Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4721
Peer-review started: September 8, 2020
First decision: January 24, 2021
Revised: January 27, 2021
Accepted: April 19, 2021
Article in press: April 19, 2021
Published online: June 26, 2021
Processing time: 270 Days and 7.8 Hours
Alport syndrome (ATS) is a rare hereditary disease caused by mutations in genes such as COL4A3, COL4A4, and COL4A5. ATS involves a spectrum of phenotypes ranging from isolated hematuria that is nonprogressive to progressive renal disease with extrarenal abnormalities. Although ATS can be combined with other diseases or syndromes, ATS combined with lupus nephritis has not been reported before.
A Chinese family with ATS was recruited for the current study. Clinical characteristics (including findings from renal biopsy) of ATS patients were collected from medical records, and potential causative genes were explored by whole-exome sequencing. A heterozygous substitution in intron 22 of COL4A3 (NM_000091 c.2657-1G>A) was found in the patients, which was further confirmed by quantitative polymerase chain reaction.
Heterozygous substitution of a COL4A3 gene splice site was identified by whole-exome sequencing, revealing the molecular pathogenic basis of this disorder. In general, identification of pathogenic genes can help to fully understand the molecular mechanism of disease and facilitate precise treatment.
Core Tip: Alport syndrome is a hereditary nephropathy that can be combined with other diseases or syndromes. We present the case of a 33-year-old man who was initially diagnosed with lupus nephritis but further diagnosed with Alport syndrome after genetic testing. He achieved complete remission after treatment with hormones and immunosuppressive agents. A variant of the splice site of intron 22 in the COL4A3 gene that cosegregated with the phenotype in the pedigree was identified by whole-exome sequencing.
- Citation: Liu HF, Li Q, Peng YQ. Alport syndrome combined with lupus nephritis in a Chinese family: A case report. World J Clin Cases 2021; 9(18): 4721-4727
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4721.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4721
Alport syndrome (ATS) is a hereditary nephropathy with a spectrum of phenotypes ranging from isolated hematuria with a nonprogressive or very slowly progressive course to progressive renal disease with extrarenal abnormalities, including hearing loss, ocular lesions, and leiomyomatosis. Approximately two-thirds of ATS cases are X-linked dominant, and 15% are autosomal recessive; approximately 20% are autosomal dominant (AD)[1-4]. ATS is a genetically heterogeneous disorder, and the pathogenesis of ATS is related to defects in the genes COL4A3, COL4A4, and COL4A5.
In the absence of treatment, renal disease progresses from microscopic hematuria to proteinuria, progressive renal insufficiency, and end-stage renal disease. The clinical diagnosis of ATS is largely based on clinical manifestations and renal histopathology. Due to its complex clinical manifestations, ATS can be misdiagnosed when the clinical manifestations are atypical. Therefore, accurate diagnosis of ATS can be established when a pathogenic variant is identified by molecular genetic testing, including polymerase chain reaction-based screening and whole-exome sequencing (WES). Indeed, with the progress in next-generation sequencing, WES has been extensively utilized in clinical practice, even for sporadic cases[5]. For example, de novo mutations in COL4A5 were identified by WES in two girls with ATS in Korea, suggesting that WES is an effective approach to obtain genetic information[6].
ATS can be combined with other diseases or syndromes. One case report described a 9-mo-old boy who was diagnosed with concomitant ATS and Klinefelter syndrome, with a homozygous single-nucleotide substitution found in the splice site of intron 41 of the COL4A5 gene by genomic DNA analysis[7]. In addition, IgA nephropathy can accompany ATS[8]. A study in 2017 involved two boys with IgA nephropathy, whose pathogenic COL4A5 mutations were identified by polymerase chain reaction-based screening[9]. Similarly, Li et al[10] detected a COL4A4 missense mutation in a Chinese child with familial hematuria with biopsyproven focal segmental glomerular sclerosis. However, the combination of ATS and lupus nephritis (LN) has not been reported to date. Here, we report a Chinese family with ATS combined with LN, and the pathogenic mutation in COL4A3 was identified by WES.
A 33-year-old Chinese man was admitted to our department for sustainable foamy urine for more than one year. He also complained of intermittent hair loss and recurrence of oral ulcers.
Approximately one year prior, the patient was hospitalized at a local hospital for the same reason, and routine urine tests indicated microscopic hematuria and proteinuria. He did not pay much attention, and there was no further diagnosis or treatment because of a lack of conscious symptoms. One month prior, his blood pressure rose to 145/91 mmHg for unknown reasons; microscopic hematuria and heavy proteinuria were again detected.
The patient had no comorbidities.
The patient's father had asymptomatic microscopic hematuria and proteinuria, as detected in a routine physical examination approximately 2 years prior. The patient had a daughter and a son; the daughter (7 years old) had asymptomatic microscopic hematuria, and the son had microscopic hematuria and proteinuria. His son had ever been diagnosed with chronic nephritis at a local hospital.
The patient's appearance was normal, without edema. His systolic and diastolic blood pressures were 141 mmHg and 90 mmHg, respectively; his pulse rate was 81 beats per minute, and his respiratory rate was 19 breaths per minute. No obvious abnormality, including growth retardation, was detected during physical examination, and no specific nervous system symptoms were recognized. The patient was also subjected to audiologic assessments, but no hearing impairments were detected, even at high frequency. Furthermore, no symptoms were found in either eye by comprehensive ophthalmic examinations.
Microscopic hematuria and proteinuria were confirmed by urine tests. The results of other tests, including routine blood tests and serum immunology, are listed in Table 1.
| Parameter | Proband | Daughter | Son | Reference range |
| Height (cm) | 177 | 145 | 140 | — |
| Weight (kg) | 75 | 38 | 35 | — |
| Blood routine tests | ||||
| WBC, 109/L | 3.0↓ | 3.5-9.5 | ||
| HGB, g/L | 91↓ | 115-150 | ||
| Platelet count, 109/L | 60↓ | 125-350 | ||
| RBC, 1012/L | 3.3↓ | 3.8-5.1 | ||
| Urine routine tests | ||||
| Specific gravity | 1.018 | 1.020 | 1.015 | 1.015-1.030 |
| Urine protein | 2+↑ | 2+↑ | 3+↑1 | 5.5-7.5 |
| Urinary occult blood | 3+↑ | 3+↑ | 3+↑1 | Negative |
| 24-h UPE, g/d | 2.45↑ | 0-0.12 | ||
| Immunology | ||||
| Complement C3, g/L | 0.21↓ | 0.9-2.1 | ||
| Anti-ANA antibody | 1:320 | Negative | ||
| Anti-Sm antibody | Positive↑ | Negative | ||
| Anti-dsDNA antibody | Positive↑ | Negative | ||
| Serum chemistry | Negative | |||
| Albumin, g/L | 31.5↓ | 40-55 | ||
| Serum creatinine, μmol/L | 121↑ | 45-105 | ||
| Cystatin-C, mg/L | 1.45↑ | 0-1.16 |
No obvious abnormality was detected by abdominal ultrasound examination, X-ray diagnosis, or electrocardiographic examination. However, heart echocardiography showed a small amount of pericardial effusion.
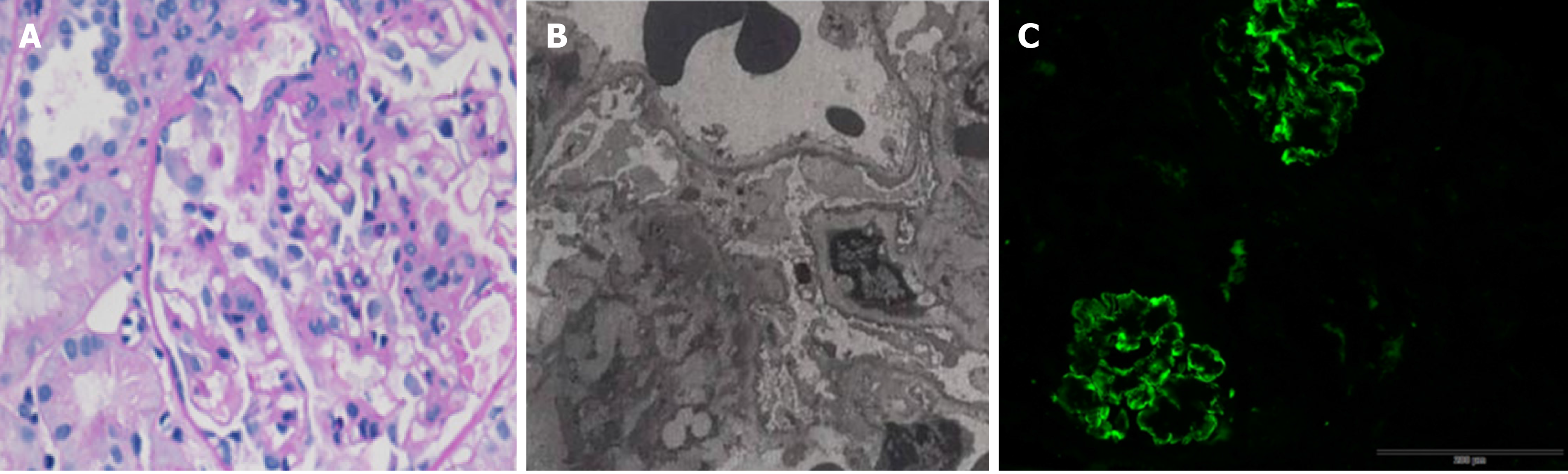
To further analyze the renal presentation, a histopathology study of renal biopsy was performed. By light microscopy, a total of 13 glomeruli were observed, with one glomerulus being enlarged and lobulated. Para-aminosailcylic acid staining and Masson staining were positive, showing mild mesangial matrix proliferation. The basement membrane was thickened. Three glomerular fibroblastic crescents and pericystic fibrosis of glomeruli were observed (Figure 1A). In addition, deposition of erythrotropin under the endothelium of the capillary loop was detected (Figure 1B). Electron microscopy revealed obvious basement membrane lesions including variable thickness and reticulation of the glomerular basement membrane, as well as irregular subepithelial protrusion of the lamina densa. Fine particles and electron-dense bodies were detected in the stratified basement membrane (Figure 1C). Immunological staining for IgG, IgA, IgM, C3, C4 C1q, К, and λ was positive in four glomeruli, with the signals being deposited in the vascular lumen and mesangial area in a granular or linear form (Figure 1).
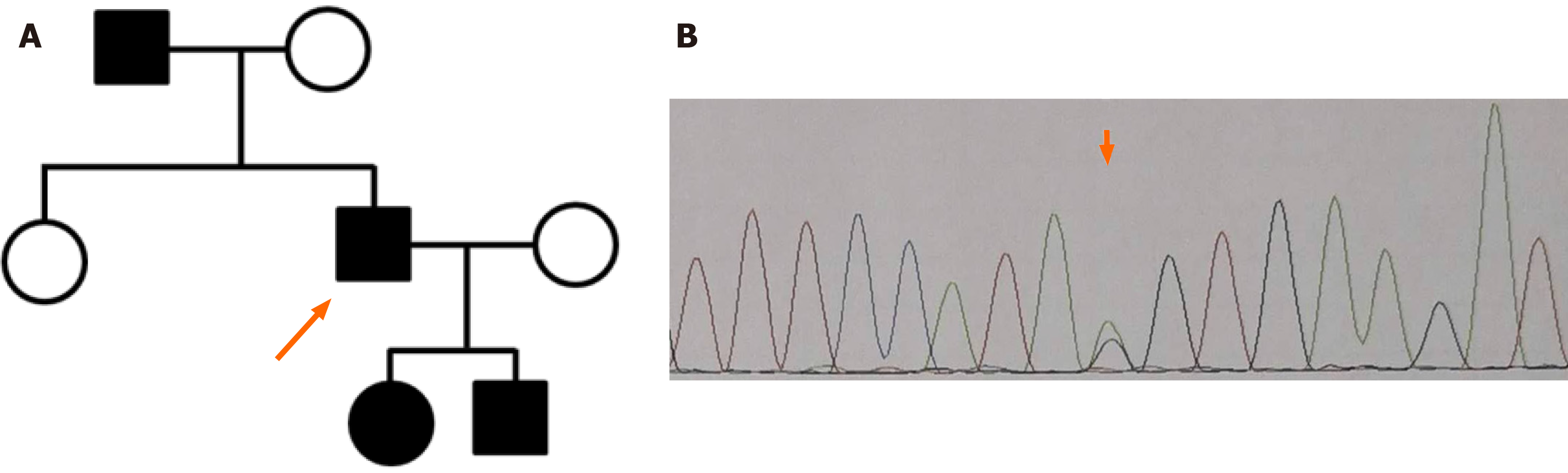
A considerable investigation of family history was performed. The patient’s father had asymptomatic microscopic hematuria and proteinuria, as detected in a routine physical examination approximately 2 years previously. As mentioned above, the patient had a daughter and a son: The former had asymptomatic microscopic hematuria, and the latter had microscopic hematuria and proteinuria; his son had been diagnosed with chronic nephritis at a local hospital. Thus, three relatives had microscopic hematuria. Therefore, a diagnosis of ATS was highly suspected (Figure 2A). For a precise conclusive diagnosis, the patient and his children were recommended to undergo genetic testing, and WES was performed. Genomic DNA was extracted from blood samples; WES was performed as previously described. After sequencing, the coverage of the target sequence was over 99.12%, and the mean sequencing depth was approximately 147. The sequencing analysis revealed a heterozygous substitution, NM_000091 c.2657-1G>A (p. V294fs) in intron 22 of the COL4A3 gene, which was confirmed by Sanger sequencing (Figure 2B). The mutation was excluded from the single nucleotide polymorphism database but was included in the ClinVar database. As this mutation is located at an evolutionarily conserved splice site, this splicing mutation is thought to lead to the skipping of exon 23. In addition, this variant is classified as “likely pathogenic” according to the American College of Medical Genetics and Genomics standards and guidelines[11] (Figure 2).
Considering the patient’s renal and heart presentations, as well as the immunological staining, he was diagnosed with ATS combined with LN, as based on the genetic test.
The patient received hormone treatment, hydroxychloroquine sulfate, and mycophenolate mofetil to delay the progression of kidney disease. He also took benazepril to control blood pressure and other traditional Chinese medicines to protect renal function.
After one week, the patient’s 24-h urinary protein excretory decreased to 1.0 g/24 h, and his hematopoietic function was greatly improved. After 3 mo, he still had sufficient renal function without obvious urine protein.
Here, we report a case of ATS combined with LN. To our knowledge, this is the first report regarding ATS combined with LN. By using WES technology, the pathogenic mutation in the COL4A3 gene was identified and confirmed by Sanger sequencing.
ATS is a rare genetic disorder that causes progressive nephritis, and its phenotypes vary from isolated hematuria with nonprogressive to progressive renal disease with extrarenal abnormalities such as sensorineural deafness and ocular abnormalities. Most cases of ATS will deteriorate to end-stage renal disease within the first three decades of life[12]. Only a small proportion of ATS cases are inherited from AD, which is caused by mutations in the COL4A3 gene. For these ATS cases, microhematuria is frequently observed, though some individuals are asymptomatic. A substantial proportion of affected individuals eventually develop proteinuria. Unlike other modes of inheritance, such as autosomal recessive or X-linked dominant inheritance, ATS cases with AD inheritance are slowly progressive, and renal insufficiency and sensorineural hearing loss may not develop until relatively late in life. In the present family, affected individuals were found in all three generations, showing AD inheritance, and a heterozygous mutation in the COL4A3 gene was detected in each affected individual. However, no hearing impairments or symptoms in eyes were found in the proband. Older than 55 years old, the proband’s father only had asymptomatic microscopic hematuria and proteinuria, which was consistent with other reports[13].
To date, more than 500 variants of the COL4A3 gene have been deposited in the ClinVar database. Among these variants, 32 are pathogenic or likely pathogenic. All of these variants, including deletion, duplication, and substitution mutations, are located at evolutionarily conserved splice sites (GT-AG rule). In the ClinVar database, this variant is classified as uncertain significance. Based on its cosegregation with the phenotype in this family and its potential influence on the protein, this variant was ultimately classified as likely pathogenic in this study.
ATS can be accompanied by other diseases or syndromes. However, ATS cases combined with LN have not yet been reported. LN is the leading cause of secondary glomerulonephritis[14-16]. LN is thought to be induced by immune system abnormalities, including a variety of cells, cytokines, and related pathways[17]. A primary event of LN is the type III hypersensitivity reaction and subsequent immune complexes, which can deposit in the mesangium and subendothelial or subepithelial space near the glomerular basement membrane of the kidney. For example, an anti-double stranded DNA (anti-dsDNA) antibody binds to DNA and forms an anti-dsDNA immune complex[18]. The current case was positive for autoantibodies such as anti-dsDNA and anti-Sm antibodies. In addition, immunological staining with an antibody against immunoglobulins and complements revealed the deposition of immune complexes (Figure 1C), and these immune complexes can lead to an inflammatory response.
Previous studies have shown that LN is a complicated disease associated with genetic and environmental factors[19]. During past decades, susceptibility genes or loci have been identified by linkage analysis, association analysis, and genome-wide association studies. These genetic factors include HLA-II, complements C2 and C4, TNF and fatigue crack growth rate genes. As a complicated disease, the contribution of genetic factors needs further study. In our case, we did not find any mutation of known genes or loci associated with LN, suggesting that environmental factors are important.
In summary, we present the case of a Chinese patient with ATS combined with LN, and the pathogenic variant in COL4A3 was identified by WES. Identification of pathogenic variants helps for understanding the relationship between the phenotypes and genotypes of ATS.
We wish to thank the patient and his family for participation in the study.
Manuscript source: Unsolicited manuscript
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pedersen EB S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Kashtan CE. Alport Syndrome: Achieving Early Diagnosis and Treatment. Am J Kidney Dis. 2021;77:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | Savige J, Ariani F, Mari F, Bruttini M, Renieri A, Gross O, Deltas C, Flinter F, Ding J, Gale DP, Nagel M, Yau M, Shagam L, Torra R, Ars E, Hoefele J, Garosi G, Storey H. Expert consensus guidelines for the genetic diagnosis of Alport syndrome. Pediatr Nephrol. 2019;34:1175-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 3. | Torra R, Furlano M. New therapeutic options for Alport syndrome. Nephrol Dial Transplant. 2019;34:1272-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Lee JM, Nozu K, Choi DE, Kang HG, Ha IS, Cheong HI. Features of Autosomal Recessive Alport Syndrome: A Systematic Review. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Yamamura T, Nozu K, Minamikawa S, Horinouchi T, Sakakibara N, Nagano C, Aoto Y, Ishiko S, Nakanishi K, Shima Y, Nagase H, Rossanti R, Ye MJ, Nozu Y, Ishimori S, Morisada N, Kaito H, Iijima K. Comparison between conventional and comprehensive sequencing approaches for genetic diagnosis of Alport syndrome. Mol Genet Genomic Med. 2019;7:e883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Han KH, Park JE, Ki CS. De novo mutations in COL4A5 identified by whole exome sequencing in 2 girls with Alport syndrome in Korea. Korean J Pediatr. 2019;62:193-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Nishida M, Hashimoto F, Kaito H, Nozu K, Iijima K, Asada D, Hamaoka K. Combined Alport syndrome and Klinefelter syndrome. Pediatr Int. 2016;58:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Hao W, Ao L, Zhang C, Zhu L, Xie D. IgA nephropathy suspected to be combined with Fabry disease or Alport syndrome: a case report. J Int Med Res. 2020;48:300060519891290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Cui J, Zhang H. Childhood IgA nephropathy combined with Alport syndrome: a report of 2 cases and literature review. Linchuang Erke Zazhi. 2017;35:9-12. |
| 10. | Li Y, Wang Y, He Q, Dang X, Cao Y, Wu X, Mo S, He X, Yi Z. Genetic mutational testing of Chinese children with familial hematuria with biopsyproven FSGS. Mol Med Rep. 2018;17:1513-1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22547] [Article Influence: 2254.7] [Reference Citation Analysis (0)] |
| 12. | Savva I, Pierides A, Deltas C. RAAS inhibition and the course of Alport syndrome. Pharmacol Res. 2016;107:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Hines SL, Agarwal A, Ghandour M, Aslam N, Mohammad AN, Atwal PS. Novel variants in COL4A4 and COL4A5 are rare causes of FSGS in two unrelated families. Hum Genome Var. 2018;5:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hou JH, Zhu HX, Zhou ML, Le WB, Zeng CH, Liang SS, Xu F, Liang DD, Shao SJ, Liu Y, Liu ZH. Changes in the Spectrum of Kidney Diseases: An Analysis of 40,759 Biopsy-Proven Cases from 2003 to 2014 in China. Kidney Dis (Basel). 2018;4:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Kostopoulou M, Adamichou C, Bertsias G. An Update on the Diagnosis and Management of Lupus Nephritis. Curr Rheumatol Rep. 2020;22:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Peleg Y, Bomback AS, Radhakrishnan J. The Evolving Role of Calcineurin Inhibitors in Treating Lupus Nephritis. Clin J Am Soc Nephrol. 2020;15:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Frangou E, Georgakis S, Bertsias G. Update on the cellular and molecular aspects of lupus nephritis. Clin Immunol. 2020;216:108445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Rekvig OP. The dsDNA, Anti-dsDNA Antibody, and Lupus Nephritis: What We Agree on, What Must Be Done, and What the Best Strategy Forward Could Be. Front Immunol. 2019;10:1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Song K, Liu L, Zhang X, Chen X. An update on genetic susceptibility in lupus nephritis. Clin Immunol. 2020;210:108272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |