Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4817
Peer-review started: January 26, 2021
First decision: February 25, 2021
Revised: February 26, 2021
Accepted: May 6, 2021
Article in press: May 6, 2021
Published online: June 26, 2021
Processing time: 136 Days and 0.4 Hours
A growing body of literature indicates that the occurrence of thalamic lesions could lead to various dysfunctions, such as somatosensory disturbances, hemiparesis, language deficits, and movement disorders. However, clinical cases describing the coexistence of these types of manifestations have not been reported. Herein, we report a patient who exhibited these rare complications secondary to thalamic hemorrhage.
A 53-year-old right-handed man experienced sudden left hemiparesis, numbness of the left side of body, and language alterations due to an acute hemorrhage located in the right basal ganglia and thalamus 18 mo ago. Approximately 17 mo after the onset of stroke, he exhibited rare complications including dysphasia, kinetic tremor confined to the left calf, and mirror movement of the left arm which are unique and interesting, and a follow-up computed tomography scan revealed an old hemorrhagic lesion in the right thalamus and posterior limb of the internal capsule.
Hypophonia may be a recognizable clinical sign of thalamus lesions; thalamus injury could cause tremor confined to the lower extremity and mimicking extremity movements.
Core Tip: Clinical cases describing the coexistence of multiple manifestations secondary to thalamic damage have not been reported. We report a patient who exhibited rare complications secondary to thalamic hemorrhage. Especially, the manifestations including dysphasia, kinetic tremor confined to the left calf, and mirror movement of the left arm are unique and interesting. This case provides new insights into thalamus damage. Hypophonia may be a recognizable clinical sign of thalamus lesions, which could help with lesion localization; thalamus injury could cause tremor confined to the lower extremity and mimicking extremity movements.
- Citation: Yu QW, Ye TF, Qian WJ. Rare coexistence of multiple manifestations secondary to thalamic hemorrhage: A case report. World J Clin Cases 2021; 9(18): 4817-4822
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4817.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4817
Anatomically, the thalamus, which lies between the forebrain and midbrain, is divided into anterior, medial, and lateral parts, and composed of various nuclei[1,2]. Due to its complex anatomical structure and connections, the thalamus serves as a pivotal relay center for the brain, subserving both sensory and motor mechanisms[3]. In recent years, thalamic stroke has increased in incidence worldwide[4] and commonly results in somatosensory dysfunction[5], hemiparesis[6], language deficits[7], and movement disorders[1]. However, the coexistence of these manifestations has been rarely reported. Especially, the case with combinations of the manifestations, including dysphasia, kinetic tremor confined to the left calf, and mirror movement of the left arm, has not been previously published. We present herein the first case of these rare complications secondary to a right thalamic hemorrhage and discuss the possible underlying neuroanatomical mechanisms after briefly reviewing the literature.
A 53-year-old right-handed male patient was referred to the rehabilitation department because of an 18-mo history of left limb weakness, left lateral paresthesia, and language alterations.
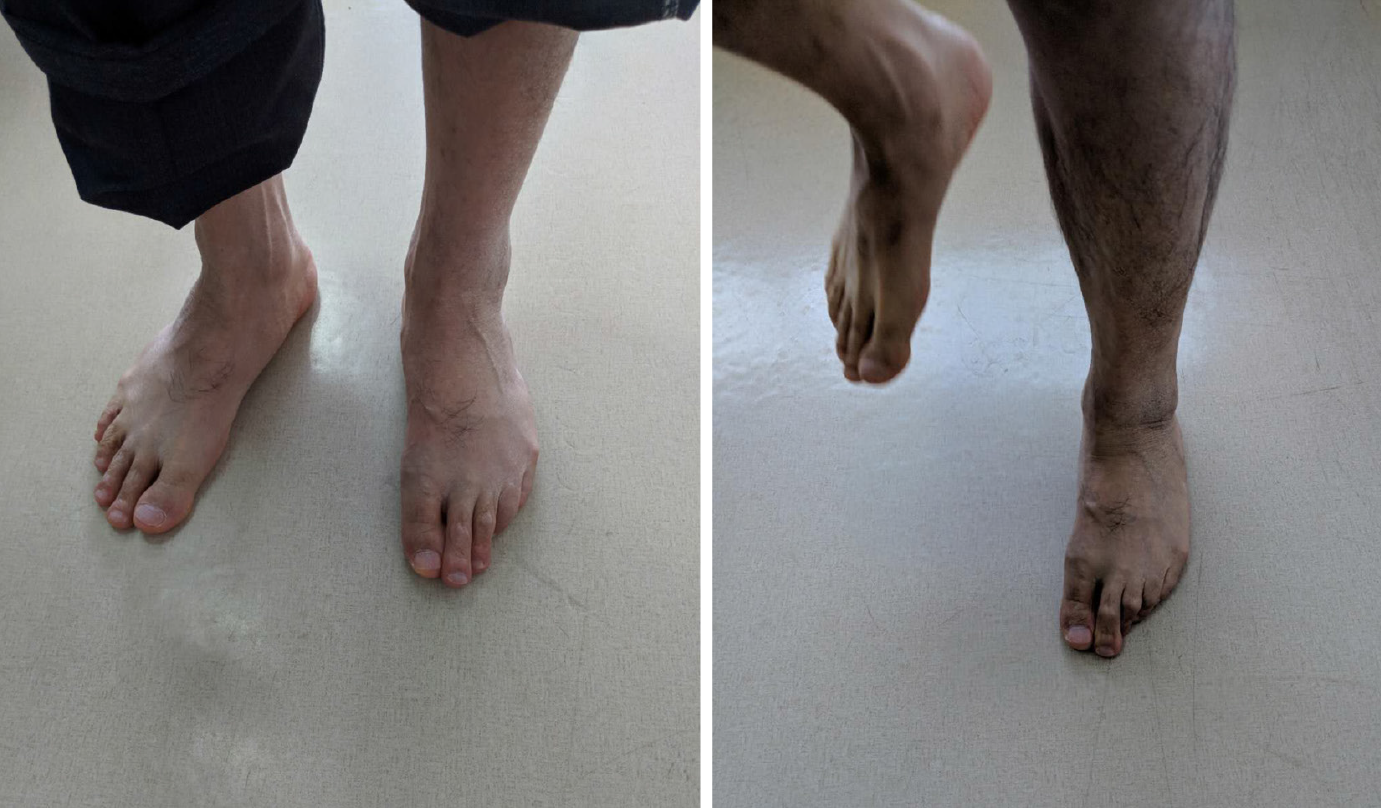
The man experienced sudden left hemiparesis, numbness of the left side of body, and language alterations 18 mo ago. He perceived a strong, uncomfortable feeling of “tugging” in his left shoulder and arm, which was aggravated with activity. The patient presented with a dysarthria characterized by hoarse voice and hypophonia, which was quite unlike his usual voice. He was unable to increase the volume of his voice when speaking. Approximately one year after the onset of the stroke, abnormal involuntary movements of the left arm and hand began to occur (Video 1). The patient was unable to extend and flex his left thumb and fingers in a coordinated manner, which prevented him from performing many basic functions, including eating, dressing, and bathing, as well as manipulating small objects. Additionally, while the patient was able to walk with minimal assistance, he experienced bradykinesia along with involuntary flexion of the toes, which was painful when walking (Figure 1, Video 2).
The patient had a free previous medical history.
The patient denied any family history of hypertension, diabetes, coronary heart disease, stroke, movement disorder diseases, or other neurologic illnesses.
The patient had partially recovered muscle strength (manual muscle testing score: 3/5 for the left arm and 4/5 for the left leg). Muscle tone in both affected limbs was slightly increased (the Modified Ashworth Scale scores were 1/5 for both the left arm and left leg). The patient’s tactile sensory perception, including sensations of pinpricks and light touch, as well as proprioceptive deficits, was pronounced, but no hyperesthesia or allodynia was present. Slight hyperreflexia in both left limbs also was present. The Babinski sign in the left foot was negative. When actively extending and flexing the left thumb and fingers, a peculiar abnormal posture of the hand, known as a “thalamic hand”, appeared, such that the patient was unable to perform the coordinated movements necessary for the rapid thumb-finger grasp (Video 3, Video 4). Additional coordination testing revealed that the patient could not complete the finger-to-nose test with the left arm due to the presence of abnormal involuntary movements (Video 1). The patient’s heel-knee-tibia test was positive for the left leg but negative for the right leg (Video 5). Interestingly, when the patient tried to take a few steps, a slow (3 Hz), regular tremor appeared in his left calf, and his left arm gradually elevated spontaneously (Video 2, Video 6). The tremor did not occur when the left leg was stationary, and the patient could stop elevation of the left arm when instructed not to lift his left arm.
Neuropsychological investigations revealed no impairment of superior cognitive function, with the Mini-Mental State Examination score and Montreal Cognitive Assessment score were both 30 points, but the patient presented severe dysarthria characterized by hoarse voice and hypophonia. He did not experience any hemispatial neglect or visual neglect.
No abnormalities were found in the patient’s laboratory examinations.
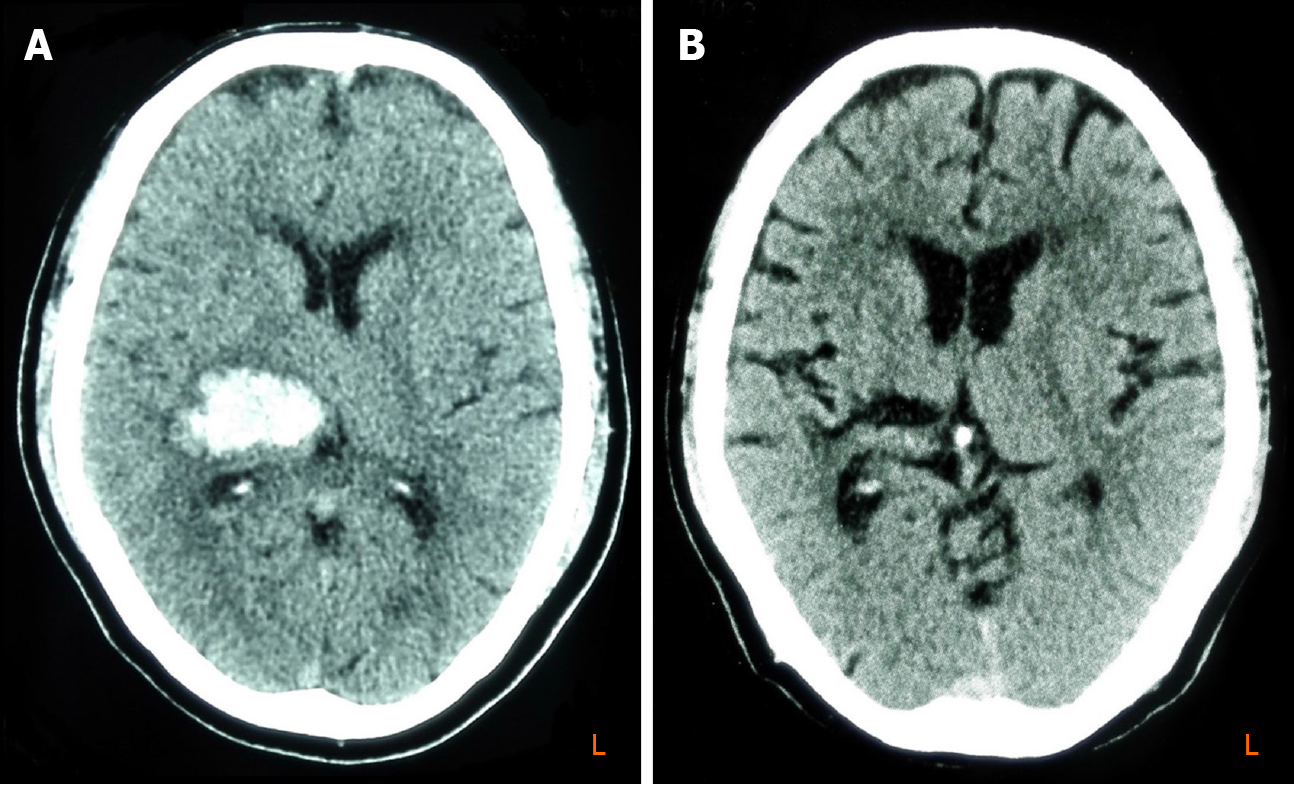
An urgent brain computed tomography (CT) scan revealed an acute hemorrhage lesion located in the right basal ganglia (Figure 2A); a follow-up CT scan performed 17 mo after the onset of the stroke demonstrated an old hemorrhage lesion involving the right thalamus and posterior limb of the internal capsule (Figure 2B).
Left hemiplegia, movement disorders, dysphasia, and hemorrhages in chronic phase.
The patient was treated with a number of drugs, including baclofen, gabapentin, and oxcarbazepine, and received nearly 6 mo of consistent rehabilitation treatment including physical therapy and occupational therapy.
The motor function in the patient’s left arm and leg improved substantially, and dysphasia relieved slightly. However, his response to these pharmaceutical treatments was unsatisfactory and left lateral paresthesia progressively worsened.
In our case, the manifestations, including dysphasia, kinetic tremor confined to the left calf, and mirror movement of the left arm, are unique and interesting. Thus, exploring the underlying neuroanatomical mechanisms correlated with these specific clinical findings is of interest. After briefly reviewing the literature, we speculate that the thalamus serves as a pivotal relay center for multiple regions of the brain and thalamic lesions lead to disruptions of the connections between thalamus and other brain structures, including the cortex, midbrain, cerebellum, and extrapyramidal system, which could be an explanation for the coexistence of these manifestations.
Several reports[8,9] have described that thalamic lesions could result in dysphasia, dysarthria, and hypophonia. It seems that the occurrence of these language deficits in patients with thalamic stroke is mainly due to lesions located in the anterior and lateral thalamus. Blacker et al[10] described two subjects with a dominant anterolateral thalamic stroke, both of whom presented with marked hypophonia as part of syndromes. They suggested that the anterolateral thalamic lesion that disrupted extrapyramidal pathways might be a major factor in producing dysphonia and that the predominantly anterior and possibly lateral thalamic lesions were responsible for hypophonia. Recently, Rodríguez-López et al[7] presented a case report of hypophonia secondary to a left thalamic hemorrhage. They speculated that a thalamo-striato-cortical loop might play an important role in controlling voice modulation, and disruptions of this loop precisely at the anterior and ventral thalamus level would result in hypophonia. While the specific role of thalamic nuclei in voice modulation is still unclear, it has been proposed that, as an extrapyramidal sign, hypophonia could be explained by the loss of input from the basal ganglia and substantia nigra to the ventral anterior and the ventral lateral nuclei[7]. Our patient presented dysphasia (a hoarse voice and hypophonia) secondary to a hemorrhage lesion involving the lateral thalamus. Perhaps, disruptions of the thalamo-striato-cortical loop at the anterior and ventral thalamus level could explain the observed speech deficits. It is worth noting that hypophonia has been mainly reported after dominant thalamus lesions[7,10] while our patient suffered right thalamic hemorrhage. In a study of right-handed Parkinson’s patients, Liotti et al[11] observed activation of the right anterior insula, caudate head, putamen, and dorsolateral prefrontal cortex after successful treatment of hypophonia. This result suggest that the right thalamo-striato-cortical loop might mediate the voice modulation, and disruptions of this loop would lead to hypophonia[7]. The precise mechanisms should be illustrated in further study.
Tremor is mainly induced by lesions affecting the basal ganglia and anterior, intermediate, and posterior thalamic nuclei[12-14]. Thalamic tremors primarily appear in the upper extremities, as thalamic tremors that are limited to the lower extremities are rarely reported. Baysal et al[15] described a 54-year-old woman who developed a tremor that primarily involved her left lower extremity approximately 2 wk after an infarction that affected the midbrain, cerebellum, and thalamus. Recently, Jung et al[16] reported a case with tremor that was limited to the left lower limb and developed 2 mo after the occurrence of a contralateral, posterolateral thalamic hemorrhage. The pathophysiological mechanisms of this clinical phenomenon are not yet understood. In 1998, a consensus statement suggested that the dopaminergic nigrostriatal and the cerebello-thalamic systems each play an important role in the occurrence of resting and action tremor[17]. Jung et al[16] hypothesized that disruption of the projections between thalamic neurons and the midbrain or cerebellum leads to kinetic tremor, and disconnection of the thalamic-striatal neurons causes resting tremor. For our patient, his tremor was confined to the left calf and appeared only during walking or taking steps. It is possible that this kinetic tremor could be explained by the hypothesis of Jung et al[16]. In addition, it is unclear why the appearance of the abnormal involuntary movements of the left arm and hand delayed and whether this phenomenon is common.
Interestingly, we also observed imitative ipsilateral extremity movements with this patient. This has rarely been mentioned in the previously published literature. In 2003, Jung et al[18] reported a case of imitative arm elevation after a recurrent right thalamic hemorrhage. A 70-year-old man spontaneously elevated his left arm when attempting to lift the affected left leg. This spontaneous elevation could not be stopped by an instruction not to lift the arm. This patient also had marked ipsilateral proprioceptive deficits. Generally, the phenomenon in which the arm imitates the ipsilateral leg is called “mirror movement” and is regarded as a result of congenital or chronic pyramidal tract injury[19]. A previous PET scan study[20] suggested that the spontaneous upper extremity elevation could be related to hypometabolism of the corticothalamic motor inhibitory pathways. Thus, Jung et al[18] believed that the imitative arm elevation could be explained by disruption of the corticothalamic motor inhibitory pathways. Additionally, marked proprioceptive sensory loss may be another mechanism of mirror movement. For example, patients with sensory alien hand syndrome often demonstrate involuntary arm elevation[21]. In addition to development of mirror movement of his left arm, our patient exhibited ipsilateral hemisensory loss that included tactile and proprioceptive deficits. Similar to other reports, we think that disruption of the corticothalamic motor inhibitory pathways and proprioceptive sensory loss may be the underlying mechanism involved in our patient.
Although the precise mechanisms associated with the clinical manifestations described in the patient are still unclear, this case provides new insights into thalamus damage. For example, hypophonia may be a recognizable clinical sign of thalamus lesions, which could help with lesion localization; thalamus injury could cause tremor confined to the lower extremity and mimicking extremity movements. Therefore, further studies employing advanced neuroimaging techniques are warranted to improve our understanding of these unique manifestations secondary to thalamic stroke.
Manuscript source: Unsolicited manuscript
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Preda SD S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Gupta N, Pandey S. Post-Thalamic Stroke Movement Disorders: A Systematic Review. Eur Neurol. 2018;79:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Powell R, Hughes T. A chamber of secrets. The neurology of the thalamus: lessons from acute stroke. Pract Neurol. 2014;14:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Chen XY, Wang Q, Wang X, Wong KS. Clinical Features of Thalamic Stroke. Curr Treat Options Neurol. 2017;19:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Teramoto S, Yamamoto T, Nakao Y, Watanabe M. Novel Anatomic Classification of Spontaneous Thalamic Hemorrhage Classified by Vascular Territory of Thalamus. World Neurosurg. 2017;104:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Chen L, Luo T, Wang K, Zhang Y, Shi D, Lv F, Li Y, Li Q, Fang W, Zhang Z, Peng J, Yang H. Effects of thalamic infarction on the structural and functional connectivity of the ipsilesional primary somatosensory cortex. Eur Radiol. 2019;29:4904-4913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Menon D, Sarojam MK, Gopal R. Unilateral Thalamic Infarct: A Rare Presentation of Deep Cerebral Venous Thrombosis. Ann Indian Acad Neurol. 2019;22:221-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Rodríguez-López C, Ayuso García B, Moreno García S. Hypophonia as a sign of thalamus lesion: a case report. Int J Neurosci. 2018;128:100-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Bogousslavsky J, Regli F, Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology. 1988;38:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 328] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Ghika-Schmid F, Bogousslavsky J. The acute behavioral syndrome of anterior thalamic infarction: a prospective study of 12 cases. Ann Neurol. 2000;48:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Blacker DJ. Softly spoken strokes: two patients with marked hypophonia as a feature of strokes involving the anterior thalamus. J Clin Neurosci. 2003;10:243-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Liotti M, Ramig LO, Vogel D, New P, Cook CI, Ingham RJ, Ingham JC, Fox PT. Hypophonia in Parkinson's disease: neural correlates of voice treatment revealed by PET. Neurology. 2003;60:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Park J. Movement Disorders Following Cerebrovascular Lesion in the Basal Ganglia Circuit. J Mov Disord. 2016;9:71-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Nowak DA, Seidel B, Reiner B. Tremor following ischemic stroke of the posterior thalamus. J Neurol. 2010;257:1934-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Krystkowiak P, Martinat P, Cassim F, Pruvo JP, Leys D, Guieu JD, Destée A, Defebvre L. Thalamic tremor: correlations with three-dimensional magnetic resonance imaging data and pathophysiological mechanisms. Mov Disord. 2000;15:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Baysal L, Acarer A, Celebisoy N. Post-ischemic Holmes' tremor of the lower extremities. J Neurol. 2009;256:2079-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Jung YJ, Lee JS, Shin WC. Surface electromyography analysis of contralateral lower extremity tremor following thalamic hemorrhage. Neurol Sci. 2015;36:1281-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13 Suppl 3:2-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 896] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 18. | Jung KH, Park SH, Chang GY. Imitative arm levitation from a recurrent right thalamic hemorrhage: a case report. Neurology. 2003;61:718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116 (Pt 5):1223-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 267] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Eidelberg D, Dhawan V, Moeller JR, Sidtis JJ, Ginos JZ, Strother SC, Cederbaum J, Greene P, Fahn S, Powers JM. The metabolic landscape of cortico-basal ganglionic degeneration: regional asymmetries studied with positron emission tomography. J Neurol Neurosurg Psychiatry. 1991;54:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 99] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Ay H, Buonanno FS, Price BH, Le DA, Koroshetz WJ. Sensory alien hand syndrome: case report and review of the literature. J Neurol Neurosurg Psychiatry. 1998;65:366-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |