Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4748
Peer-review started: November 9, 2020
First decision: December 3, 2020
Revised: December 16, 2020
Accepted: April 23, 2021
Article in press: April 23, 2021
Published online: June 26, 2021
Processing time: 214 Days and 3.1 Hours
Duodenal papillary tumor is a rare tumor of the digestive tract, accounting for about 0.2% of gastrointestinal tumors and 7% of periampullary tumors. The clinical manifestations of biliary obstruction are most common. Some benign tumors or small malignant tumors are often not easily found because they have no obvious symptoms in the early stage. Surgical resection is the only treatment for duodenal papillary tumors. At present, the methods of operation for duodenal papillary tumors include pancreatoduodenectomy, duodenectomy, ampullectomy, and endoscopic resection.
A 47-year-old man was admitted to because of a duodenal mass that had been discovered 2 mo previously. Electronic gastroscopy at another hospital revealed a duodenal papillary mass that had been considered to be a high-grade intraepithelial neoplasia. Therefore, we conducted a multidisciplinary group discussion and decided to perform a pancreas-preserving duodenectomy and a R0 resection was successfully performed. After surgery, the patient underwent a follow-up period of 5 yr. No recurrence or metastasis occurred.
According to our experience with a duodenal papillary tumor, compared with pancreaticoduodenectomy, the use of pancreas-preserving duodenectomy can preserve pancreatic function, maintain gastrointestinal structure and function, reduce tissue damage and complications, and render the postoperative recovery faster. Pancreas-preserving duodenectomy for treatment of a duodenal papillary tumor is feasible under strict control of surgical indications.
Core Tip: Duodenal papillary tumor is a rare digestive tract tumor because of its special anatomical location. The clinical manifestations of biliary obstruction are most common. Some benign tumors or small malignant tumors are often not easily found because they have no obvious symptoms in the early stage. Surgical resection is the only treatment for duodenal papillary tumors. With the advancement of surgical technology, reducing trauma and improving the quality of life have attracted more and more attention, and the surgical methods have changed accordingly. This case highlights that, under the condition of strictly controlling the indications of operation, pancreas-preserving duodenectomy for treatment of duodenal papillary tumors is feasible.
- Citation: Wu B, Chen SY, Li Y, He Y, Wang XX, Yang XJ. Pancreas-preserving duodenectomy for treatment of a duodenal papillary tumor: A case report. World J Clin Cases 2021; 9(18): 4748-4753
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4748.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4748
Duodenal papillary tumor is a rare tumor of the digestive tract, and most researchers believe that for benign tumors in that area, the long-term effect of local mass resection is similar to that of pancreaticoduodenectomy. There is still much debate over its indications and effects because of the lack of comparative analysis on long-term effects of local resections and pancreaticoduodenectomies. Therefore, we report a patient with pancreas-preserving duodenectomy and review the relevant literature to improve our understanding of this disease.
A duodenal mass that had been discovered 2 mo previously
A 47-year-old man was admitted to our hospital on November 20, 2015 because of a duodenal mass that had been discovered 2 mo previously. Electronic gastroscopy at another hospital revealed a duodenal papillary mass that had been considered to be a high-grade intraepithelial neoplasia and was left untreated. The patient reported having no significant, recent weight loss. We carried out relevant examinations, confirmed the diagnosis, and took further treatment measures.
The patient was healthy before.
The patient and families were healthy before.
The patient’s vital signs were: Temperature, 36.2 ℃; pulse rate, 75 bpm; respiratory rate, 20 breaths/min; and blood pressure, 110/70 mmHg. The physical examination revealed a normal countenance; absence of superficial lymph node enlargement and heart abnormalities; a flat abdomen; liver and spleen not palpated below the costal margin; hypogastric region tenderness; no shifting dullness absent; bowel sounds of 4 times/min, with no increase or decrease; and gurgles and abdominal vascular murmurs not audible.
Laboratory test results included: White blood cell count, 4.5 × 109/L; erythrocyte count, 4.42 × 1012/L; hemoglobin, 144 g/L; platelet count, 130 × 109/L; normal liver and kidney function; prothrombin time, 14 s; fibrinogen, 3.09 g/L; tumor marker CA-125, 7.19 U/mL (normal < 35 U/mL); CA-199, 7.07 U/mL (normal < 37 U/mL); alpha-fetoprotein, 3.17 ng/mL (normal 0.89-8.78 ng/mL); and CEA, 1.67 ng/mL (normal < 5 ng/mL). The rest of the parameters were within the normal range.
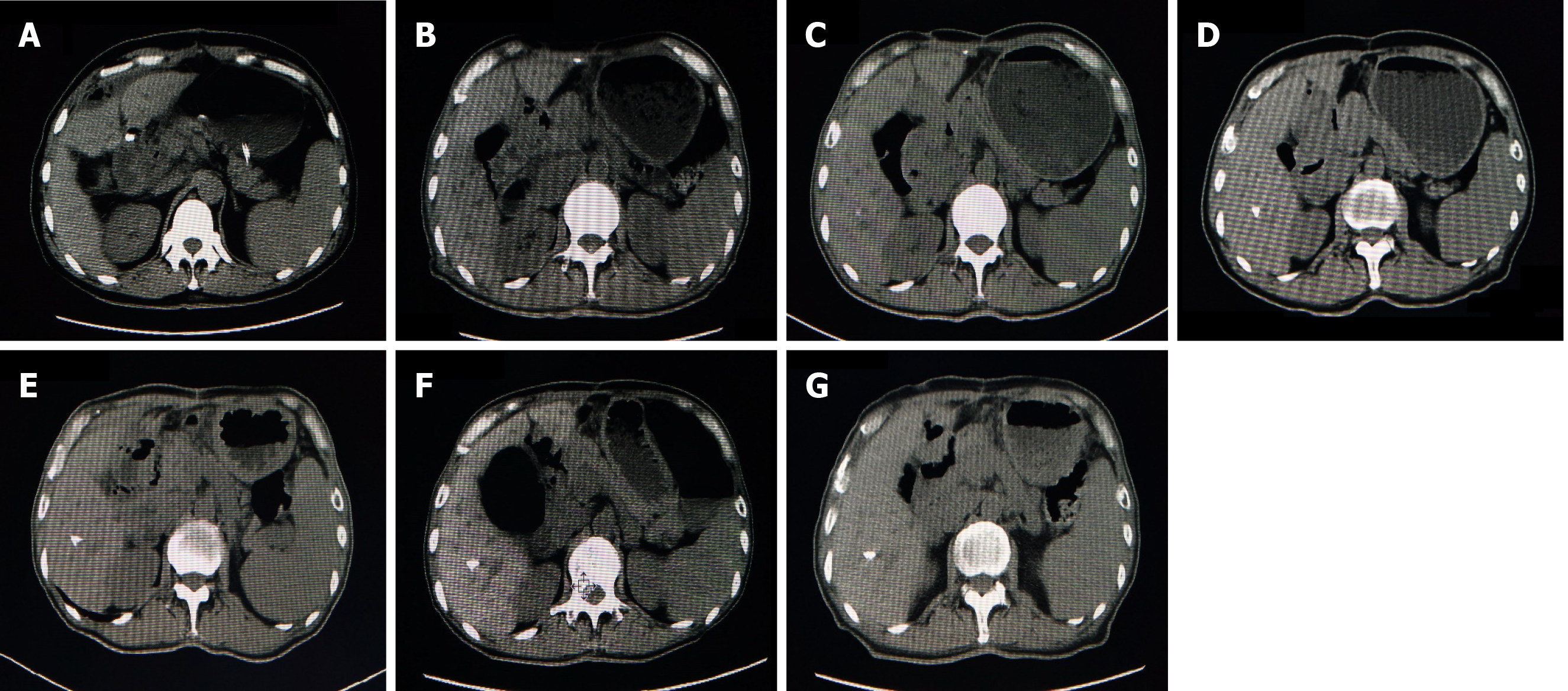
Abdominal computed tomography showed a space-occupying lesion at the head of the pancreas and the duodenal bulb (Figure 1A). Due to the aforementioned imaging findings, a preliminary diagnosis of a duodenal papillary space-occupying lesion was made.
Two days before the operation, the patient was fed a liquid diet. After fasting for 8 h before surgery, an intravenous injection of 2 g cefazoxime sodium was given 30 min before surgery to prevent infection. A patient-controlled analgesia pump was provided postoperatively. The patient was treated by hemostasis, nutrition, and water and electrolyte supplementation. Routine blood and biochemical indexes were monitored.
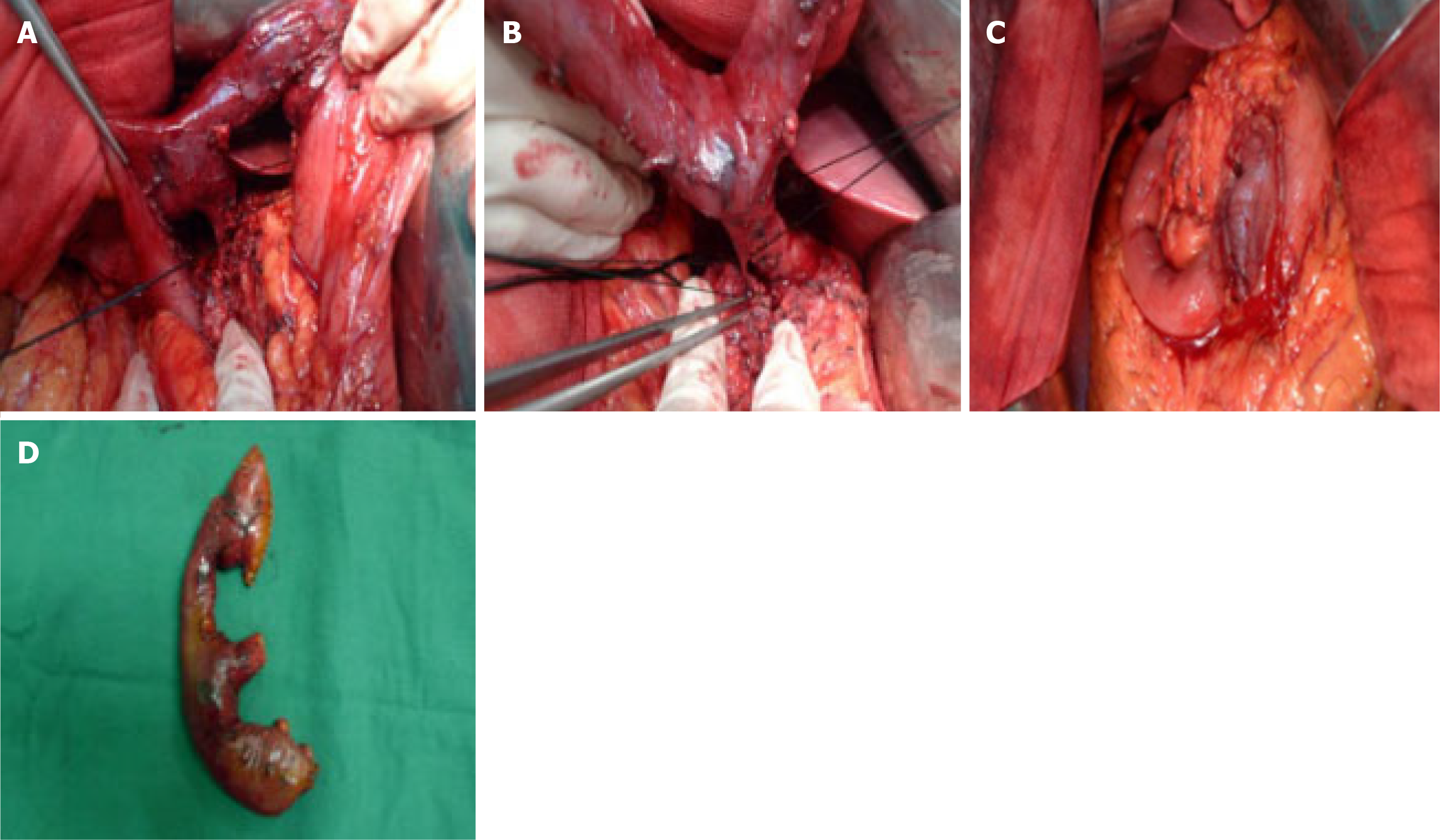
After general anesthesia, the patient was placed in a supine position, and then disinfected and trocar inserted. A laparoscopic exploration was then performed. As the gallbladder wall tension was not increased, the hepatoduodenal lymph nodes were not significantly enlarged, and there were no other significant findings in the peritoneal and pelvic cavities, we decided to perform a pancreas-preserving duodenectomy with a median abdominal incision. The gastrocolic ligament was opened to expose the duodenum, which was dissociated along the upper edge of the duodenum until the ligament of Treitz. The branch vessels entering the upper end of the duodenal bulb and the pancreas were ligated using USP 0 sutures. On turning the descending part of duodenum to the right and freeing the common bile duct and pancreatic duct on the inner side of the descending part of the duodenum (Figure 2A and B), a 3 cm × 3 cm solid mass with a hard, firm texture was palpable. The mass was not found to involve the pancreas and hepatoduodenal ligament. The common bile duct was freed along the duodenal papilla upward, until no significant mass invasion could be found. The common bile duct was severed at the pancreas side and the pancreatic duct was freed from the head of the pancreas toward the inner side until no significant mass invasion was found. After the distal part of the stomach, duodenum, and the tissues surrounding the pancreas were freed, the stomach was transected 6 cm from the pylorus using an endoscopic linear cutter and the jejunum was also transected 5 cm under the ligament of Treitz using a 6-0 linear cutter. After removing the specimen, the distal part of the jejunum was lifted from behind the colon to the level of the porta hepatis. An incision with a length equivalent to the opening of the bile duct was made on the contralateral edge of the mesojejunum, 5 cm from the jejunal stump. Bilioenteric anastomosis was performed through full-thickness continuous sutures using 6-0 prolene sutures. The residual pancreatic body and tail were freed and lifted for placement of an infusion tube scalp needle with an opening on the side. The main pancreatic duct and jejunal mucosa were continuously sutured using 5-0 proline sutures, and the head of the pancreas and the seromuscular layer of the jejunum were sutured in an interrupted manner, using USP 0 sutures. A Frankenman 25 stapler was used for Billroth I in situ anastomosis between the pyloric part and the jejunum, and the serous layer at the anastomosis site was sutured using USP 0 sutures (Figure 2C). The operation was successful and the tumor was completely removed. The operative time was 295 min, and the intraoperative blood loss was 100 mL. There were no obvious postoperative complications, and the patient was discharged 14 d after the operation.
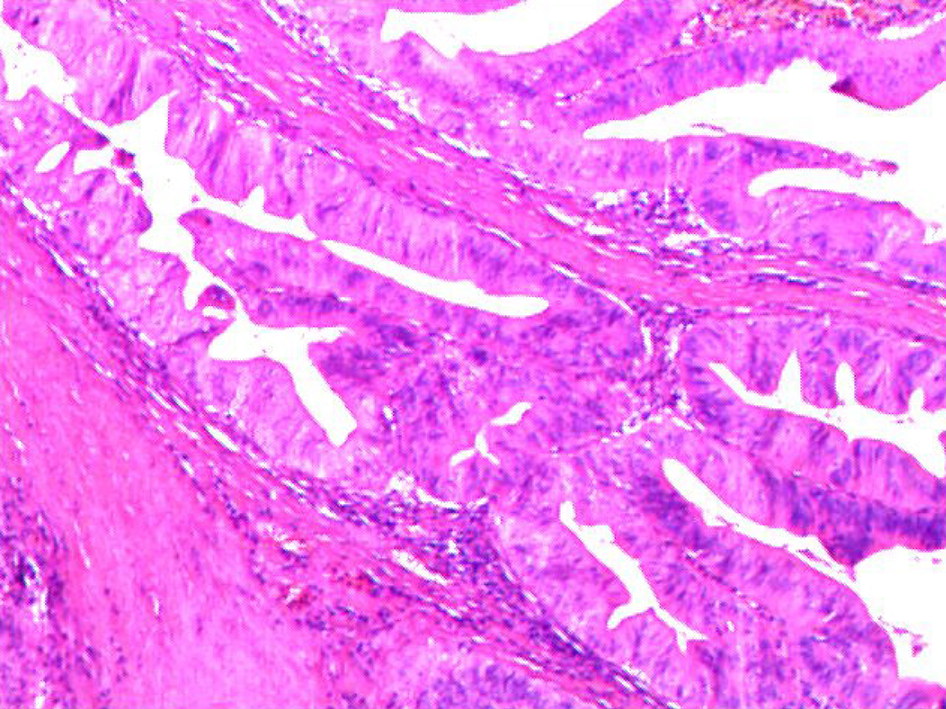
The macroscopic examination of the surgical specimen showed a cancerous tumor with a white-gray color and a hard texture in the duodenal papilla (Figure 2D). A pathological diagnosis of adenocarcinoma of the duodenal papilla with infiltration in the full thickness of the intestinal wall was made. The stumps of the pancreatic duct and bile duct and the lymph nodes were negative and thus an R0 resection was achieved. No atypical cells were found in the pancreatic tissues surrounding the pancreatic duct and bile duct (Figure 3) (pT2N0M0).
A patient-controlled analgesia pump was provided postoperatively. The patient was treated by hemostasis, nutrition, and water and electrolyte supplementation. Routine blood and biochemical indexes were monitored. The patient refused to receive chemotherapy, and no obvious abnormalities were found in relevant examinations.
Adenocarcinoma of duodenal papilla.
Pancreas-preserving duodenectomy.
The patient underwent a follow-up period of 5 yr, computed tomography examination was performed after 1-5 yr, and no recurrence or metastasis was found (Figure 1B-G).
Duodenal papillary tumor is a rare benign or malignant disease in the digestive tract[1]. Surgical resection is the only treatment for duodenal papillary tumors. At present, the methods of operation for duodenal papilla tumor include pancreatoduodenectomy, duodenectomy, ampullectomy and endoscopic resection. From the design and implementation of the first case of pancreatoduodenectomy in 1935, Whipple operation has been a classic procedure for the treatment of tumors in the ampulla, head of the pancreas, and duodenal papilla[2]. However, with surgical technique improvements and more research on duodenal papilla tumors, the operative methods are evolving towards the direction of minimally invasive surgery. As the selection of operative methods is directly related to a patient’s prognosis and postoperative quality of life, it is critical for doctors to be familiar with the indications for different operative methods. Currently, although the approach of local tumor resection in the treatment of duodenal papillary tumors is attracting increasing attention from experts and scholars in China and other countries, there is still much debate over its indications and effects. Studies have demonstrated that the indications of local tumor resections include: Benign tumors at the duodenal papilla; pathologically confirmed malignant transformation of benign tumors; tumors with a diameter ≤ 2 cm, no infiltration, a margin with negative biopsy results, and no lymph node metastasis; well differentiated duodenal papillary cancer with a diameter < 1 cm and no lymph node metastasis (T1-2N0M0); for older patients with multiple complications and higher surgical risks, local tumor resection can be adopted to improve the quality of life[3,4]. Most researchers believe that for benign tumors and early malignant tumors (T1-2N0M0) in that area, the long-term outcomes of local mass resection are similar to those of pancreaticoduodenectomy. Pancreaticoduodenectomies are still the primary treatment method for malignant tumors because of the lack of comparative analysis on long-term outcomes of pancreas-preserving duodenectomy and pancreaticoduodenectomies. Although the mortality rate after pancreatoduodenectomy is less than 5%, the rate of postoperative complications is still as high as 30%-50%, among which pancreatic fistula is the most common one, with a rate as high as 20%-26%[5]. However, Rattner et al[6] suggested that pancreaticoduodenectomy is associated with better long-term outcomes than local tumor resection and that the indications of local tumor resection need to be strictly controlled. According to Asbun et al[7], the effect of local tumor resection is better than or the same as that of pancreaticoduodenectomy for stage T1 duodenal papillary cancer. Tarazi et al[8] studied the relationship between the operative method for ampullary cancer and a patient’s prognosis and reported a 5-year survival rate of 37.8% for pancreaticoduodenectomy and a 5-year survival rate of 40.9% for local tumor resection, with no statistically significant difference between the two rates. A follow-up study conducted at the Department of Hepatobiliary Surgery of The First Affiliated Hospital of Zhengzhou University reported a 5-year survival rate of 73.3% in 15 patients with duodenal malignant tumors who had undergone local resections, which was a satisfactory result[9]. In addition, it has been reported that the effect of radical resection can be achieved when local resection is performed for benign tumors of the duodenal papilla, benign tumors with a pathologically confirmed malignant transformation, and early papillary cancer that is localized in the mucosa without lymph node metastasis[10]. For early malignant tumors, intraoperative frozen examination showed negative resection margins around the tumor and at the base, and pancreas-preserving duodenectomy could be performed as a safe and effective surgical method[11]. Therefore, we report a patient with pancreas-preserving duodenectomy and provide case support for the long-term outcomes of pancreas-preserving duodenectomy to duodenal papillary tumors.
According to our experience with a duodenal papillary tumor, compared with pancreaticoduodenectomy, the use of pancreas-preserving duodenectomy can preserve pancreatic function, maintain gastrointestinal structure and function, reduce tissue damage and complications, and render the postoperative recovery faster. Pancreas-preserving duodenectomy for the treatment of a duodenal papillary tumor is feasible under strict control of surgical indications.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rangarajan M S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Wang FS, Gao ZJ, Liu YF. Recent advances in diagnosis and treatment of primary duodenal tumors. Zhonghua Xiaohuaxue Zazhi. 2014;22:5221-5227. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampu1 La of Vater. Ann Surg. 1935;102:765. [RCA] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 896] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 3. | Futakawa N, Kimura W, Wada Y, Muto T. Clinicopathological characteristics and surgical procedures for carcinoma of the papilla of Vater. Hepatogastroenterology. 1996;43:260-267. [PubMed] |
| 4. | Mansukhani VM, Desai GS, Mouli S, Shirodkar K, Shah RC, Palepu J. Transduodenal ampullectomy for ampullary tumors. Indian J Gastroenterol. 2017;36:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Kapoor VK. Complications of pancreato-duodenectomy. Rozhl Chir. 2016;95:53-59. [PubMed] |
| 6. | Rattner DW, Fernandez-del Castillo C, Brugge WR, Warshaw AL. Defining the criteria for local resection of ampullary neoplasms. Arch Surg. 1996;131:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Asbun HJ, Rossi RL, Munson JL. Local resection for ampullary tumors. Is there a place for it? Arch Surg. 1993;128:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Tarazi RY, Hermann RE, Vogt DP, Hoerr SO, Esselstyn CB Jr, Cooperman AM, Steiger E, Grundfest S. Results of surgical treatment of periampullary tumors: a thirty-five-year experience. Surgery. 1986;100:716-723. [PubMed] |
| 9. | Shen XW, Tang Z, Zhao YF. The application of local resection for duodenal papillary tumor. Henan Yixue Yanjiu. 2013;22:817-819. [DOI] [Full Text] |
| 10. | Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 197] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Xia H, Lu YB, Zhou J, Zhang ZP, Chen HX, Sun WJ. Clinical analysis of cancer of the duodenal papilla: a report of 80 cases. Zhonghua Putong Waike Zazhi. 2018;27:468-473. [DOI] [Full Text] |