Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4728
Peer-review started: September 30, 2020
First decision: December 13, 2020
Revised: December 16, 2020
Accepted: February 1, 2021
Article in press: February 1, 2021
Published online: June 26, 2021
Processing time: 254 Days and 7.6 Hours
Cockayne syndrome (CS) is a rare inherited disease characterized by progressive motor symptoms including muscle weakness, joint contracture, ataxia, and spasticity. Botulinum neurotoxin type A has been used for conditions such as dystonia and spasticity, but it has rarely been used in patients with CS.
We report a 6-year-and-9-mo old girl diagnosed with CS who received an injection of botulinum neurotoxin type A to manage her difficulty with walking. A total dose of 210 units of botulinum neurotoxin type A was administered into the bilateral tibialis posterior and gastrocnemius muscles. To evaluate the treatment effects on spasticity, joint contracture, pain, and ataxia, measurement tools including the Modified Ashworth Scale, the passive range of motion, the Faces Pain Scale-Revised, and the Scale for the Assessment and Rating of Ataxia, were employed. The first week after the injection, the Modified Ashworth Scale score for the plantar flexors and foot invertors improved bilaterally, along with advancements in the passive range of motion of the bilateral ankles and a lower score for the Faces Pain Scale-Revised. These treatment effects persisted to the 8th week post-injection, but returned to baseline values at the 12th week post-injection, except for the pain scale.
Botulinum toxin injection can thus be considered as a treatment option for lower extremity spasticity, joint contracture, and pain derived from CS.
Core Tip: Cockayne syndrome (CS) is a rare inherited disease, and symptoms such as spasticity in CS are uncommon. No studies in the literature have addressed the effect of botulinum toxin injection in managing gait problems and spasticity in patients with CS. In this article, we report a patient aged 6 years and 9 mo with CS who responded well to botulinum toxin type A administration.
- Citation: Hsu LC, Chiang PY, Lin WP, Guo YH, Hsieh PC, Kuan TS, Lien WC, Lin YC. Botulinum toxin injection for Cockayne syndrome with muscle spasticity over bilateral lower limbs: A case report. World J Clin Cases 2021; 9(18): 4728-4733
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4728.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4728
Cockayne syndrome (CS) is a rare autosomal recessive disorder caused by an ERCC8 or ERCC6 gene mutation[1]. CS is characterized by progressive multisystem degeneration of the central nervous system, vision, hearing, and the musculoskeletal systems[1]. Patients typically present within the first two years of life with delayed developmental milestones, short stature, microcephaly, premature cataracts, hearing loss, tremor, ataxia, spasticity, joint contracture, and muscle weakness[1,2]. Progression may cause gradual deterioration of ambulation in patients with CS[1]. In addition, ataxia, joint contracture, spasticity, and muscle weakness also impede normal motor development[3].
Spasticity is defined as increased muscle tone resulting in abnormal resistance to passive movements[4]. Lesions in the upper motor neurons may cause spasticity[4]. Botulinum neurotoxin type A (BoNT-A), which blocks acetylcholine release from the nerve terminal, has been used to relieve dystonia and spasticity in a variety of disease entities[5].
No studies in the literature have addressed the effect of botulinum toxin injections in the management of gait problems in patients with CS. In this article, we report a patient aged 6 years and 9 mo with CS who responded well to BoNT-A administration.
A 6-year-and-9-mo old girl presented with spasticity over the lower extremities since early childhood.
Other notable characteristics of the patient included abnormal developmental milestones, microcephaly, myopia, exotropia, hearing impairment, ataxia, joint contracture, and spasticity over four limbs, which was worse in the lower extremities. She was unable to ambulate independently due to scissoring and equinus in gait. CS was diagnosed with an ERCC6 gene mutation at the age of 4 years and 6 mo.
On tracing back her medical history, she was born without significant findings on physical examination. She was brought to clinical attention at the age of 2 years and 5 mo due to her short status. Her body height at the time was 77 cm (< 3%), with a body weight of 10.5 kg (< 10%). Generalized hypotonia and microcephaly were also noted. She had delayed developmental milestones, with sitting at 9 mo, crawling at 16 mo, and meaningful words like “papa” and “mama” at 2 years of age. A formal evaluation revealed global developmental delay including in cognitive, language, and motor domains, and rehabilitation programs were initiated at the age of 2 years and 9 mo. Despite 11 mo of rehabilitation, a moderate delay persisted on the Wechsler Preschool and Primary Scale of Intelligence-Fourth Edition at the age of 3 years and 4 mo. Wide-based gait and spasticity over the muscles of the bilateral lower extremities also progressed.
Her parents had no similar symptoms and there was no history of the disease in her family. Also, the patient had no remarkable personal history.
When the patient visited our clinics at the age of 4 years and 9 mo, spasticity over the bilateral plantar flexors and foot invertors with limited ankle dorsiflexion and ankle eversion were noted.
There were no remarkable laboratory examinations in this patient, except that the ERCC6 gene mutation was confirmed at the age of 4 years and 6 mo.
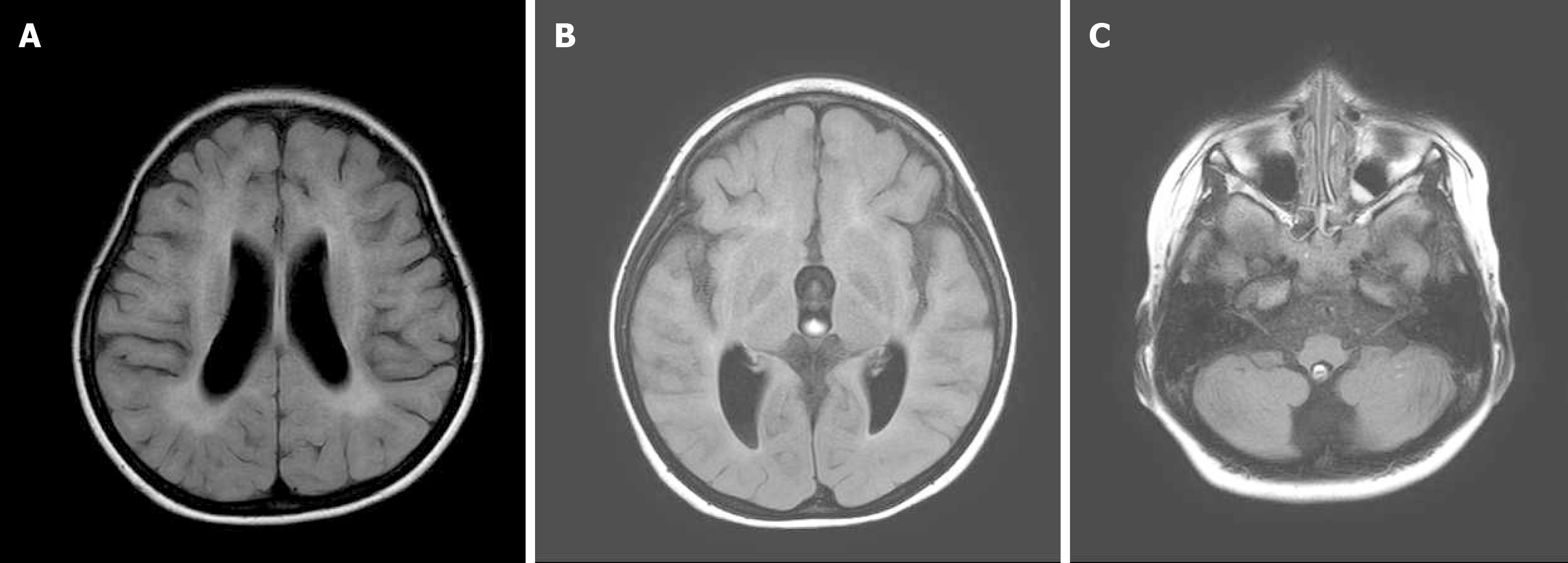
Magnetic resonance imaging (MRI) of the brain without contrast medium at the age of 1 year and 9 mo was normal, but follow-up imaging (Figure 1) at the age of 4 years and 2 mo revealed atrophic bilateral thalami, midbrain, pons, and cerebellar hemispheres.
She was diagnosed with CS and ERCC6 gene mutation at the age of 4 years and 6 mo.
For spasticity of her legs, we injected a total dose of 210 units (15 units/kg) of Botox (OnabotulinumtoxinA; Allergan, Inc., Irvine, CA, United States), with 75 units in each gastrocnemius and 30 units in each tibialis posterior muscle. Techniques including stretching exercises for major joints such as the hips, knees, and ankles were also provided to the patient’s parents.
Outcome measures for the therapeutic effects of BoNT-A injection included the Modified Ashworth Scale (MAS), passive range of motion (PROM), the Scale for the Assessment and Rating of Ataxia (SARA) and the Faces Pain Scale-Revised (FPS-R). The MAS is widely used for evaluating spasticity and has good reliability[6]. PROM is a quantitative assessment of joint contracture[7]. The SARA is a clinical scale developed by Schmitz-Hübsch to evaluate cerebellar ataxia[8]. The scale includes gait, stance, sitting, speech disturbances, finger chase, nose-finger test, fast alternating hand movement, and the heel-shin slide. It has been reported to be applicable in children older than 4 years of age[8]. The FPS-R is a reliable tool to measure pain intensity in children aged 5 years and older[9]. The results of the assessments are summarized in Table 1.
| Outcome measures | Date | ||||||
| Pre-injection | Post-injection week 1 | Post-injection week 2 | Post-injection week 4 | Post-injection week 8 | Post-injection week 12 | ||
| MAS (0-4) | Plantar flexors | R: 3 | R: 2 | R: 2 | R: 2 | R: 2 | R: 3 |
| L: 3 | L: 2 | L: 2 | L: 2 | L: 2 | L: 3 | ||
| Foot invertors | R: 3 | R: 2 | R: 2 | R: 2 | R: 2 | R: 3 | |
| L: 3 | L: 2 | L: 2 | L: 2 | L: 2 | L: 3 | ||
| PROM | Ankle plantar flexion | R: 45°-50° | R: 35°-50° | R: 35°-50° | R: 35°-50° | R: 35°-50° | R: 45°-50° |
| L: 40°-50° | L: 30°-50° | L: 30°-50° | L: 30°-50° | L: 30°-50° | L: 40°-50° | ||
| Inversion | R: 50°-60° | R: 40°-60° | R: 40°-60° | R: 40°-60° | R: 40°-60° | R: 50°-60° | |
| L: 45°-60° | L: 35°-60° | L: 35°-60° | L: 35°-60° | L: 35°-60° | L: 45°-60° | ||
| FPS-R | 6 | 4 | 4 | 4 | 4 | 4 | |
| SARA | 15 | 14 | 14 | 14 | 14 | 15 | |
Toe-walking gait improved one week after injection of BoNT-A. The MAS of bilateral ankle plantar flexors and foot invertors decreased from 3 to 2, the SARA decreased from 15 to 14, and the FPS-R decreased from 6 to 4. The therapeutic effects persisted to the eighth week post-injection. All the parameters returned to values comparable to the pre-injection state on the 12th week post-injection with the exception of the FPS-R score, which remained good at 4.
Although CS was first described in 1936, there is no cure for this syndrome. The goal of management for subjects with CS is to maximize quality of life including pain alleviation and disability reduction in patients, as well as support for their care-givers[10]. In terms of the central nervous system, CS commonly involves the cerebrum, cerebellum, basal ganglia, brainstem, and the spinal cord[1], causing neuronal loss, demyelination, or calcification. This patient was diagnosed with CS at the age of 4 years and 6 mo by genetic testing that revealed the ERCC6 gene mutation. An MRI at 4-years-and-2-mo of age found white matter changes over the periventricular region and atrophic thalami, brain stem, and cerebellum. These findings may explain why the girl presented with both pyramidal tract signs such as muscle weakness with spasticity and extrapyramidal tract syndromes such as ataxia, tremor, myoclonus, and dystonia.
To the best of our knowledge, from the literature, only one 4 year-old patient with CS has received botulinum toxin injection for pain management in a study examining the use of botulinum toxin for the treatment of CS[11]. The pain related to severe spasticity was significantly abated two weeks after OnabotulinumtoxinA injection to the bilateral hip adductors, 50 units on each side[11]. In our case, the pain score was quantified using FPS-R and was found to have improved after the BoNT-A injection. The American Food and Drug Administration approved BoNT-A treatment for chronic migraines in 2010, and BoNT-A has also been used to reduce pain in spastic conditions[12]. Animal models indicate that the botulinum toxin, particularly BoNT-A, may inhibit the release of various pain-modulating neurotransmitters, including glutamate, substance P, calcitonin gene-related peptide, and pain-sensing transmembrane receptors on the neuronal plasma membrane[13]. The duration of pain relief has varied between reports and is still under research.
Spasticity occurs in only 28% of all patients with CS[10]. Our case is the first report of botulinum toxin administration for improvement of gait disturbance in patients with CS. Botulinum toxin type A (BoNT-A) is one of the neurotoxins produced by Clostridium botulinum. BoNT-A can block acetylcholine release at cholinergic nerve endings of skeletal muscle. It results in temporary chemical denervation and reversible paralysis of striated muscles for a period of two to six months[14,15]. BoNT-A has proven effective in patients with spasticity in many upper motor neuron diseases[5]. Repeated BoNT-A injections every three months have proven to be safe and effective for focal muscle spasticity in children[16]. Joint contractures are related not only to tightness of soft-tissue but also to increases in muscle tone[7], which may be the reason for advancements in range of motion after botulinum toxin intervention. In addition to the BoNT-A injection, the patient also engaged in stretching exercises for both ankles on a daily basis, which has been reported to increase ankle range of motion either alone or in combination with other therapies[17].
To date, there is little evidence of pharmacologic effects in cerebellar ataxia[18]. No studies have evaluated the application of botulinum toxin in subjects with cerebellar ataxia. The SARA score improved from 15 points to 14 points after BoNT-A treatment in this patient, which may be attributed to the “heel-shin slide” item in the SARA. Amendment in lower limb spasticity and range of motion may be the reason underlying the improvement in the SARA score of our patient.
BoNT-A treatment for uncommon neurogenic syndromes such as Moyamoya disease and CS in general provides pain relief for at least 2 wk[11]. Other influences such as improvements in gait or fine motor skills have been reported to last from 2 wk to 5 mo in progressive conditions such as familial spastic paraplegia, Pelizaeus-Merzbacher disease, leukodystrophy, and Huntington’s disease[11]. The age at which patients began receiving treatment with BoNT-A ranged from 2 years old to 19 years old, and the average dosage was between 0.4 units/kg and 7.4 units/kg[11]. According to the updated European consensus in 2009, the recommended total dose of Botox for children is 1-20 units/kg, and the maximal total dose is 400 units[19]. Our case received the first course of treatment with BoNT-A at the age of 6 years with a dose of 15 units/kg, without perceivable adverse effects. Pain relief persisted for at least 3 mo, and improvement in spasticity lasted for 2 mo, which was comparable to the reported results of other neurogenic disorders treated with BoNT-A[11].
In summary, BoNT-A local injection of the lower extremities improved spasticity, joint contracture, pain, gait, and balance in our case of CS. It is likely a feasible alternative for patients with CS and the above-mentioned conditions.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shen F S-Editor: Fan JR L-Editor: Webster JR P-Editor: Xing YX
| 1. | Karikkineth AC, Scheibye-Knudsen M, Fivenson E, Croteau DL, Bohr VA. Cockayne syndrome: Clinical features, model systems and pathways. Ageing Res Rev. 2017;33:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Rapin I, Weidenheim K, Lindenbaum Y, Rosenbaum P, Merchant SN, Krishna S, Dickson DW. Cockayne syndrome in adults: review with clinical and pathologic study of a new case. J Child Neurol. 2006;21:991-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Nance MA, Berry SA. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992;42:68-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 531] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 4. | Camargo CHF, Teive HAG. Use of botulinum toxin for movement disorders. Drugs Context. 2019;8:212586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, Armstrong MJ, Gloss D, Potrebic S, Jankovic J, Karp BP, Naumann M, So YT, Yablon SA. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 411] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 6. | Meseguer-Henarejos AB, Sánchez-Meca J, López-Pina JA, Carles-Hernández R. Inter- and intra-rater reliability of the Modified Ashworth Scale: a systematic review and meta-analysis. Eur J Phys Rehabil Med. 2018;54:576-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 7. | Salazar R, Montes J, Dunaway Young S, McDermott MP, Martens W, Pasternak A, Quigley J, Mirek E, Glanzman AM, Civitello M, Gee R, Duong T, Mazzone ES, Main M, Mayhew A, Ramsey D, Muni Lofra R, Coratti G, Fanelli L, De Sanctis R, Forcina N, Chiriboga C, Darras BT, Tennekoon GI, Scoto M, Day JW, Finkel R, Muntoni F, Mercuri E, De Vivo DC. Quantitative Evaluation of Lower Extremity Joint Contractures in Spinal Muscular Atrophy: Implications for Motor Function. Pediatr Phys Ther. 2018;30:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Bürk K, Sival DA. Scales for the clinical evaluation of cerebellar disorders. Handb Clin Neurol. 2018;154:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 2010;126:e1168-e1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 10. | Wilson BT, Stark Z, Sutton RE, Danda S, Ekbote AV, Elsayed SM, Gibson L, Goodship JA, Jackson AP, Keng WT, King MD, McCann E, Motojima T, Murray JE, Omata T, Pilz D, Pope K, Sugita K, White SM, Wilson IJ. The Cockayne Syndrome Natural History (CoSyNH) study: clinical findings in 102 individuals and recommendations for care. Genet Med. 2016;18:483-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Pidcock FS. Botulinum toxin type A treatment in neurogenetic syndromes. Pediatr Rehabil. 2005;8:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Bartolo M, Chiò A, Ferrari S, Tassorelli C, Tamburin S, Avenali M, Azicnuda E, Calvo A, Caraceni AT, Defazio G, DE Icco R, Formisano R, Franzoni S, Greco E, Jedrychowska I, Magrinelli F, Manera U, Marchioni E, Mariotto S, Monaco S, Pace A, Saviola D, Springhetti I, Tinazzi M, DE Tanti A; Italian Consensus Conference on Pain in Neurorehabilitation (ICCPN). Assessing and treating pain in movement disorders, amyotrophic lateral sclerosis, severe acquired brain injury, disorders of consciousness, dementia, oncology and neuroinfectivology. Evidence and recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Eur J Phys Rehabil Med. 2016;52:841-854. [PubMed] |
| 13. | Oh HM, Chung ME. Botulinum Toxin for Neuropathic Pain: A Review of the Literature. Toxins (Basel). 2015;7:3127-3154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Fonfria E, Maignel J, Lezmi S, Martin V, Splevins A, Shubber S, Kalinichev M, Foster K, Picaut P, Krupp J. The Expanding Therapeutic Utility of Botulinum Neurotoxins. Toxins (Basel). 2018;10:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Sätilä H. Over 25 Years of Pediatric Botulinum Toxin Treatments: What Have We Learned from Injection Techniques, Doses, Dilutions, and Recovery of Repeated Injections? Toxins (Basel). 2020;12:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Koman LA, Brashear A, Rosenfeld S, Chambers H, Russman B, Rang M, Root L, Ferrari E, Garcia de Yebenes Prous J, Smith BP, Turkel C, Walcott JM, Molloy PT. Botulinum toxin type a neuromuscular blockade in the treatment of equinus foot deformity in cerebral palsy: a multicenter, open-label clinical trial. Pediatrics. 2001;108:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Young R, Nix S, Wholohan A, Bradhurst R, Reed L. Interventions for increasing ankle joint dorsiflexion: a systematic review and meta-analysis. J Foot Ankle Res. 2013;6:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Marquer A, Barbieri G, Pérennou D. The assessment and treatment of postural disorders in cerebellar ataxia: a systematic review. Ann Phys Rehabil Med. 2014;57:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Heinen F, Desloovere K, Schroeder AS, Berweck S, Borggraefe I, van Campenhout A, Andersen GL, Aydin R, Becher JG, Bernert G, Caballero IM, Carr L, Valayer EC, Desiato MT, Fairhurst C, Filipetti P, Hassink RI, Hustedt U, Jozwiak M, Kocer SI, Kolanowski E, Krägeloh-Mann I, Kutlay S, Mäenpää H, Mall V, McArthur P, Morel E, Papavassiliou A, Pascual-Pascual I, Pedersen SA, Plasschaert FS, van der Ploeg I, Remy-Neris O, Renders A, Di Rosa G, Steinlin M, Tedroff K, Valls JV, Viehweger E, Molenaers G. The updated European Consensus 2009 on the use of Botulinum toxin for children with cerebral palsy. Eur J Paediatr Neurol. 2010;14:45-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |