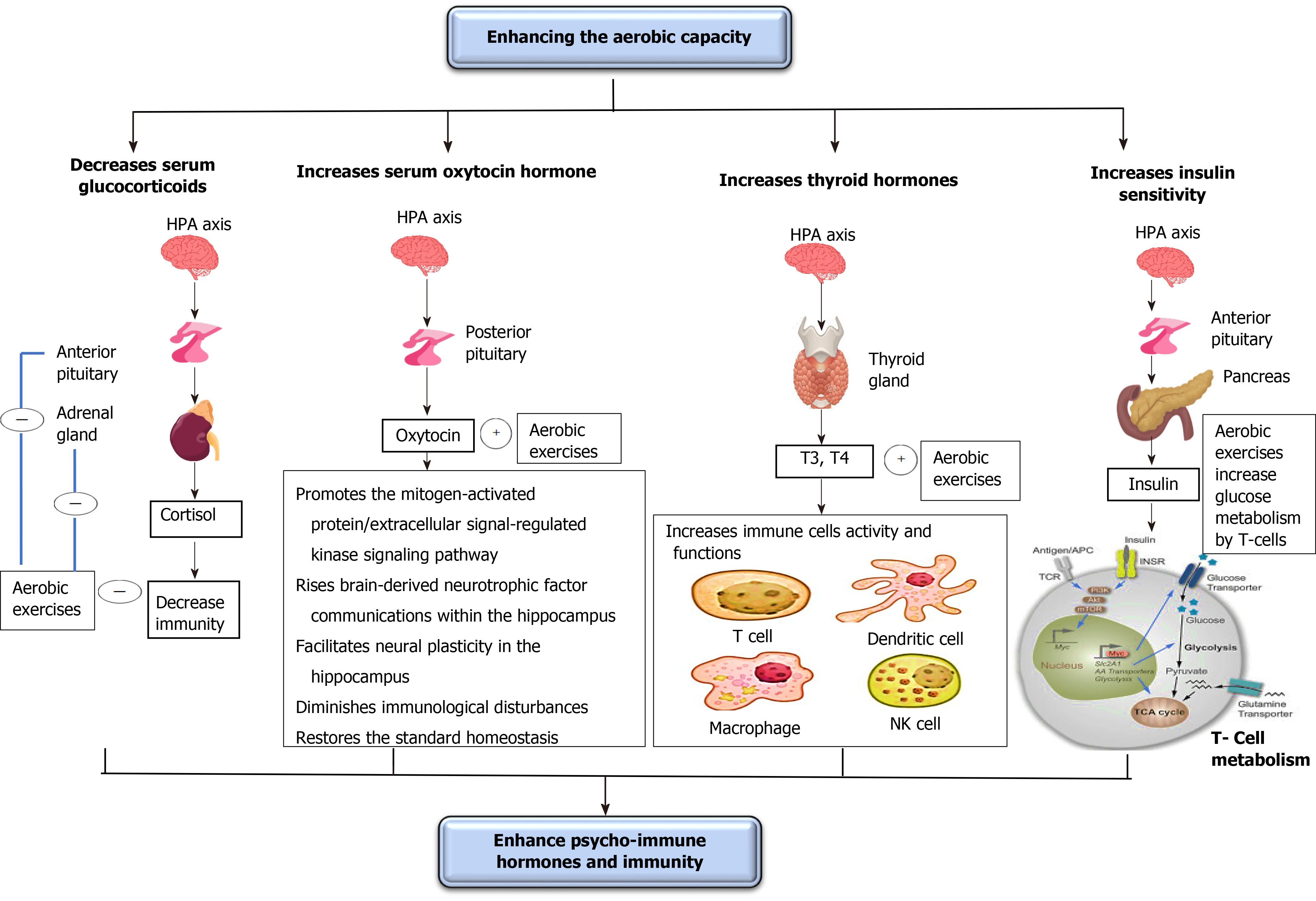
Psychological problems usually occur in both healthy people throughout the lockdown (who have difficulty going to psychiatrists) and patients with COVID-19 (who are waiting for the death to come at any moment)[70]. These psychological problems can significantly decrease immunity and cause the development of several disorders including diabetes and hypertension. Thus, controlling these psychological problems is a must to either decrease COVID-19 associated disorders and death rates or prevent the development of other disorders (diabetes and hypertension). This subtopic discusses the influence of enhancing oxygenation on renormalizing the psycho-immune hormones, mostly those involved in the fight or flight reaction, including glucocorticoids (GCs), oxytocin, thyroid (THs), and insulin hormones through clarifying the impact of regulation of these hormones on enhancing immune functions essential to counter COVID-19. The effects of increasing the oxygenation on psycho-immune hormones are illustrated in Figure 3.
GC release starts by stimulating the hypothalamus to secret corticotropin-releasing hormone (CRH) and arginine vasopressin. CRH-containing neurons, present in the hypothalamus (paraventricular nucleus), attach to the noradrenergic areas in the spinal cord and brainstem. The locus coeruleus of the brainstem directly connects with the autonomic nervous system neurons in the brainstem and spinal cord[71]. Stress activates the locus coeruleus to stimulate the sympathetic activity through triggering α1-adrenoceptors and inhibit the parasympathetic activity through activation of α2-adrenoceptors[71]. The sympathetic nervous system activation increases the secretion of CRH from the paraventricular nucleus of the hypothalamus causing a stimulation of the hypothalamic-pituitary-adrenal axis to secrete adrenocorticotropic hormone (ACTH) from the anterior pituitary gland[71].
Adrenocorticotropic hormone, in turn, activates the release of GCs from the adrenal cortex[72]. These GCs act as transcript factors to control cell functions, even after the stoppage of acute stresses. GCs can overwhelm immunity and avert the central secretion of inflammation mediators (leukotrienes and prostaglandins); this happens as a result of the decreasing effect of GCs on the action and function of immune cells (T-lymphocytes, B-lymphocytes, macrophages, neutrophils, basophils, eosinophils, and mast cells)[73,74].
Short periods of aerobic exercise can significantly decrease serum GCs, arginine vasopressin, and CRH. Lu et al[75] studied the influence of aerobic exercise on GC receptor message on blood leukocytes in healthy and asthmatic young persons[75]. They detected that regular mild aerobic exercise performed for 8 wk significantly declined the GC receptor message on blood leukocytes. Hill et al[76] investigated the influence of aerobic exercise on serum GCs in moderately skilled athletes[76]. They observed that the performance of low aerobic exercise for 30 min significantly reduced serum GCs. Silva et al[77] examined the influence of aerobic exercise on GC receptor communication in rats[77]. They observed that the performance of mild aerobic exercise for 60 min with 50% of maximal exercise capacity significantly reduced the communication of the GC receptors in rats. Fediuc et al[78] investigated the influence of aerobic exercise on sensitivity of the hypothalamic-pituitary-adrenal axis and serum GCs in Sprague-Dawley rats[78]. They found that the performance of mild intensity aerobic exercise for 5 wk significantly declined both serum GCs and sensitivity of the hypothalamic-pituitary-adrenal axis.
OX hormone is produced from magnocellular and parvocellular neurons inside the paraventricular nucleus and the supraoptic nucleus of the hypothalamus and then is transmitted by axonal transport to the posterior pituitary where it deposits until its secretion into the blood. OX hormone receptors exist in many regions of the brain, including the cerebral cortex, hypothalamus, hippocampus, amygdala, and nucleus accumbens[79].
The increase in serum OT levels can help in decreasing stress, anxiety, and depression; however, the actual mechanisms by which the OT system decreases these disorders are still unclear[79]. However, the importance of increasing oxygenation on enhancing OT function still needs more investigations particularly on humans.
Numerous studies have shown that OT has a significant antidepressant function in diminishing anxiety and stress[80-84]. The antidepressant function of OT may occur due to enhancing the mitogen-activated protein/extracellular signal-regulated kinase signaling path and intensifying brain-derived neurotrophic factor communications within the hippocampus; this enhancing the neural plasticity function in the hippocampus[79]. Also, OT plays an important role in diminishing immunological disturbances and returning the standard homeostasis because its relationship with several usual immune cytokines, such as IL-1β, in addition to prostaglandins, endocannabinoids, nitric oxide, and nitric oxide[85].
Short periods of aerobic exercise can increase blood OT, which promotes both decreasing stress, anxiety, and depression and increasing immune functions. Yüksel et al[86] examined the influence of aerobic exercise on serum OT in female mice[86]. They observed that the performance of mild aerobic exercise for 6 wk significantly elevated serum OT and this helped in reducing anxiety. Arabacı Tamer et al[87] studied the influence of aerobic exercise on oxytocinergic motion in rats[87]. They detected that the daily repetitive moderate aerobic exercise for 30 min for 5 d significantly reversed the artificially produced negative-regulated oxytocinergic motion. Martins et al[88] studied the influence of aerobic exercise on serum OT in mice[88]. They detected that the performance of mild-aerobic exercise for 3 mo significantly elevated serum OT.
The TH gland activity usually decreases in stressful circumstances. In stress, serum 3,5,3′,5′-tetraiodothyronine thyroxine (T4) and 3,5,3′- triiodothyronine (T3) decay, and the TH-stimulating hormone (TSH) release ceases as a consequence of the rise of serum GCs[89,90]. TH hormones, principally T3 and T4, are indispensable hormones in controlling numerous functions in human bodies, such as oxygen utilization, protein, lipid, and carbohydrate absorptions, adolescence, intermittent bleeding, and stress lessening[90].
In humans, various neurons in the paraventricular nucleus of the hypothalamus, mainly parvocellular zone, stabilize the hypothalamic-pituitary-TH axis activity. The hypothalamic-pituitary-TH axis triggers the release of thyrotropin-releasing hormone into the median eminence. The thyrotropin-releasing hormone triggers the frontal pituitary gland to release TSH. TSH voyages throughout peripheral vessels to activate the release of T3 and T4 from the TH gland. T4 generally transmutes into T3 by deiodinase enzymes present in the utmost body tissues. TH hormones have adverse feedback function by exciting T hormone receptors present in the pituitary and hypothalamus to stop the release of TSH[89,91].
The decrease in serum TH hormones, mainly T3 and T4, can decrease the function of various immune cells (e.g., lymphocytes, monocytes, natural killer cells, and macrophages), consequently decreasing the activity of various infection-related processes, including chemotaxis, phagocytosis, cytokines release, and reactive oxygen species production. The real mechanisms behind this relationship are still unclear[92,93]. Preceding studies have demonstrated that patients with hyperthyroidism usually have a rise in the activity of macrophages, leukocytes, and lymphocytes[94,95]. Other studies have demonstrated opposite outcomes in patients with hypothyroidism[96,97].
Short periods of aerobic exercise can significantly increase serum T3. Altaye et al[98] investigated the influence of continued aerobic exercise on the alteration in serum TH hormones in teenagers with intelligence debilities[98]. They detected that routine mild aerobic exercise for 3 mo significantly raised serum T3, T4, and TSH. Fathi et al[99] examined the influence of aerobic exercise on TH hormones in obese postmenopausal females[99]. They observed that mild aerobic exercise implemented for 8 wk significantly increased serum T3, T4, and TSH levels. Rone et al[100] studied the influence of serum T3 in males[100]. They observed that athletes had greater serum T3 than sedentary controls during kinetic analysis.
Insulin is considered a peptide hormone released by the β-cells present in the pancreatic islets of Langerhans. Insulin maintains normal serum glucose by facilitating the digesting of cellular glucose to facilitate carbohydrate, protein, and lipid metabolism and promote cell splitting by its mitogenic characteristics[101]. Stress causes an increase in serum insulin; if the stress continues for longer periods, it causes insulin exhaustion, serum glucose elevation, and diabetes mellitus (DM) occurrence[101,102]. Insulin resistance upsurge is a compensatory mechanism to sustain ordinary serum glucose. The dysfunction of this compensatory mechanism causes an increase in serum glucose and the development of DM. Insulin also has a direct connection with numerous hormones, such as growth hormone, GCs, glucagon, norepinephrine, and epinephrine because all these hormones participate in the control of glucose and carbohydrate digestion and undergoes the same neural control[103]. Several studies have revealed there is a direct relationship between prolonged psychological stress and the depletion of insulin and the development of DM[104-106]. Also, several studies have reported that DM leads to a decline in immunity strength, though the actual mechanisms by which low insulin level or increased insulin resistance causes a decrease in immunity strength are not clear yet[107-109]. It might be that insulin has a crucial role in glucose metabolism, which is vital in delivering vitality to immune cells against viral infection.
Enhancing oxygenation can increase insulin levels and enhance glucose metabolism. The increase in insulin production might be caused by the influence of increasing the oxygenation on lowering serum GCs. There is strong evidence in the literature to support the beneficial role of implementing aerobic exercise on increasing glucose metabolism and insulin sensitivity[110-114]. Winnick et al[110] studied the influence of aerobic exercise on the sensitivity of insulin in overweight persons having type II diabetes[110]. They observed that the performance of aerobic exercise for a week significantly elevated insulin sensitivity. Schwaab et al[111] compared the influence of anaerobic and aerobic training on glucose tolerance in persons with type II diabetes and coronary artery illnesses[111]. They observed that aerobic exercise implemented for 16 wk, unlike anaerobic exercise, significantly reduced glucose tolerance. Karstoft et al[112] examined the influence of mild aerobic exercise on physical fitness, glycemic control, and body structures in persons with type II diabetes[112]. They observed that alternating mild and high aerobic exercise implemented for 4 mo significantly elevated insulin sensitivity and reduced glycemic control. Nassis et al[113] investigated the influence of aerobic exercise on insulin sensitivity between overweight girls[113]. They observed that the performance of mild aerobic exercise for 12 wk significantly reduced insulin sensitivity.
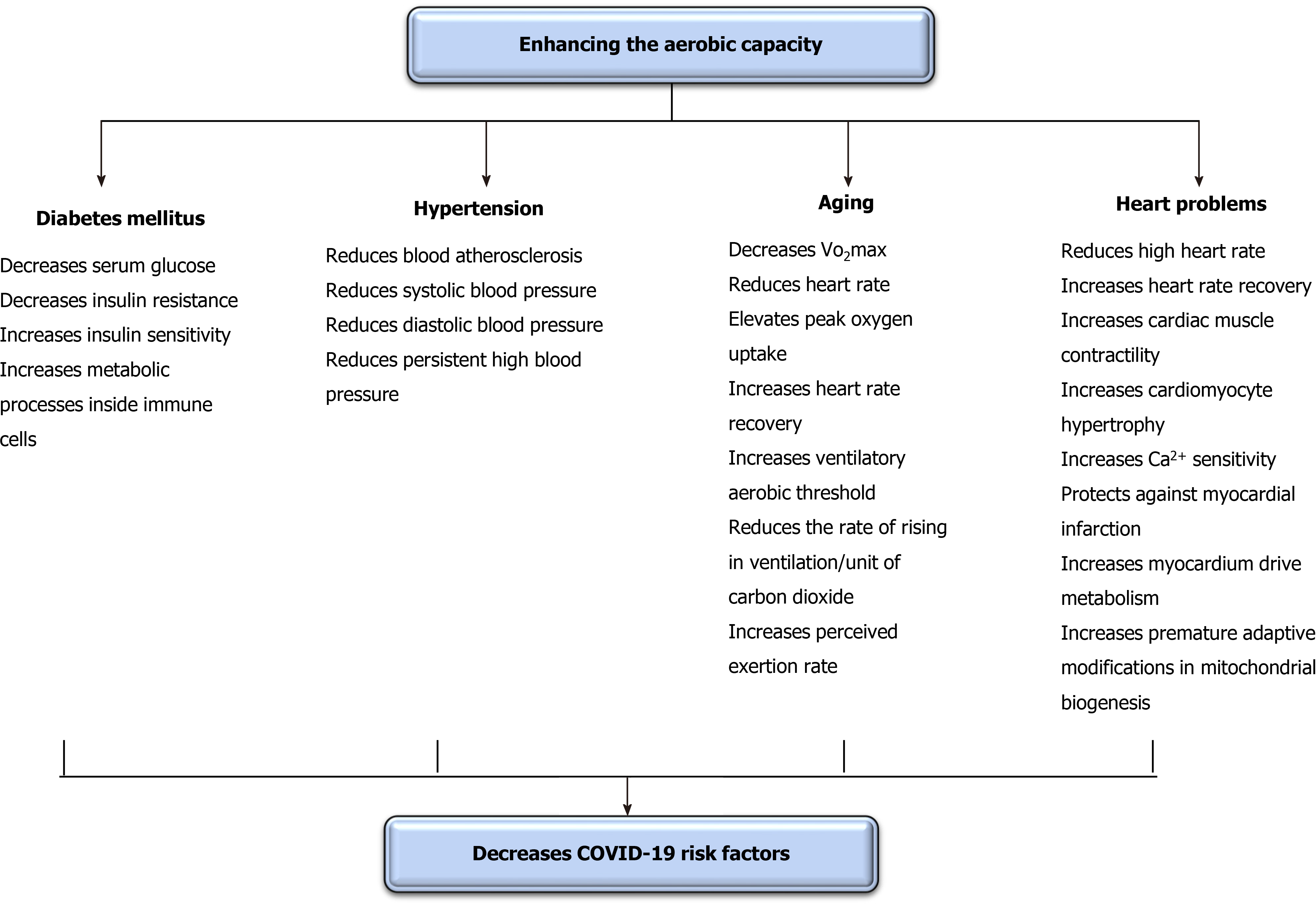
Brief periods of aerobic exercise can significantly regulate COVID-19 risk factors. Interestingly, this effect in some studies occurred after a single session only. Thus, this subtopic mainly focuses on the instantaneous and short-term influence of enhancing oxygenation on COVID-19 risk factors as these risk factors can increase spread and progress rates[47]. The communal risk factors occurring with COVID-19 are hypertension, aging, heart problems, and diabetes[115]. The defensive role of enhancing oxygenation on risk factors of COVID-19 is illustrated in Figure 4.
DM negatively affects numerous body structures[116], including immunity[117] and pulmonary functions[118]. Enhancing oxygenation can cause an immediate decline in serum glucose in both types of DM. Yardley et al[119] studied the immediate influence of aerobic vs anaerobic exercise on serum glucose in individuals with type I DM[119]. They observed that 45 min of mild aerobic exercise significantly reduced serum glucose more than resistance exercise. However, this reduction quickly returned to the pre-exercise level following the session. Bacchi et al[120] studied the immediate influence of aerobic vs anaerobic exercise on serum glucose in individuals with type II DM[120]. They observed that, unlike anaerobic exercise, mild aerobic exercise significantly reduced serum glucose during the session and for the following entire night. Yokoyama et al[121] studied the temporary influence of aerobic exercise on stiffness of arteries in peoples with type II DM[121]. They observed that 45 min of mild aerobic exercise implemented for 3 wk significantly reduced the stiffness of common carotid and femoral arteries, and this decrease was accompanied with an improvement in tissue resistance to insulin.
Short periods of aerobic exercise can yield an acute substantial decrease in high blood pressure (BP)[122]. Ciolac et al[123] inspected the influence of aerobic exercise on BP in persons with lasting untreated hypertension[123]. They observed that just a seldom session of aerobic exercise significantly declined ambulatory BP. Lund et al[124] inspected the influence of aerobic exercise on ambulatory BP amid woman cleaners[124]. They observed that one session of aerobic exercise significantly reduced ambulatory BP, and this reduction continued for 25 h after the session. Guimarães et al[125] studied the influence of immediate aerobic exercise in warm water on general BP in people with resistant hypertension[125]. They detected that performing mild aerobic exercise for 2 wk significantly declined systolic BP and diastolic BP and cardiovascular size either subsequent the exercise or through the subsequent 24 h. Nascimento et al[126] studied the instant and extended influence of aerobic exercise on BP in people with resilient hypertension[126]. They detected that mild aerobic exercise implemented for 8 wk significantly decreased BP.
Aging significantly reduces bodily function and activity exposing these structures to dysfunction and failure. Older patients with COVID-19 commonly have greater death rates than younger patients. Thus, reducing the influence of aging on body structures could assist in reducing these death rates. Giallauria et al[127] studied the influence of mild aerobic exercise on recovery of normal heart rate in older adults. They observed that the performance of mild aerobic exercise for 8 wk significantly elevated peak oxygen uptake, heart rate recovery, and ventilatory aerobic threshold and reduced the rate of rising in ventilation/unit of carbon dioxide release anticipating that mild aerobic exercise can modify the autonomic nervous system activity by raising the vagal/sympathetic balance. Chapman et al[128] studied the acute influence of mild aerobic exercise on cardiovascular fitness in older adults. They observed that a routine of mild aerobic exercise for 6 wk significantly enhanced both perceived exertion rate and VO2max.
Heart and pulmonary problems usually come with together. Thus, heart problems could increase the declined rate in lung function and disorders (heart-lung interaction)[129-131]. Enhancing oxygenation can cause significant short-term enhancements in heart disorders. The immediate and chronic enhancements in heart, functions, rates, and volumes were demonstrated in the preceding paragraph[54,127,128]. Animal studies have demonstrated that the performance of mild aerobic exercise significantly improves heart functions. Wisløff et al[132] studied the short-term influence of aerobic exercise on cardiac muscle contractility, cardiomyocyte hypertrophy, and Ca2+ sensitivity in rats after an artificially-induced myocardial infarction. This study detected that a routine of mild aerobic exercise performed for 8 wk significantly enhanced cardiac contractility and Ca2+ sensitivity and reduced the hypertrophy of cardiomyocytes. Tao et al[133] studied the impact of aerobic exercise in ending the artificially-produced acute myocardial infarction in mice. They observed that the performance of mild aerobic exercise for 3 wk significantly protected rats from acute myocardial infarction by enhancing myocardium driven metabolism and inducing premature adaptive modifications in mitochondrial biogenesis.
This subtopic intends to scrutinize the previous studies that examined the influences of aerobic exercise on immunity amid healthy individuals as an effort to afford aerobic exercise prescriptions for patients with COVID-19. In this section, we analyzed eleven studies[19,20,37,46,134-140] performed on healthy subjects and accomplished an increase in immunological markers[141]. The recommendations and prescriptions of aerobic exercise for patients with COVID-19 are shown in Table 1.
| Type of exercise | Intensity of exercise | Duration of exercise | Frequency of exercise | |
| Aerobic exercise recommendation and prescriptions | Cycling | 55%-85% VO2max | 18-60 min | 2-3 sessions/wk |
| Light running | 60%-80% MHR |
The type of aerobic exercise implemented in the discussed studies were chiefly running and cycling. Most of the included studies implemented pedaling[19,134-138,140], while the other studies implemented either running[37,139] or both pedaling and running[20,46]. Based on these findings, treadmill biking or running would be the appropriate type of aerobic exercise in patients with COVID-19, Also for older patients or patients who have balance problems, the bicycle may be a decent selection for them[142].
The intensity of aerobic exercise performed in the previous studies was measured by maximum heart rate (MHR) or VO2max. In the studies that were implemented for short times, most of them implemented aerobic exercise using VO2max as the main indicator to determine its intensity, and mostly the intensity ranged from 55% to 85% VO2max, including two studies used 55% VO2max[19,134], two studies used 55%-85% VO2max[136,140], one study used 60% VO2max[137], and one study used MHR instead of VO2max at 60%-70% MHR[139]. In the studies that were implemented for prolonged times, most of them implemented aerobic exercise using MHR the main indicator to determine its intensity, and mostly the intensity ranged from 60%-80% MHR, including one study used 60%-75% MHR[37], one study used 70%-80% MHR[135], one study used 70% VO2max[20], and one study used 75% VO2max[138]. Based on the aforementioned intensities, implementing aerobic exercise at an intensity range from 55% to 85% VO2max would be the recommended intensity in patients with COVID-19. The starting intensity should be determined personally to avoid the development of exhaustion[143] because exhaustion can adversely affect immunity[144]. Thus, aerobic exercise should be graded starting from light activity through performing extended warming-up and cooling-down, then a rise in the intensity should followed.
The duration of aerobic exercise performed in the earlier studies fluctuated from 18-80 min. In the studies that studied the immediate effects of aerobic exercise, the time of the session fluctuated from 18 to 60 min[136,140], two studies implemented exercise for 45 min[134,139], and two studies implemented exercise for 60 min[19,137]. In the studies that tested the long-term effect of aerobic exercise, the duration of the session ranged from 30 to 80 min, including one study implemented exercise for 30 min[138], one study implemented exercise for 45 min[135], one study implemented exercise for 50 min[37], one study implemented exercise for 60 min[46], and one study used a specific distance (5 km) instead of a duration[20]. Therefore, 20-60 min of aerobic exercise can be a fitting time of the aerobic exercise session for patients with COVID-19. If the patient is sedentary and cannot perform the session time without exhaustion, day-to-day several short bursts of aerobic exercise could be used.
The frequency of aerobic exercise performed in the earlier studies fluctuated from 1 session/wk to 3 sessions/wk. In the studies that studied the short-term effects of aerobic exercise, exercise frequencies included: 4 studies implemented aerobic exercise for only 1 session[136], 1 study implemented aerobic exercise for 2 sessions (one session/wk)[19], and 1 study implemented aerobic exercise for 3 sessions for only a week[134]. In the studies that studied the long-term effect of aerobic exercise, exercise frequencies included: 1 study implemented aerobic exercise for 2 sessions/wk for 8 wk[46], one study implemented aerobic exercise for 3 sessions/wk for 5 wk[20], one study implemented aerobic exercise for 3 sessions/wk for 9 wk[138], one study implemented aerobic exercise for 3 sessions/wk for 10 wk[135], and one study implemented aerobic exercise for 3 sessions/wk for 12 wk[37]. Based on the aforementioned findings, the frequency of 3 sessions/wk can be helpful and safe in patients with COVID-19.
Limitations of this review include that most comprised studies were conducted on animals as a result of the deficiency of human studies addressing these intended effects, predominantly infection-related ones. Also, the searched databases were the most cited ones to ensure the quality of the included studies.
Improving oxygenation in patients with COVID-19 is crucial because it has a significant role in improving immune and pulmonary functions. In addition, it helps in decreasing COVID-19 risk factors that increase the severity of symptoms. Finally, it helps in controlling hormones that are involved in bodily infection-fighting mechanisms. To increase immunity, particularly immunological markers, patients with COVID-19 should implement a continuous aerobic exercise session at an intensity of 55%-80% of VO2max or 60%-80% of MHR for 2-3 sessions/wk with a duration of 18-60 min/session and using cycling ergometer or light running because these specifications could enhance immunity without developing fatigue or exhaustion.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: West J S-Editor: Fan JR L-Editor: Filipodia P-Editor: Xing YX
| 1. | World Health Organization. Corona- virus disease (COVID-19) outbreak. [cited 15 January 2021]. Available from: https://covid19.who.int/. |
| 2. |
Guan WJ, Ni ZY, Hu Y, Liang WH, Zhong NS.
Clinical Characteristics of Coronavirus Disease 2019 in China. |
| 3. | He F, Deng Y, Li W. Coronavirus Disease 2019 (COVID-19): What we know? J Med Virol. 2020;2019:0-2. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 4. | Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). 2021 Apr 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–.. [PubMed] |
| 5. | Mohamed AA, Alawna M. Role of increasing the aerobic capacity on improving the function of immune and respiratory systems in patients with coronavirus (COVID-19): A review. Diabetes Metab Syndr. 2020;14:489-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28:465-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 626] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 7. | Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. Coronavirus infections and immune responses. J Med Virol. 2020;92:424-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1056] [Cited by in RCA: 1168] [Article Influence: 233.6] [Reference Citation Analysis (0)] |
| 8. | Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ Jr, Baric RS, Enjuanes L, Gallagher T, McCray PB Jr, Perlman S. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci USA. 2014;111:4970-4975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 9. | Yang Y, Xiong Z, Zhang S, Yan Y, Nguyen J, Ng B, Lu H, Brendese J, Yang F, Wang H, Yang XF. Bcl-xL inhibits T-cell apoptosis induced by expression of SARS coronavirus E protein in the absence of growth factors. Biochem J. 2005;392:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Mubarak A, Alturaiki W, Hemida MG. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Infection, Immunological Response, and Vaccine Development. J Immunol Res. 2019;2019:6491738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 11. | Pascal KE, Coleman CM, Mujica AO, Kamat V, Badithe A, Fairhurst J, Hunt C, Strein J, Berrebi A, Sisk JM, Matthews KL, Babb R, Chen G, Lai KM, Huang TT, Olson W, Yancopoulos GD, Stahl N, Frieman MB, Kyratsous CA. Pre- and postexposure efficacy of fully human antibodies against Spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci USA. 2015;112:8738-8743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 12. | Chen J, Lau YF, Lamirande EW, Paddock CD, Bartlett JH, Zaki SR, Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84:1289-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 323] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 13. | Manni ML, Robinson KM, Alcorn JF. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev Respir Med. 2014;8:25-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 352] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 15. | Dutzan N, Abusleme L. T Helper 17 Cells as Pathogenic Drivers of Periodontitis. Adv Exp Med Biol. 2019;1197:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Gonçalves CAM, Dantas PMS, Dos Santos IK, Dantas M, da Silva DCP, Cabral BGAT, Guerra RO, Júnior GBC. Effect of Acute and Chronic Aerobic Exercise on Immunological Markers: A Systematic Review. Front Physiol. 2019;10:1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Lippi G, Banfi G, Montagnana M, Salvagno GL, Schena F, Guidi GC. Acute variation of leucocytes counts following a half-marathon run. Int J Lab Hematol. 2010;32:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Lippi G, Salvagno GL, Danese E, Skafidas S, Tarperi C, Guidi GC, Schena F. Mean platelet volume (MPV) predicts middle distance running performance. PLoS One. 2014;9:e112892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Li TL, Cheng PY. Alterations of immunoendocrine responses during the recovery period after acute prolonged cycling. Eur J Appl Physiol. 2007;101:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Lira FS, Dos Santos T, Caldeira RS, Inoue DS, Panissa VLG, Cabral-Santos C, Campos EZ, Rodrigues B, Monteiro PA. Short-Term High- and Moderate-Intensity Training Modifies Inflammatory and Metabolic Factors in Response to Acute Exercise. Front Physiol. 2017;8:856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Reis Gonçalves CT, Reis Gonçalves CG, de Almeida FM, Lopes FD, dos Santos Durão AC, dos Santos FA, da Silva LF, Marcourakis T, Castro-Faria-Neto HC, Vieira RP, Dolhnikoff M. Protective effects of aerobic exercise on acute lung injury induced by LPS in mice. Crit Care. 2012;16:R199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Ababneh M, Alrwashdeh M, Khalifeh M. Recombinant adenoviral vaccine encoding the spike 1 subunit of the Middle East Respiratory Syndrome Coronavirus elicits strong humoral and cellular immune responses in mice. Vet World. 2019;12:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Ali MT, Morshed MM, Gazi MA, Musa MA, Kibria MG, Uddin MJ, Khan MA, Hasan S. Computer aided prediction and identification of potential epitopes in the receptor binding domain (RBD) of spike (S) glycoprotein of MERS-CoV. Bioinformation. 2014;10:533-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Niu P, Zhang S, Zhou P, Huang B, Deng Y, Qin K, Wang P, Wang W, Wang X, Zhou J, Zhang L, Tan W. Ultrapotent Human Neutralizing Antibody Repertoires Against Middle East Respiratory Syndrome Coronavirus From a Recovered Patient. J Infect Dis. 2018;218:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Niu P, Zhao G, Deng Y, Sun S, Wang W, Zhou Y, Tan W. A novel human mAb (MERS-GD27) provides prophylactic and postexposure efficacy in MERS-CoV susceptible mice. Sci China Life Sci. 2018;61:1280-1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Chen Z, Bao L, Chen C, Zou T, Xue Y, Li F, Lv Q, Gu S, Gao X, Cui S, Wang J, Qin C, Jin Q. Human Neutralizing Monoclonal Antibody Inhibition of Middle East Respiratory Syndrome Coronavirus Replication in the Common Marmoset. J Infect Dis. 2017;215:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Shishido SN, Varahan S, Yuan K, Li X, Fleming SD. Humoral innate immune response and diease. Cin iImunolo. 2012;144:142-158. [RCA] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 648] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 28. | Baker S, Kessler E, Darville-Bowleg L, Merchant M. Different mechanisms of serum complement activation in the plasma of common (Chelydra serpentina) and alligator (Macrochelys temminckii) snapping turtles. PLoS One. 2019;14:e0217626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Smith JK, Chi DS, Krish G, Reynolds S, Cambron G. Effect of exercise on complement activity. Ann Allergy. 1990;65:304-310. [PubMed] |
| 30. | Karacabey K, Peker, Saygın, Cıloglu F, Ozmerdivenli R, Bulut V. Effects of acute aerobic and anaerobic exercise on humoral immune factors in elite athletes. Biotechnol Biotechnol Equip. 2005;19:175-180. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Karacabey K, Saygin O, Ozmerdivenli R, Zorba E, Godekmerdan A, Bulut V. The effects of exercise on the immune system and stress hormones in sportswomen. Neuro Endocrinol Lett. 2005;26:361-366. [PubMed] |
| 32. | Wolach B, Eliakim A, Gavrieli R, Kodesh E, Yarom Y, Schlesinger M, Falk B. Aspects of leukocyte function and the complement system following aerobic exercise in young female gymnasts. Scand J Med Sci Sports. 1998;8:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | MacKinnon LT. Special feature for the Olympics: effects of exercise on the immune system: overtraining effects on immunity and performance in athletes. Immunol Cell Biol. 2000;78:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Rodríguez A, Tjärnlund A, Ivanji J, Singh M, García I, Williams A, Marsh PD, Troye-Blomberg M, Fernández C. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine. 2005;23:2565-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Hines MT, Schott HC 2nd, Bayly WM, Leroux AJ. Exercise and immunity: a review with emphasis on the horse. J Vet Intern Med. 1996;10:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Cunningham-Rundles C. Lung disease, antibodies and other unresolved issues in immune globulin therapy for antibody deficiency. Clin Exp Immunol. 2009;157 Suppl 1:12-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Mohamed G, Taha M. Comparison between the effects of aerobic and resistive training on immunoglobulins in obese women. Bull Fac Phys Ther. 2016;21:11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 1718] [Article Influence: 245.4] [Reference Citation Analysis (0)] |
| 39. | Rasmussen F, Mikkelsen D, Hancox RJ, Lambrechtsen J, Nybo M, Hansen HS, Siersted HC. High-sensitive C-reactive protein is associated with reduced lung function in young adults. Eur Respir J. 2009;33:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Singhal T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr. 2020;87:281-286. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2006] [Cited by in RCA: 1573] [Article Influence: 314.6] [Reference Citation Analysis (0)] |
| 41. | Zhang J, Zhou L, Yang Y, Peng W, Wang W, Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11-e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 42. | Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80:1055-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 983] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 43. | Marklund P, Mattsson CM, Wåhlin-Larsson B, Ponsot E, Lindvall B, Lindvall L, Ekblom B, Kadi F. Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J Appl Physiol (1985). 2013;114:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | de Gonzalo-Calvo D, Dávalos A, Montero A, García-González Á, Tyshkovska I, González-Medina A, Soares SM, Martínez-Camblor P, Casas-Agustench P, Rabadán M, Díaz-Martínez ÁE, Úbeda N, Iglesias-Gutiérrez E. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J Appl Physiol (1985). 2015;119:124-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Zheng G, Qiu P, Xia R, Lin H, Ye B, Tao J, Chen L. Effect of Aerobic Exercise on Inflammatory Markers in Healthy Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Aging Neurosci. 2019;11:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 46. | Okita K, Nishijima H, Murakami T, Nagai T, Morita N, Yonezawa K, Iizuka K, Kawaguchi H, Kitabatake A. Can exercise training with weight loss lower serum C-reactive protein levels? Arterioscler Thromb Vasc Biol. 2004;24:1868-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30123] [Article Influence: 6024.6] [Reference Citation Analysis (3)] |
| 48. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17648] [Article Influence: 3529.6] [Reference Citation Analysis (0)] |
| 49. | Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. 2020;15:700-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 927] [Cited by in RCA: 1000] [Article Influence: 200.0] [Reference Citation Analysis (0)] |
| 50. | Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 856] [Article Influence: 171.2] [Reference Citation Analysis (0)] |
| 51. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 52. | Baumann FT, Zimmer P, Finkenberg K, Hallek M, Bloch W, Elter T. Influence of endurance exercise on the risk of pneumonia and Fever in leukemia and lymphoma patients undergoing high dose chemotherapy. A pilot study. J Sports Sci Med. 2012;11:638-642. [PubMed] |
| 53. | Williams PT. Dose-response relationship between exercise and respiratory disease mortality. Med Sci Sports Exerc. 2014;46:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Stravinskas Durigon T, MacKenzie B, Carneiro Oliveira-Junior M, Santos-Dias A, De Angelis K, Malfitano C, Kelly da Palma R, Moreno Guerra J, Damaceno-Rodrigues NR, Garcia Caldini E, de Almeida FM, Aquino-Santos HC, Rigonato-Oliveira NC, Leal de Oliveira DB, Aimbire F, Ligeiro de Oliveira AP, Franco de Oliveira LV, Durigon EL, Hiemstra PS, Vieira RP. Aerobic Exercise Protects from Pseudomonas aeruginosa-Induced Pneumonia in Elderly Mice. J Innate Immun. 2018;10:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Neuman MI, Willett WC, Curhan GC. Physical activity and the risk of community-acquired pneumonia in US women. Am J Med 2010; 123: 281.e7-281. e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Olivo CR, Miyaji EN, Oliveira ML, Almeida FM, Lourenço JD, Abreu RM, Arantes PM, Lopes FD, Martins MA. Aerobic exercise attenuates pulmonary inflammation induced by Streptococcus pneumoniae. J Appl Physiol (1985). 2014;117:998-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Rigonato-Oliveira NC, Mackenzie B, Bachi ALL, Oliveira-Junior MC, Santos-Dias A, Brandao-Rangel MAR, Delle H, Costa-Guimaraes T, Damaceno-Rodrigues NR, Dulley NR, Benetti MA, Malfitano C, de Angelis C, Albertini R, Oliveira APL, Abbasi A, Northoff H, Vieira RP. Aerobic exercise inhibits acute lung injury: from mouse to human evidence Exercise reduced lung injury markers in mouse and in cells. Exerc Immunol Rev. 2018;24:36-44. [PubMed] |
| 58. | Vieira RP, Oliveira-Junior MC, Teixeira RW, Greiffo FR, Silva VR, Pereira PR, Sousa ASA, Albertini R, Oliveira LVF. The crucial role of IL-10 in the anti-inflammatory effects of aerobic exercise in a model LPS-induced ARDS. Eur Respir J. 2013;42. |
| 59. | Shi Y, Liu T, Nieman DC, Cui Y, Li F, Yang L, Shi H, Chen P. Aerobic Exercise Attenuates Acute Lung Injury Through NET Inhibition. Front Immunol. 2020;11:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 60. | Guimarães I, Padilha G, Lopes-Pacheco M, Marques P, Antunes M, Rocha N, Assis E, Farianeto H, Magalhaes R, Xisto D. The impact of aerobic exercise on lung inflammation and remodeling in experimental emphysema. Eur Respir J. 2011;38. |
| 61. | Park J, Han D. Effects of high intensity aerobic exercise on treadmill on maximum-expiratory lung capacity of elderly women. J Phys Ther Sci. 2017;29:1454-1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Taskin H, Atalay OT, Kurtca MP, Kabul EG, Cobankara V. The effects of aerobic training on respiratory muscle strength and exercise capacity in ankylosing spondylitis patients. Eur Respir Soc. 2018;PA1444. [DOI] [Full Text] |
| 63. | Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A, Neri LM. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9:17181-17198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 298] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 64. | Trefler S, Rodríguez A, Martín-Loeches I, Sanchez V, Marín J, Llauradó M, Romeu M, Díaz E, Nogués R, Giralt M. Oxidative stress in immunocompetent patients with severe community-acquired pneumonia. A pilot study. Med Intensiva. 2014;38:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Gagnon DD, Dorman S, Ritchie S, Mutt SJ, Stenbäck V, Walkowiak J, Herzig KH. Multi-Day Prolonged Low- to Moderate-Intensity Endurance Exercise Mimics Training Improvements in Metabolic and Oxidative Profiles Without Concurrent Chromosomal Changes in Healthy Adults. Front Physiol. 2019;10:1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Toledo AC, Magalhaes RM, Hizume DC, Vieira RP, Biselli PJ, Moriya HT, Mauad T, Lopes FD, Martins MA. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur Respir J. 2012;39:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | da Cunha MJ, da Cunha AA, Ferreira GK, Baladão ME, Savio LE, Reichel CL, Kessler A, Netto CA, Wyse AT. The effect of exercise on the oxidative stress induced by experimental lung injury. Life Sci. 2013;92:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Leite MR, Ramos EM, Kalva-Filho CA, Freire AP, de Alencar Silva BS, Nicolino J, de Toledo-Arruda AC, Papoti M, Vanderlei LC, Ramos D. Effects of 12 weeks of aerobic training on autonomic modulation, mucociliary clearance, and aerobic parameters in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:2549-2557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Borghi-Silva A, Arena R, Castello V, Simões RP, Martins LE, Catai AM, Costa D. Aerobic exercise training improves autonomic nervous control in patients with COPD. Respir Med. 2009;103:1503-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Amro M, Mohamed A, Alawna M. Effects of increasing aerobic capacity on improving psychological problems seen in patients with COVID-19: a review. Eur Rev Med Pharmacol Sci. 2021;25:2808-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 71. | Won E, Kim YK. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr Neuropharmacol. 2016;14:665-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 72. | Beurel E, Nemeroff CB. Interaction of stress, corticotropin-releasing factor, arginine vasopressin and behaviour. Curr Top Behav Neurosci. 2014;18:67-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Oppong E, Cato AC. Effects of Glucocorticoids in the Immune System. Adv Exp Med Biol. 2015;872:217-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 74. | Goppelt-Struebe M, Wolter D, Resch K. Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br J Pharmacol. 1989;98:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Lu KD, Cooper D, Haddad F, Zaldivar F, Kraft M, Radom-Aizik S. Glucocorticoid receptor expression on circulating leukocytes in healthy and asthmatic adolescents in response to exercise. Pediatr Res. 2017;82:261-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008;31:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 375] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 77. | Silva RA, Almeida FM, Olivo CR, Saraiva-Romanholo BM, Martins MA, Carvalho CRF. Exercise reverses OVA-induced inhibition of glucocorticoid receptor and increases anti-inflammatory cytokines in asthma. Scand J Med Sci Sport. 2016;26:82-92. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol (1985). 2006;100:1867-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 79. | Matsushita H, Latt HM, Koga Y, Nishiki T, Matsui H. Oxytocin and Stress: Neural Mechanisms, Stress-Related Disorders, and Therapeutic Approaches. Neuroscience. 2019;417:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 80. | Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, Grauer S, Pulicicchio C, Resnick L, Rahman Z, Sukoff Rizzo SJ, Luo B, Beyer CE, Logue SF, Marquis KL, Hughes ZA, Rosenzweig-Lipson S. Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology. 2010;58:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 81. | Matsushita H, Matsuzaki M, Han XJ, Nishiki TI, Ohmori I, Michiue H, Matsui H, Tomizawa K. Antidepressant-like effect of sildenafil through oxytocin-dependent cyclic AMP response element-binding protein phosphorylation. Neuroscience. 2012;200:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 83. | Fang A, Treadway MT, Hofmann SG. Working hard for oneself or others: Effects of oxytocin on reward motivation in social anxiety disorder. Biol Psychol. 2017;127:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, Phan KL. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology. 2014;39:2061-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 85. | Wang P, Yang HP, Tian S, Wang L, Wang SC, Zhang F, Wang YF. Oxytocin-secreting system: A major part of the neuroendocrine center regulating immunologic activity. J Neuroimmunol. 2015;289:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 86. |
Yüksel O, Ateş M, Kızıldağ S, Yüce Z, Koç B, Kandiş S, Güvendi G, Karakılıç A, Gümüş H, Uysal N.
Regular Aerobic Voluntary Exercise Increased Oxytocin in Female Mice: The Cause of Decreased Anxiety and Increased Empathy-Like Behaviors |
| 87. | Arabacı Tamer S, Üçem S, Büke B, Güner M, Karaküçük AG, Yiğit N, Şirvancı S, Çevik Ö, Ercan F, Yeğen BÇ. Regular moderate exercise alleviates gastric oxidative damage in rats via the contribution of oxytocin receptors. J Physiol. 2020;598:2355-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Martins AS, Crescenzi A, Stern JE, Bordin S, Michelini LC. Hypertension and exercise training differentially affect oxytocin and oxytocin receptor expression in the brain. Hypertension. 2005;46:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 89. | Helmreich DL, Parfitt DB, Lu XY, Akil H, Watson SJ. Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology. 2005;81:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 90. | Ranabir S, Reetu K. Stress and hormones. Indian J Endocrinol Metab. 2011;15:18-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 91. | Helmreich DL, Tylee D. Thyroid hormone regulation by stress and behavioral differences in adult male rats. Horm Behav. 2011;60:284-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 92. | Jara EL, Muñoz-Durango N, Llanos C, Fardella C, González PA, Bueno SM, Kalergis AM, Riedel CA. Modulating the function of the immune system by thyroid hormones and thyrotropin. Immunol Lett. 2017;184:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 93. | Klein JR. The immune system as a regulator of thyroid hormone activity. Exp Biol Med (Maywood). 2006;231:229-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | Marino F, Guasti L, Cosentino M, De Piazza D, Simoni C, Piantanida E, Cimpanelli M, Klersy C, Bartalena L, Venco A, Lecchini S. Thyroid hormone regulation of cell migration and oxidative metabolism in polymorphonuclear leukocytes: clinical evidence in thyroidectomized subjects on thyroxine replacement therapy. Life Sci. 2006;78:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Wolach B, Lebanon B, Jedeikin A, Shapiro MS, Shenkman L. Neutrophil chemotaxis, random migration, and adherence in patients with hyperthyroidism. Acta Endocrinol (Copenh). 1989;121:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 96. | Klecha AJ, Genaro AM, Gorelik G, Barreiro Arcos ML, Silberman DM, Schuman M, Garcia SI, Pirola C, Cremaschi GA. Integrative study of hypothalamus-pituitary-thyroid-immune system interaction: thyroid hormone-mediated modulation of lymphocyte activity through the protein kinase C signaling pathway. J Endocrinol. 2006;189:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 97. | Klecha AJ, Genaro AM, Lysionek AE, Caro RA, Coluccia AG, Cremaschi GA. Experimental evidence pointing to the bidirectional interaction between the immune system and the thyroid axis. Int J Immunopharmacol. 2000;22:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Altaye KZ, Mondal S, Legesse K, Abdulkedir M. Effects of aerobic exercise on thyroid hormonal change responses among adolescents with intellectual disabilities. BMJ Open Sport Exerc Med. 2019;5:e000524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Fathi M, Ziaaldini MM, Khairabadi S, Hejazi K. Effect of Aerobic Exercise on Thyroid Hormones and Quality of Life in Obese Postmenopausal Women. Med Lab J. 2018;12:5-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 100. | Rone JK, Dons RF, Reed HL. The effect of endurance training on serum triiodothyronine kinetics in man: physical conditioning marked by enhanced thyroid hormone metabolism. Clin Endocrinol (Oxf). 1992;37:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 101. | Fargion S, Dongiovanni P, Guzzo A, Colombo S, Valenti L, Fracanzani AL. Iron and insulin resistance. Aliment Pharmacol Ther. 2005;22 Suppl 2:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Kamba A, Daimon M, Murakami H, Otaka H, Matsuki K, Sato E, Tanabe J, Takayasu S, Matsuhashi Y, Yanagimachi M, Terui K, Kageyama K, Tokuda I, Takahashi I, Nakaji S. Association between Higher Serum Cortisol Levels and Decreased Insulin Secretion in a General Population. PLoS One. 2016;11:e0166077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 103. | Cox DJ, Taylor AG, Nowacek G, Holley-Wilcox P, Pohl SL, Guthrow E. The relationship between psychological stress and insulin-dependent diabetic blood glucose control: preliminary investigations. Health Psychol. 1984;3:63-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 53] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol. 2017;13:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 279] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 105. | Afrisham R, Paknejad M, Soliemanifar O, Sadegh-Nejadi S, Meshkani R, Ashtary-Larky D. The Influence of Psychological Stress on the Initiation and Progression of Diabetes and Cancer. Int J Endocrinol Metab. 2019;17:e67400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 106. | Harris ML, Oldmeadow C, Hure A, Luu J, Loxton D, Attia J. Stress increases the risk of type 2 diabetes onset in women: A 12-year longitudinal study using causal modelling. PLoS One. 2017;12:e0172126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 107. | Shu CJ, Benoist C, Mathis D. The immune system's involvement in obesity-driven type 2 diabetes. Semin Immunol. 2012;24:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 108. | Ferlita S, Yegiazaryan A, Noori N, Lal G, Nguyen T, To K, Venketaraman V. Type 2 Diabetes Mellitus and Altered Immune System Leading to Susceptibility to Pathogens, Especially Mycobacterium tuberculosis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 109. | de Candia P, Prattichizzo F, Garavelli S, De Rosa V, Galgani M, Di Rella F, Spagnuolo MI, Colamatteo A, Fusco C, Micillo T, Bruzzaniti S, Ceriello A, Puca AA, Matarese G. Type 2 Diabetes: How Much of an Autoimmune Disease? Front Endocrinol (Lausanne). 2019;10:451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 110. | Winnick JJ, Sherman WM, Habash DL, Stout MB, Failla ML, Belury MA, Schuster DP. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J Clin Endocrinol Metab. 2008;93:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 111. | Schwaab B, Kafsack F, Markmann E, Schütt M. Effects of aerobic and anaerobic exercise on glucose tolerance in patients with coronary heart disease and type 2 diabetes mellitus. Cardiovasc Endocrinol Metab. 2020;9:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 112. | Karstoft K, Winding K, Knudsen SH, Nielsen JS, Thomsen C, Pedersen BK, Solomon TP. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2013;36:228-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 113. | Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, Chrousos GP, Sidossis LS. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 114. | Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 115. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5518] [Article Influence: 1103.6] [Reference Citation Analysis (1)] |
| 116. | Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1026] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 117. | Graves DT, Kayal RA. Diabetic complications and dysregulated innate immunity. Front Biosci. 2008;13:1227-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 118. | Ardigo D, Valtuena S, Zavaroni I, Baroni MC, Delsignore R. Pulmonary complications in diabetes mellitus: the role of glycemic control. Curr Drug Targets Inflamm Allergy. 2004;3:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Yardley JE, Kenny GP, Perkins BA, Riddell MC, Balaa N, Malcolm J, Boulay P, Khandwala F, Sigal RJ. Resistance versus aerobic exercise: acute effects on glycemia in type 1 diabetes. Diabetes Care. 2013;36:537-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 120. | Bacchi E, Negri C, Trombetta M, Zanolin ME, Lanza M, Bonora E, Moghetti P. Differences in the acute effects of aerobic and resistance exercise in subjects with type 2 diabetes: results from the RAED2 Randomized Trial. PLoS One. 2012;7:e49937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Koyama H, Shoji T, Inaba M, Nishizawa Y. Short-term aerobic exercise improves arterial stiffness in type 2 diabetes. Diabetes Res Clin Pract. 2004;65:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 122. | Hamer M. The anti-hypertensive effects of exercise: integrating acute and chronic mechanisms. Sports Med. 2006;36:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 123. | Ciolac EG, Guimarães GV, D'Avila VM, Bortolotto LA, Doria EL, Bocchi EA. Acute aerobic exercise reduces 24-h ambulatory blood pressure levels in long-term-treated hypertensive patients. Clinics (Sao Paulo). 2008;63:753-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 124. | Lund Rasmussen C, Nielsen L, Linander Henriksen M, Søgaard K, Krustrup P, Holtermann A, Korshøj M. Acute effect on ambulatory blood pressure from aerobic exercise: a randomised cross-over study among female cleaners. Eur J Appl Physiol. 2018;118:331-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 125. | Guimarães GV, Cruz LG, Tavares AC, Dorea EL, Fernandes-Silva MM, Bocchi EA. Effects of short-term heated water-based exercise training on systemic blood pressure in patients with resistant hypertension: a pilot study. Blood Press Monit. 2013;18:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 126. | Nascimento LS, Santos AC, Lucena J, Silva L, Almeida A, Brasileiro-Santos MS. Acute and chronic effects of aerobic exercise on blood pressure in resistant hypertension: study protocol for a randomized controlled trial. Trials. 2017;18:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Giallauria F, Del Forno D, Pilerci F, De Lorenzo A, Manakos A, Lucci R, Vigorito C. Improvement of heart rate recovery after exercise training in older people. J Am Geriatr Soc. 2005;53:2037-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 128. | Chapman SB, Aslan S, Spence JS, Defina LF, Keebler MW, Didehbani N, Lu H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci. 2013;5:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 129. | Agostoni P, Cattadori G, Bussotti M, Apostolo A. Cardiopulmonary interaction in heart failure. Pulm Pharmacol Ther. 2007;20:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 130. | Lee HM, Liu MA, Barrett-Connor E, Wong ND. Association of lung function with coronary heart disease and cardiovascular disease outcomes in elderly: The Rancho Bernardo study. Respir Med. 2014;108:1779-1785. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 131. | Olson TP, Beck KC, Johnson BD. Pulmonary function changes associated with cardiomegaly in chronic heart failure. J Card Fail. 2007;13:100-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 132. | Wisløff U, Loennechen JP, Currie S, Smith GL, Ellingsen Ø. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res. 2002;54:162-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 133. | Tao L, Bei Y, Lin S, Zhang H, Zhou Y, Jiang J, Chen P, Shen S, Xiao J, Li X. Exercise Training Protects Against Acute Myocardial Infarction via Improving Myocardial Energy Metabolism and Mitochondrial Biogenesis. Cell Physiol Biochem. 2015;37:162-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 134. | Edwards KM, Burns VE, Ring C, Carroll D. Individual differences in the interleukin-6 response to maximal and submaximal exercise tasks. J Sports Sci. 2006;24:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 135. | LaPerriere A, Antoni MH, Ironson G, Perry A, McCabe P, Klimas N, Helder L, Schneiderman N, Fletcher MA. Effects of aerobic exercise training on lymphocyte subpopulations. Int J Sports Med. 1994;15 Suppl 3:S127-S130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Moyna NM, Acker GR, Weber KM, Fulton JR, Robertson RJ, Goss FL, Rabin BS. Exercise-induced alterations in natural killer cell number and function. Eur J Appl Physiol Occup Physiol. 1996;74:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Kurokawa Y, Shinkai S, Torii J, Hino S, Shek PN. Exercise-induced changes in the expression of surface adhesion molecules on circulating granulocytes and lymphocytes subpopulations. Eur J Appl Physiol Occup Physiol. 1995;71:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 138. | Mitchell JB, Paquet AJ, Pizza FX, Starling RD, Holtz RW, Grandjean PW. The effect of moderate aerobic training on lymphocyte proliferation. Int J Sports Med. 1996;17:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 139. | Nehlsen-Cannarella SL, Nieman DC, Jessen J, Chang L, Gusewitch G, Blix GG, Ashley E. The effects of acute moderate exercise on lymphocyte function and serum immunoglobulin levels. Int J Sports Med. 1991;12:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 140. | Moyna NM, Acker GR, Weber KM, Fulton JR, Goss FL, Robertson RJ, Rabin BS. The effects of incremental submaximal exercise on circulating leukocytes in physically active and sedentary males and females. Eur J Appl Physiol Occup Physiol. 1996;74:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 141. | Alawna M, Amro M, Mohamed AA. Aerobic exercises recommendations and specifications for patients with COVID-19: a systematic review. Eur Rev Med Pharmacol Sci. 2020;24:13049-13055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 142. | Traynor K. Planning an Exercise Regimen for the Sedentary Patient: What a Cardiologist Needs to Know - American College of Cardiology. Am Coll Cardiol. 2016;. |
| 143. | Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH. Sedentary behavior, physical activity, and markers of health in older adults. Med Sci Sports Exerc. 2013;45:1493-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 144. | Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8:201-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 704] [Article Influence: 117.3] [Reference Citation Analysis (0)] |