Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4852
Peer-review started: February 8, 2021
First decision: March 29, 2021
Revised: April 2, 2021
Accepted: April 20, 2021
Article in press: April 20, 2021
Published online: June 26, 2021
Processing time: 122 Days and 20.5 Hours
Metabolic associated fatty liver disease frequently occurs in patients with hypopituitarism and growth hormone (GH) deficiency. Some patients may develop to hepatopulmonary syndrome (HPS). HPS has a poor prognosis and liver transplantation is regarded as the only approach to cure it.
A 29-year-old man presented with progressive dyspnea for 1 mo. At the age of 10 years, he was diagnosed with panhypopituitarism associated with pituitary stalk interruption syndrome. Levothyroxine and hydrocortisone were given since then. To achieve ideal height, he received GH treatment for 5 years. The patient had an oxygen saturation of 78% and a partial pressure of arterial oxygen of 37 mmHg with an alveolar–arterial oxygen gradient of 70.2 mmHg. Abdominal ultrasonography showed liver cirrhosis and an enlarged spleen. Perfusion lung scan demonstrated intrapulmonary arteriovenous right-to-left shunt. HPS (very severe) was our primary consideration. His hormonal evaluation revealed GH deficiency and hypogonadotropic hypogonadism when thyroid hormone, cortisol, and desmopressin were administrated. After adding with long-acting recombinant human GH and testosterone for 14 mo, his liver function and hypoxemia were improved and his progressive liver fibrosis was stabilized. He was off the waiting list of liver transplantation.
Clinicians should screen HPS patients' anterior pituitary function as early as possible and treat them primarily with GH cocktail accordingly.
Core Tip: Liver transplantation is currently known to be the only way to cure hepatopulmonary syndrome (HPS). Even after the successful transplantation surgery, metabolic associated fatty liver disease (MAFLD) always recurs in patients with hypopituitarism who do not receive appropriate hormone replacement therapy. We present herein a case of HPS (very severe) induced by panhypopituitarism that was recovered by complete hormone replacement without surgery, especially growth hormone and testosterone. This case report highlights the importance of screening anterior pituitary function in patients with MAFLD or HPS as early as possible. The growth hormone cocktail therapy, especially growth hormone and testosterone, is expected to avoid liver transplantation.
- Citation: Ji W, Nie M, Mao JF, Zhang HB, Wang X, Wu XY. Growth hormone cocktail improves hepatopulmonary syndrome secondary to hypopituitarism: A case report. World J Clin Cases 2021; 9(18): 4852-4858
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4852.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4852
The incidence of metabolic associated fatty liver disease (MAFLD) in patients with hypopituitarism is significantly higher than that in gender and age matched healthy population (70.6% vs 32.5%)[1]. MAFLD usually occurs after hypopituitarism in the next 6-8 year[2]. The deficiency of insulin-like growth factors-1 (IGF-1) is associated with increasing histological severity of MAFLD and the presence of cirrhosis, independent of age, body mass index (BMI), and diabetes[3].
A 29-year-old man was admitted to our hospital in August 2018, complaining of worsening generalized weakness and dyspnea.
The patient could hardly walk without oxygen mask, presenting with cyanosis and clubbing fingers for 16 mo, and progressive dyspnea for 1 mo.
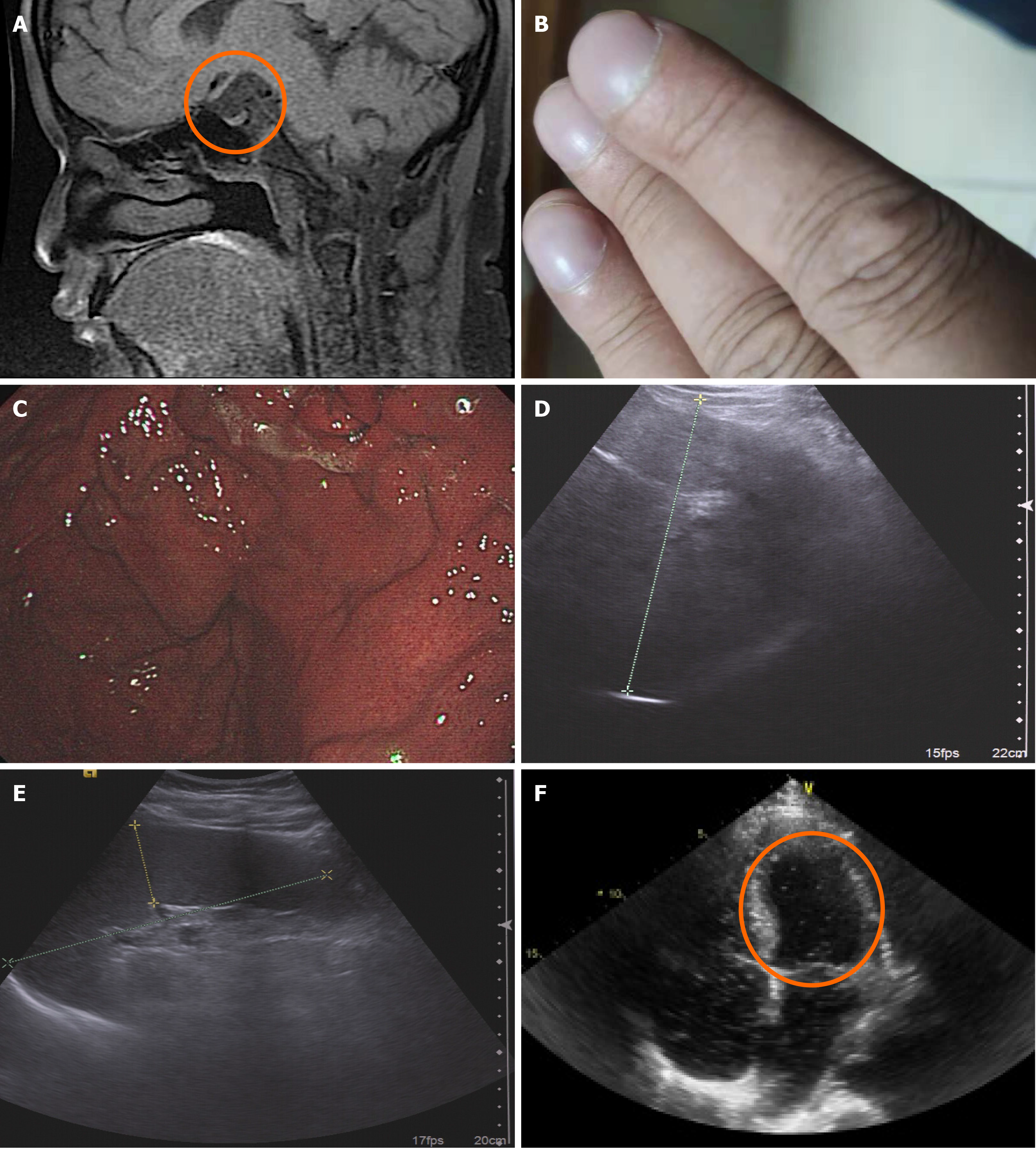
The patient denied any history of pulmonary and cardiac disorders, and alcohol abuse. When he was 10 years old, his height was 125 cm (-2.5 SD compared to age and sex matched boys). Further investigation found multiple pituitary hormone deficiency (low thyroxin and thyroid-stimulating hormone, low cortisol and adrenocorticotropin hormone at 8 am, and low IGF-1 levels). Hypopituitarism was diagnosed based on laboratory findings. Magnetic resonance imaging showed aplasia of the pituitary gland, interrupted pituitary stalk, and ectopic posterior bright spot (Figure 1A). A diagnosis of pituitary stalk interruption syndrome was made. Levothyroxine 50 μg per day and hydrocortisone 20 mg per day were started. His poor compliance made him take these medicines occasionally. To achieve ideal height, his recombinant human GH (rhGH) treatment was started at the age of 14 years, and lasted for 5 years. At the age of 23 years, he was diagnosed with nonalcoholic fatty liver disease. It gradually deteriorated to cirrhosis in the following 6 years.
The patient was 187 cm in height, 105 kg in weight, and 117 cm in waist circumference. His BMI was 30 kg/m2. Oxygen saturation was 78% on room air, and could increase to 92% with oxygen mask. His blood pressure was 128/79 mmHg and resting heart rate was 66 bpm with a regular rhythm. Physical examination revealed acanthosis nigricans, barrel chest with clear bilateral respiratory sounds, cyanotic lips, and clubbed fingers (Figure 1B). Hepatomegaly was palpable 4 cm beneath the xiphoid and 8 cm beneath the right costal margin. Splenomegaly was also palpable. Gynecomastia (Tanner stage IV) and prepubertal testicular size of 3 mL could be observed.
Laboratory results indicated multiple pituitary hormones deficiency. Pulmonary function test showed decreased infiltration of oxygen from pulmonary alveoli into the blood. Common causes for cirrhosis, especially hepatitis B virus infection, were ruled out by serological evaluation (Table 1). Activated partial thromboplastin time and international normalized ratio were within normal range, but prothrombin time was 14.9 s (normal range: 11-13 s).
| Hormone data | Value | Respiratory data | Value | HBV serologic testing | Value |
| TSH (0.380-4.340 μU/mL) | 4.595 | FVC | 3.15 L | HBsAg | - |
| FT3 (1.80-4.10 pg/mL) | 1.62 | %VC | 55% | HBsAb | - |
| FT4 (0.81-1.89 pg/mL) | 4.05 | FEV1.0 | 2.63L | HBeAg | - |
| GH (< 2.0 ng/mL) | < 0.05 | FEV1.0% | 84% | HBeAb | - |
| IGF-1 (ng/mL) | 32 (115-307) | %DLCO | 36.5% | HBcAb | - |
| ACTH (8 am, pg/mL) | 10.3 | ||||
| Cortisol (8 am, 4.26-24.85 μg/dL) | 20.06 | ||||
| FSH (1.27-19.26 U/L) | 0.35 | ||||
| LH (1.24-8.62 U/L) | 0.21 | ||||
| Testosterone (1.75-7.81 ng/mL) | < 0.1 | ||||
| Estradiol (< 47 pg/mL) | 6.8 | ||||
| Progesterone (0.10-0.84 ng/mL) | < 0.08 | ||||
| Prolactin (2.6-13.1 ng/mL) | 14.18 |
Gastric varices were observed by gastroscopy (Figure 1C). Abdominal ultrasound showed cirrhosis and hypersplenotrophy without ascites (Figure 1D and E). There was no evidence of an intracardiac shunt on echocardiography, and the ejection fraction was 52%. Transthoracic contrast echocardiography revealed opacification of the left chambers of the heart by micro-bubbles five heartbeats after the appearance of microbubbles in the right atrium, suggesting intrapulmonary shunting (Figure 1F).
Cirrhosis (Child-Pugh A), hepatopulmonary syndrome (HPS) (very severe) secondary to MAFLD (fatty liver index[4], 95), and panhypopituitarism.
Long-acting rhGH (1.5 mg, subcutaneously injected per week), oral testosterone (testosterone undecanoate, 40 mg, three times per day), thyroid hormone (levothyroxine, 75 μg per day), glucocorticoid (hydrocortisone, 20 mg per day), and desmopressin (50 μg per night) were administered.
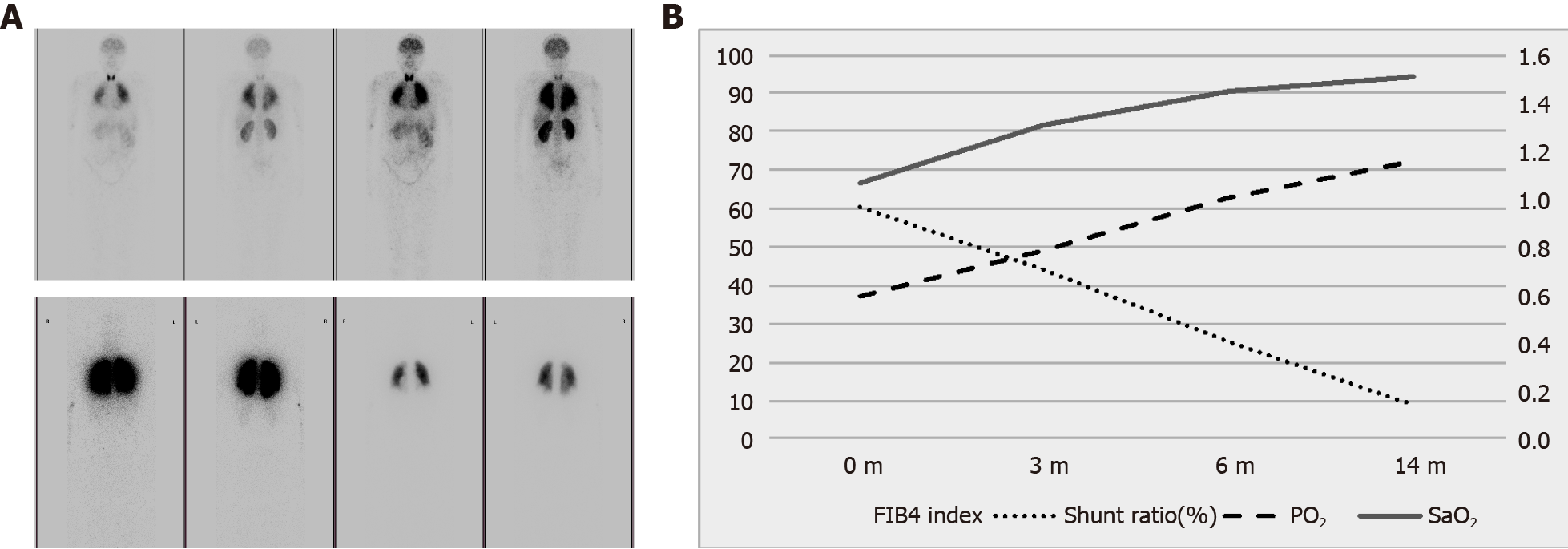
After 1 mo of treatment, the patient was able to climb up to the third floor without an oxygen mask. After continuing hormone replacement therapy for 14 mo, his intrapulmonary shunting returned to normal range (Figure 2A, bottom). His progressive liver fibrosis was stabilized and even slightly ameliorated according to fibrosis 4 (FIB-4) index (Figure 2B). His portal vein was not dilated any more (1.7 cm→1.5 cm), indicating progressive-free portal hypertension. Blood oxygenation was significantly enhanced. Serum aspartate aminotransferase and alanine aminotransferase levels gradually decreased to normal. The levels of gamma-glutamyl transferase, total bilirubin, direct bilirubin, and lipid profile were all improved significantly (Table 2). His body weight decreased by 5 kg and waist circumference reduced by 2 cm. He was thus taken off from the waiting list of liver transplantation.
| Blood gas analysis on room air | Baseline | Post-treatment | Liver function | Baseline | Post-treatment | Metabolic parameters | Baseline | Post-treatment |
| pH (7.35-7.45) | 7.42 | 7.42 | Alb (35-52g/L) | 43 | 44 | TG (0.45-1.70 mmol/L) | 2.14 | 1.36 |
| PCO2 (35-45 mmHg) | 34.0 | 34.6 | Tbil (5.1-22.2 μmol/L) | 37.0 | 30.4 | TC (2.85-5.70 mmol/L) | 6.71 | 5.92 |
| PO2 (83-108 mmHg) | 37.0 | 71.1 | Dbil (0-6.8 μmol/L) | 9.6 | 7.2 | LDL-c (< 3.37 mmol/L) | 4.70 | 4.43 |
| SaO2 (95%-99%) | 78% | 93.1% | GGT (0-40 U/L) | 70 | 64 | HDL-c (0.93-1.81 mmol/L) | 0.91 | 0.80 |
| BE (-3.0 ± 3.0 mmol/L) | -7.2 | -2.0 | ALP (45-125 U/L) | 65 | 80 | Fasting-insulin (5.2 - 17.2 μU/mL) | 29.1 | 26.6 |
| HCO-3 (22.0-27.0 mmol/L) | 17.7 | 22.7 | ALT (9-50 U/L) | 58 | 47 | Fasting blood glucose (3.9-6.1 mmol/L) | 6.00 | 5.60 |
| P(A-a)O2 (5.0-15.0 mmHg) | 70.2 | 35.7 | AST (15-40 U/L) | 45 | 32 | HbA1c (4.5%-6.3%) | 7.4% | 7.1% |
| Intrapulmonary shunt ratio(1.0%-10.0%) | 64.4% | 9.0% | PIIINP (< 15.00 ng/mL) | 13.50 | 15.21 | Na (135-145 mmol/L) | 142 | 138 |
| CIV (< 95.00 ng/mL) | 356.30 | 46.88 | CRP (< 3.00 mg/L) | 5.12 | 0.79 | |||
| HA (< 120.00 ng/mL) | 73.50 | 51.83 |
HPS, first described in 1977[5], is a hypoxemia state caused by pulmonary vascular dilatation based on advanced chronic liver diseases. Major clinical symptoms are dyspnea, cyanosis, and abdominal distension[6]. Here we report a case with HPS caused by congenital panhypopituitarism in detail. Clinical symptom and laboratory results were remarkably improved by pituitary hormone replacement therapy, especially growth hormone and testosterone.
Liver transplantation is currently known to be the only way to cure HPS[7]. Remission of HPS-related symptoms can be achieved within 6-12 mo after liver transplantation[6,8]. Oxygen partial pressure could be significantly improved after surgery, from 57 mmHg to 87 mmHg[9]. For patients with GH deficiency, MAFLD would relapse 2-18 mo after liver transplantation[10-12]. However, the importance of GH therapy on metabolism and cirrhosis did not draw enough attention in patients with HPS.
The patient initially received thyroxin and cortisol. However, these therapies were not enough to prevent HPS occurrence. When rhGH and testosterone were administered, the clinical manifestations and laboratory data were dramatically improved in 6 mo. This indicates the essential role of GH cocktail in liver and pulmonary pathology. The effect of GH was also described before. In an 11-year-old boy with panhypopituitarism caused by mature teratoma in the Sellar area, liver transplantation improved the intrapulmonary shunt rate from 57.5% to 25.4%. However, adipose accumulated in the liver again in 12 mo after surgery. Subsequently, low-dose rhGH (0.3 mg/d) therapy reversed the grafted fatty liver[10]. A randomized, double-blind, and placebo-controlled study showed that a relative low dose of GH (0.2 mg/d) for 7 d may improve symptom of cirrhosis[13]. A prospective randomized study showed that consecutive rhGH 1.3 mg/d for 4 wk can significantly improve the prognosis of patients with liver failure, suggesting that rhGH can extend the life expectancy[14].
The central mechanism of hypoxia in HPS is intrapulmonary vascular dilatation due to a large amount of endogenous vasodilators in circulation, like carbon monoxide and nitric oxide (NO)[6]. Torii et al[15] reported a similar case to confirm amelioration of MAFLD by liver biopsy and FIB-4 index. They believe that hormone imbalance influences the occurrence of HPS. However, repeated liver puncture is not suitable for detecting liver condition in patients with long-term follow-up. We evaluated liver fibrosis synthetically and non-invasively by FIB-4 index, serum HA, PШNP, CIV, and ultrasound. We also probed into the effect of GH, IGF-1, and testosterone on HPS by literature review as follows. GH can reduce adipose deposition in the liver by directly inhibiting lipogenesis and indirectly activating hormone-sensitive lipase. IGF-1 can induce cell senescence and inactivate hepatic stellate cells, improving liver cirrhosis[7]. GH/IGF-1 was known to have antioxidative effects and improve mitochondrial function[16,17], which may prevent steatohepatitis inflammation from hepatic steatosis[18]. GH replacement alone only reduces the proportion of proteins which reincorporate back into protein. Testosterone decreases the substrate for NO synthesis by reducing protein oxidation in the presence of GH. Significantly, the interaction of testosterone and GH occurs mainly in the liver, resulting in a greater stimulation of whole-body protein synthesis[19]. Testosterone stimulates protein anabolism by reducing protein oxidation only in the presence of GH[20].
In summary, GH deficiency increases the risk of steatohepatitis and induces cirrhosis and HPS. GH and testosterone replacement therapy remarkably improves symptom of HPS by reducing adipose deposition in the liver and NO production. Since HPS patients are primarily admitted to gastrointestinal, respiratory, or liver transplantation clinics, clinicians should screen patients' anterior pituitary function as early as possible and treat them primarily with growth hormone cocktail accordingly.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang Y S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Hong JW, Kim JY, Kim YE, Lee EJ. Metabolic parameters and nonalcoholic fatty liver disease in hypopituitary men. Horm Metab Res. 2011;43:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology. 2004;39:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Dichtel LE, Corey KE, Misdraji J, Bredella MA, Schorr M, Osganian SA, Young BJ, Sung JC, Miller KK. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2017;8:e217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 5. | Kennedy TC, Knudson RJ. Exercise-aggravated hypoxemia and orthodeoxia in cirrhosis. Chest. 1977;72:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 422] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 7. | Takahashi Y. The Role of Growth Hormone and Insulin-Like Growth Factor-I in the Liver. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 8. | Gupta S, Castel H, Rao RV, Picard M, Lilly L, Faughnan ME, Pomier-Layrargues G. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010;10:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 10. | Fujio A, Kawagishi N, Echizenya T, Tokodai K, Nakanishi C, Miyagi S, Sato K, Fujimori K, Ohuchi N. Long-term survival with growth hormone replacement after liver transplantation of pediatric nonalcoholic steatohepatitis complicating acquired hypopituitarism. Tohoku J Exp Med. 2015;235:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Justino H, Sanders K, McLin VA. Rapid progression from hepatopulmonary syndrome to portopulmonary hypertension in an adolescent female with hypopituitarism. J Pediatr Gastroenterol Nutr. 2010;50:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Jonas MM, Krawczuk LE, Kim HB, Lillehei C, Perez-Atayde A. Rapid recurrence of nonalcoholic fatty liver disease after transplantation in a child with hypopituitarism and hepatopulmonary syndrome. Liver Transpl. 2005;11:108-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Donaghy A, Ross R, Wicks C, Hughes SC, Holly J, Gimson A, Williams R. Growth hormone therapy in patients with cirrhosis: a pilot study of efficacy and safety. Gastroenterology. 1997;113:1617-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Li N, Zhou L, Zhang B, Dong P, Lin W, Wang H, Xu R, Ding H. Recombinant human growth hormone increases albumin and prolongs survival in patients with chronic liver failure: a pilot open, randomized, and controlled clinical trial. Dig Liver Dis. 2008;40:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Torii N, Ichihara A, Mizuguchi Y, Seki Y, Hashimoto E, Tokushige K. Hormone-replacement Therapy for Hepatopulmonary Syndrome and NASH Associated with Hypopituitarism. Intern Med. 2018;57:1741-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Kokoszko A, Dabrowski J, Lewiński A, Karbownik-Lewińska M. Protective effects of GH and IGF-I against iron-induced lipid peroxidation in vivo. Exp Toxicol Pathol. 2008;60:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med (Maywood). 2002;227:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3125] [Article Influence: 115.7] [Reference Citation Analysis (36)] |
| 19. | Mauras N, Rini A, Welch S, Sager B, Murphy SP. Synergistic effects of testosterone and growth hormone on protein metabolism and body composition in prepubertal boys. Metabolism. 2003;52:964-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Birzniece V, Meinhardt UJ, Umpleby MA, Handelsman DJ, Ho KK. Interaction between testosterone and growth hormone on whole-body protein anabolism occurs in the liver. J Clin Endocrinol Metab. 2011;96:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |