Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4668
Peer-review started: February 6, 2021
First decision: March 16, 2021
Revised: March 23, 2021
Accepted: April 20, 2021
Article in press: April 20, 2021
Published online: June 26, 2021
Processing time: 124 Days and 23.4 Hours
Sarcopenia is a nutrition-related disease and has a profound effect on the long-term overall survival (OS) of patients with gastric cancer. Its diagnostic criterion is critical to clinical diagnosis and treatment. However, previous research reported widely differing sarcopenia prevalence due to different criteria. AWGS2019 and EWGSOP2 are the two latest and widely adopted criteria.
To compare the effects of AWGS2019 and EWGSOP2 on the long-term OS of Chinese gastric cancer patient after radical gastrectomy.
An observational study was conducted from July 2014 to January 2017, which included 648 consecutive gastric cancer patients who underwent radical gastrectomy. The sarcopenia elements (skeletal muscle index, handgrip strength, and gait speed) were measured within 1 mo or 7 d before surgery. The patients were followed at fixed intervals to gain the outcomes. Multivariate Cox regression analysis was performed to determine the association between sarcopenia and the long-term OS of these patients according to the two criteria separately. The predictive performance of the models with AWGS2019 and EWGSOP2 were evaluated by the concordance index (C-index) and area under the time-dependent receiver operating characteristic curve (AUC). The Akaike information criterion (AIC) was applied to compare model fits.
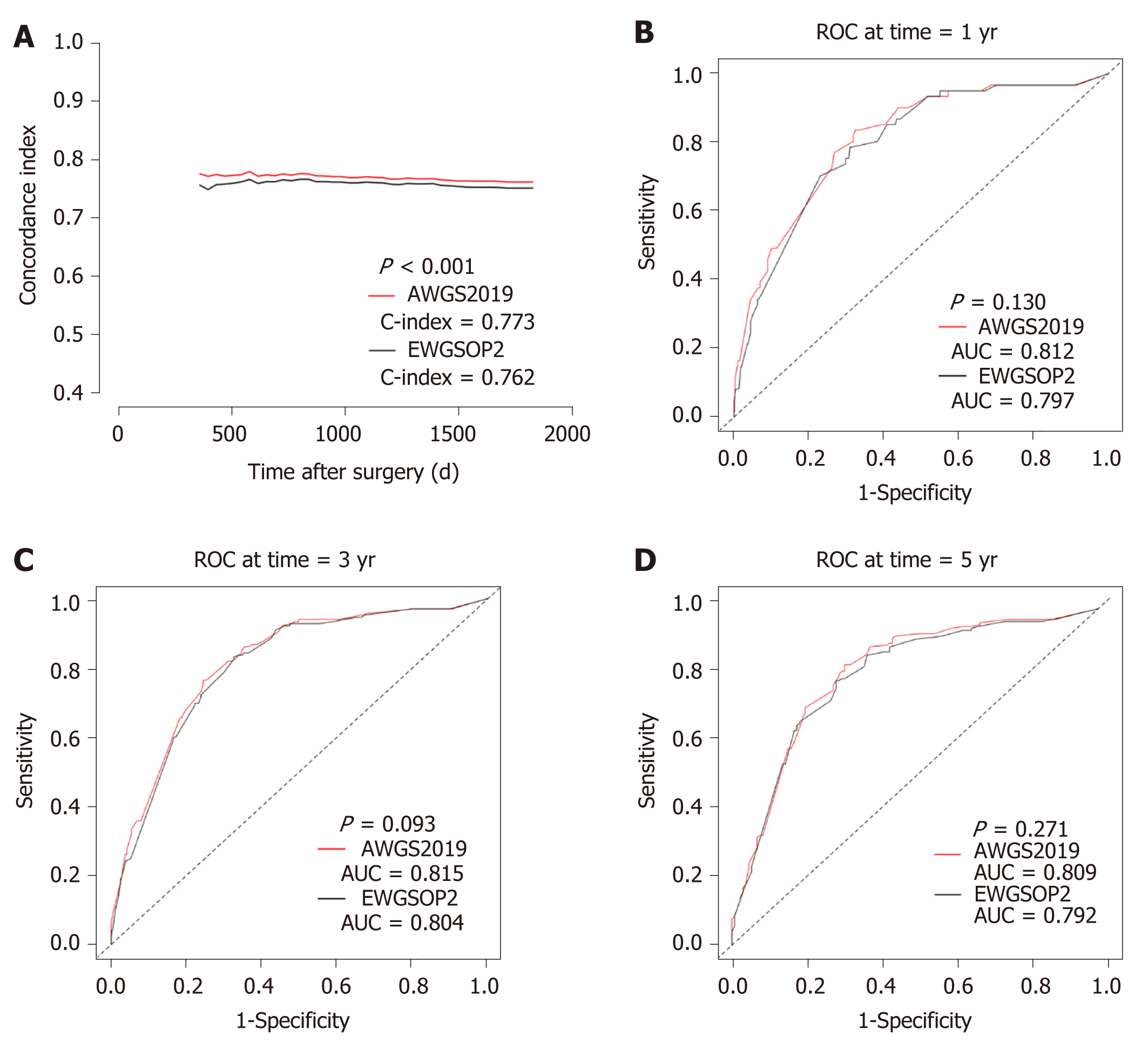
The prevalence of sarcopenia was 20.5% and 11.3% according to AWGS2019 and EWGSOP2, respectively. Sarcopenia was an independent risk factor for the long-term OS no matter based on AWGS2019 or EWGSOP2, but AWGS2019-sarcopenia in multivariate model had a higher hazard ratio (HR) [2.150 (1.547-2.988)] than EWGSOP2-sarcopenia [HR 1.599 (1.092-2.339)]. Meanwhile, the model with AWGS2019-sarcopenia [C-index 0.773 (0.742-0.804); AIC 2193.7; time-dependent AUC 0.812 (0.756-0.867) for 1-year OS, 0.815 (0.778-0.852) for 3-year OS, and 0.809 (0.759-0.859) for 5-year OS] had better predictive power and model fits than the model with EWGSOP2-sarcopenia [C-index 0.762 (0.729-0.795); AIC 2215.2; time-dependent AUC 0.797 (0.741-0.854) for 1-year OS, 0.804 (0.767-0.842) for 3-year OS, and 0.799 (0.748-0.850) for 5-year OS].
Sarcopenia is an independent risk factor for the long-term OS in Chinese gastric cancer patients undergoing radical gastrectomy. The prediction model with AWGS2019-sarcopenia has better predictive power and model fits than the prediction model with EWGSOP2-sarcopenia. AWGS2019 may be more appropriate for diagnosing sarcopenia in these Chinese patients than EWGSOP2.
Core Tip: Stomach cancer is an important contributor to the global burden of cancer, especially to Asian countries, while sarcopenia is a nutrition-related disease and has a profound effect on patients with gastric cancer. Early diagnosis of sarcopenia may contribute to improving the prognosis of these patients. Even though a significant difference between Asian people and European people, EWGSOP2 is more widely adopted than AWGS2019 in Asian countries. This study is the first to determine which method is more appropriate for Chinese patients and may guide Asian clinicians to make clinical decision based on our own criterion.
- Citation: Wu WY, Dong JJ, Huang XC, Chen ZJ, Chen XL, Dong QT, Bai YY. AWGS2019 vs EWGSOP2 for diagnosing sarcopenia to predict long-term prognosis in Chinese patients with gastric cancer after radical gastrectomy . World J Clin Cases 2021; 9(18): 4668-4680
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4668.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4668
Gastric cancer is the sixth leading type of cancer and the second leading cause of cancer-related death worldwide[1]. Surgical resection is currently the primary option for early-stage patients. However, radical gastrectomy is often associated with an unsatisfactory clinical prognosis, especially for malnutrition patients. Nutrition-related disease “sarcopenia” is characterized by skeletal muscle mass and/or quality and/or strength decline, and has been recognized for its profound effect on human health, which occurs with aging and/or immobility[2]. Several studies found that sarcopenia was an independent risk factor for the long-term survival in gastric cancer patient[3]. From these studies, we found extensively differing sarcopenia prevalence due to various criteria.
To standardize definition and diagnosis, in 2010 the EWGSOP published the first guidelines[4]. In order to address ethnic differences in anatomy (about height and weight) and lifestyle, AWGS2014 put forward their diagnostic criterion[5]. Nowadays, the latest versions EWGSOP2 and AWGS2019 are the two extensively adopted criteria. These two standards have different diagnostic procedures and cut-off points. To our knowledge, the European criterion is more widely applied than the Asian criterion, even in Asian country. The study of AWGS2019 vs EWGSOP2 for diagnosing sarcopenia in Chinese patients with gastric cancer after radical gastrectomy has not been reported.
Considering that the diagnostic criteria for sarcopenia are critical to clinical diagnosis and treatment, we conducted this study to confirm the effect of the two up-to-date sarcopenia criteria from AWGS and EWGSOP on the long-term overall survival (OS) of Chinese gastric cancer patient after radical gastrectomy, with an aim to determine which method is more appropriate for Asian patients.
From July 2014 to January 2017, consecutive gastric cancer patients who had undergone elective radical gastrectomy at the First Affiliated Hospital of Wenzhou Medical University were identified and relevant patient data were maintained in a prospectively collected database. The inclusion criteria were: (1) Received elective radical surgery for gastric cancer; (2) postoperative histologically confirmed gastric carcinoma; and (3) available abdominal computed tomography (CT) images within 1 mo before surgery. The exclusion criteria were: (1) Cancer metastasis; (2) remnant gastric cancer; and (3) motor system diseases that make patients unable to complete the measurement of grip strength and gait speed. All the surgery procedures were performed routinely following the Japanese gastric cancer treatment guidelines 2010 (version 3)[6]. This study was approved by the Ethical Review Board of the First Affiliated Hospital of Wenzhou Medical University (No. 2014063).
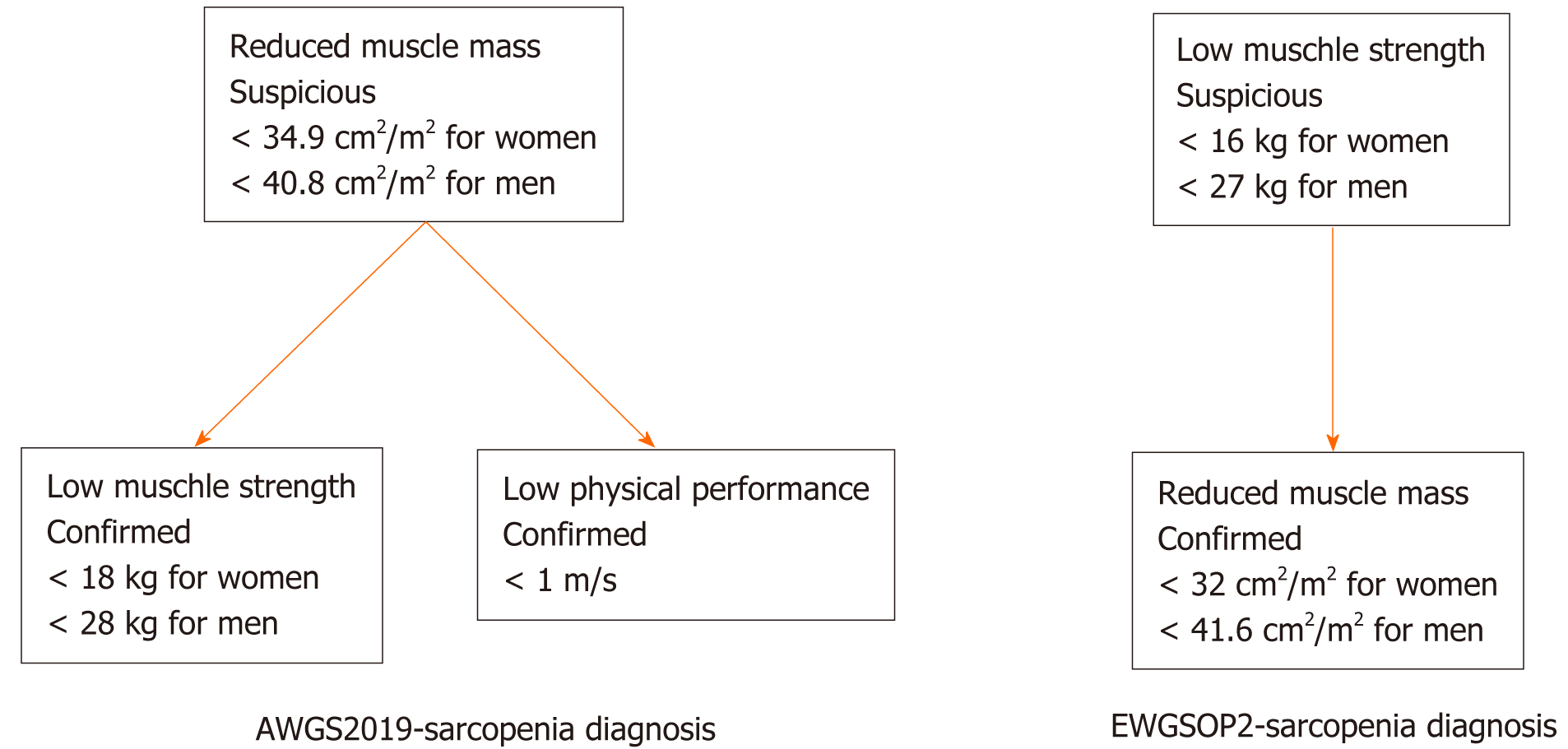
Abdominal CT was performed routinely for all the patients within 1 mo before surgery, and the CT films were stored in the Picture Archiving and Communication System. Lumbar vertebra (L3) muscle area was evaluated on a single image at CT workstation (GE ADW 4.5) with tissue specific HU thresholds of -29 to 150. All the images analyses were performed by two independent trained radiology residents who were blinded to relevant clinical information. Entire muscle areas were normalized by the square of stature and are reported as lumbar skeletal muscle index (SMI, cm2/m2). Low SMI was defined according to EWGSOP2 (< 32 cm2/m2 for women and < 41.6 cm2/m2 for men) and AWGS2019 (< 34.9 cm2/m2 for women and < 40.8 cm2/m2 for men) separately[7,8].
Hand-grip strength had an advantage of simple measurement and was extensively utilized to assess muscle strength, which had a moderate correlation with strength of other body compartments. Hand-grip strength of the dominant hand was measured with an electronic hand dynamometer (EH101; Camry, Guangdong Province, China). Low hand-grip strength was defined according to EWGSOP2 (< 16 kg for women and < 27 kg for men) and AWGS2019 (< 18.0 kg for women and < 28.0 kg for men) separately[2,9].
Six-meter usual walking speed test was chosen to measure physical performance. The observer instructed patients to walk as usual from standing position behind the starting line. Timing started from the first foot movement and ended with first foot crossed the finish line of 6 m. Low gait speed was defined < 1.0 m/s according to AWGS2019[9].
These two tests were conducted three times on hospitalized patients within 7 d before surgery, and the maximum value would be recorded.
AWGS2019-sarcopenia diagnosis required documentation of both of low muscle mass (L3-SMI, < 34.9 cm2/m2 for women and < 40.8 cm2/m2 for men) and either low muscle strength (Hand-grip strength, < 18.0 kg for women and < 28.0 kg for men) or low physical performance (6-m usual walking speed, < 1.0 m/s). EWGSOP2-sarcopenia diagnosis was suspected in the presence of low muscle strength (hand-grip strength, < 16 kg for women and < 27 kg for men) and confirmed by documentation of reduced muscle mass (L3-SMI, < 32 cm2/m2 for women and < 41.6 cm2/m2 for men). Figure 1 exhibits the diagnostic process[2,9].
The following data were prospectively collected when the patient was enrolled in this study: (1) Preoperative social and disease characteristics, including age, gender, body mass index (BMI), albumin, hypertension, diabetes, American Society of Anesthesiologists (ASA) grade, and Charlson comorbidity index (CCI); (2) surgery and tumor details, including laparoscopy-assisted surgery, surgery duration, type of resection, extent of lymph node dissection, anastomosis, TNM stage; and (3) postoperative outcomes, including duration of hospital stay, postoperative complications (within 30 d after surgery), readmission (within 30 d after discharge), and time to flatus.
All patients received routine reviews within 30 d after surgery. In the first 2 years, follow-up investigations were scheduled every 3 mo. Thereafter, telephone interviews or outpatient visits were performed every 6 mo. OS was calculated from the date of surgery to the death date of any cause. The last follow-up date was January 2020.
Continuous normally distributed and homogeneous variables were compared using the analysis of t-test. For continuous, non-normally distributed or non-homogeneous variables, the Manne Whitney U test was performed. The chi-square test or Fisher exact test was conducted on categorical data. Survival curves were estimated using the Kaplan Meier method and compared using log-rank tests. Univariate Cox regression analysis was used to assess the relationship between the long-term OS and variables and compute the hazard ratios (HRs) of all potential baseline predictors. Once two or more remaining features after univariate analyses were correlated (Pearson’s correlation coefficient > 0.80), the feature with the smaller P-value would be retained[10]. Variables with a trend (P < 0.10) in the univariate analysis were selected to be evaluated through multivariable Cox regression analysis using forward stepwise selection (P < 0.05) to confirm the relationship between sarcopenia and the long-term OS. Multivariable COX regression survival models with significant predictors were developed. The Harrell concordance index (C-index) and area under the time-dependent receiver operating characteristic curve (AUC) was calculated for the models which could be interpreted as the measure of the model’s predictive performance[11,12]. The Akaike information criterion (AIC) was calculated to compare model fits. Higher C-index and time-dependent AUC indicates better predictive performance, while lower AIC indicates better model. All the analyses were performed using SPSS statistics version 25.0 or R version 3.6.3.
Initially, 683 consecutive patients met the inclusion criteria. Due to palliative surgery, physical deformities, or being unwilling to participate in the study, 35 patients were excluded. Finally, 648 participants were included in the analyses. The median age of the patients was 64.3 ± 10.9 years, and the majority were men (male:female ratio = 3:1).
Based on AWGS2019, the prevalence of sarcopenia was 20.5% (133/648), while the prevalence was 11.3% (73/648) according to EWGSOP2. To intuitively reveal the impacts of the revised diagnostic criteria, we compared the clinical parameters for AWGS2019-sarcopenia patients with EWGSOP2-sarcopenia patients (Table 1). Compared with AWGS2019-sarcopenia patients, EWGSOP2-sarcopenia patients had a higher percentage of males (P = 0.018). The two groups were comparable in terms of age, BMI, albumin, ASA grade, CCI, and other characteristics.
| Factor | AWGS2019 (n = 133) | EWGSOP2 (n = 73) | P value |
| Age | 71.4 ± 8.8 | 73.3 ± 8.2 | 0.121 |
| Gender, n (%) | 0.018a | ||
| Female | 42 (31.6) | 12 (16.4) | |
| Male | 91 (68.4) | 61 (83.6) | |
| BMI | 20.4 ± 2.6 | 20.0 ± 2.5 | 0.303 |
| Albumin | 35.9 ± 4.6 | 34.8 ± 4.3 | 0.098 |
| Hypertension, n (%) | 0.539 | ||
| No | 93 (69.9) | 54 (74.0) | |
| Yes | 40 (30.1) | 19 (26.0) | |
| Diabetes, n (%) | 0.908 | ||
| No | 114 (85.7) | 63 (86.3) | |
| Yes | 19 (14.3) | 10 (13.7) | |
| ASA grade, n (%) | 0.239 | ||
| I | 8 (6.0) | 4 (5.5) | |
| II | 94 (70.7) | 44 (60.3) | |
| III | 31 (23.3) | 25 (34.2) | |
| CCI | 5 (5-6) | 5 (5-6) | 0.109 |
| Operative duration | 205.5 ± 55.9 | 195.0 ± 42.9 | 0.166 |
| TNM stage, n (%) | 0.806 | ||
| I | 23 (17.3) | 11 (15.1) | |
| II | 28 (21.1) | 18 (24.7) | |
| III | 82 (61.7) | 44 (60.3) | |
| Laparoscopic surgery, n (%) | 0.494 | ||
| No | 116 (87.2) | 66 (90.4) | |
| Yes | 17 (12.8) | 7 (9.6) | |
| Type of resection, n (%) | 0.922 | ||
| Subtotal | 72 (54.1) | 39 (53.4) | |
| Total | 61 (45.9) | 34 (46.6) | |
| Anastomosis method, n (%) | 0.881 | ||
| B1 | 41 (30.8) | 25 (34.2) | |
| B2 | 27 (20.3) | 14 (19.2) | |
| RY | 65 (48.9) | 34 (46.6) | |
| Lymph node dissection, n (%) | 0.073 | ||
| D1 | 18 (13.5) | 4 (5.5) | |
| D2 | 115 (86.5) | 69 (94.5) | |
| Differentiation, n (%) | 0.438 | ||
| Differentiated | 30 (22.6) | 20 (27.4) | |
| Undifferentiated | 103 (77.4) | 53 (72.6) | |
| Total complications, n (%) | 40 (30.1) | 25 (34.2) | 0.538 |
| Pneumonia | 17 (12.8) | 10 (13.7) | 0.852 |
| Leakage | 5 (3.8) | 3 (4.1) | 0.901 |
| Wound infection | 4 (3.0) | 2 (2.7) | 0.913 |
| Intestinal obstruction | 3 (2.3) | 1 (1.4) | 0.659 |
| Gastrointestinal dysfunction | 6 (4.5) | 4 (5.5) | 0.757 |
| Intra-abdominal infection | 7 (5.3) | 5 (6.8) | 0.642 |
| Intra-abdominal hemorrhage | 4 (3.0) | 3 (4.1) | 0.676 |
| Venous thrombosis | 2 (1.5) | 1 (1.4) | 0.939 |
| First time to flatus | 4 (3-5) | 4 (3-6) | 0.594 |
| Hospital stay | 16 (12-21) | 16 (12-22) | 0.566 |
| Readmission within 30 d | 8 (6.0%) | 3 (4.1%) | 0.561 |
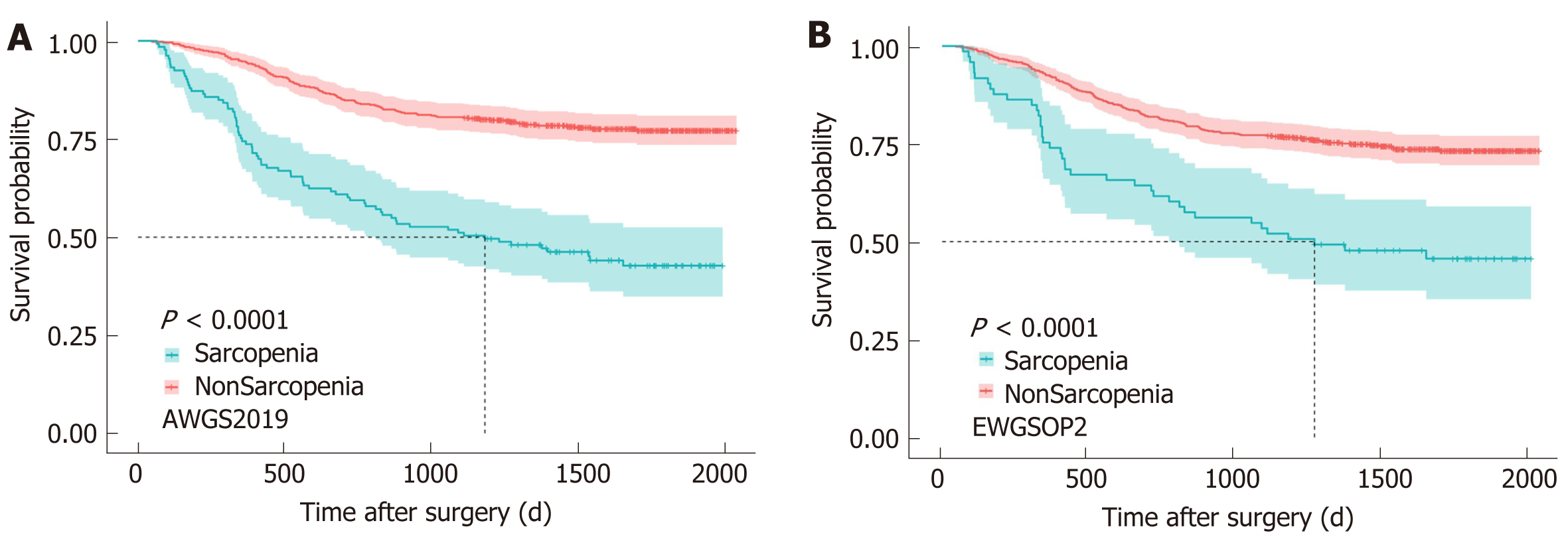
The median follow-up time was 1630 d. According to AWGS2019, 1-year (365 d), 3-year (1095 d), and 5-year (1825 d) OS rates were 74.4% (0% censored), 51.1% (0% censored), and 13.5% (30.8% censored), respectively, among patients with sarcopenia, and 94.6% (0% censored), 80.6% (0% censored), and 21.7% (56.1% censored) in patients without sarcopenia. According to EWGSOP2, 1-year (365 d), 3-year (1095 d), and 5-year (1825 d) OS rates were 75.3% (0% censored), 53.4% (0% censored), and 15.1% (30.1% censored), respectively, among patients with sarcopenia, and 92.3% (0% censored), 77.2% (0% censored), and 20.5% (53.6% censored) in patients without sarcopenia.
Kaplan Meier analyses showed that no matter based on AWGS2019 (Figure 2A) or EWGSOP2 (Figure 2B), sarcopenia patients experienced a significantly shorter OS than patients without sarcopenia (log-rank, P < 0.001). A multivariate Cox model revealed that AWGS2019-sarcopenia [HR, 2.150 (1.547-2.988), P = 0.001], TNM stage {II/I [HR, 1.660 (0.887-3.108), P = 0.113]; III/I [HR, 4.751 (2.753-8.200), P = 0.001]}, anastomosis method {B2/B1 [HR, 1.414 (0.904-2.210), P = 0.129]; RouY/B1 [HR, 1.614 (1.109-2.348), P = 0.012]}, histologic type [HR, 1.899 (1.263-2.856), P = 0.002], and two postoperative complications {pneumonia [HR, 1.990 (1.222-3.241), P = 0.006] and leakage [HR, 3.018 (1.557-5.853), P = 0.001]} were independently associated with poorer OS (P < 0.05), while the other variables (age, BMI, albumin, ASA grade, CCI, operation duration, laparoscopic surgery, type of resection, total complications, intestinal obstruction, and hospital stay) were excluded due to no statistical difference (P > 0.05). When we replaced AWGS2019-sarcopenia with EWGSOP2-sarcopenia, EWGSOP2-sarcopenia remained in the model [HR, 1.599 (1.092-2.339), P = 0.016] (Table 2). The HR of EWGSOP2-sarcopenia and AWGS2019-sarcopenia was 1.599 (1.092-2.339) and 2.150 (1.547-2.988), respectively.
| Factor | Univariable analysis | Multivariate analysis | ||||
| AWGS2019 | EWGSOP2 | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| AWGS2019 | 3.311 (2.470-4.439) | 0.001b | 2.150 (1.547-2.988) | 0.001b | ||
| EWGSOP2 | 2.566 (1.803-3.651) | 0.001b | 1.599 (1.092-2.339) | 0.016a | ||
| Age | 1.036 (1.021-1.051) | 0.001b | ||||
| Gender | ||||||
| Male/female | 1.038 (0.745-1.448) | 0.824 | ||||
| BMI | 0.934 (0.890-0.980) | 0.005b | ||||
| Albumin | 0.930 (0.904-0.957) | 0.001b | ||||
| Hypertension | ||||||
| Yes/No | 1.290 (0.948-1.755) | 0.105 | ||||
| Diabetes | ||||||
| Yes/No | 0.765 (0.476-1.229) | 0.268 | ||||
| ASA grade | ||||||
| II/I | 1.301 (0.773-2.190) | 0.323 | ||||
| III/I | 2.402 (1.366-4.225) | 0.002b | ||||
| CCI | 1.288 (1.146-1.448) | 0.001b | ||||
| Operative duration | 1.003 (1.001-1.006) | 0.017a | ||||
| TNM stage | ||||||
| II/I | 2.494 (1.349-4.610) | 0.004b | 1.660 (0.887-3.108) | 0.113 | 1.636 (0.872-3.067) | 0.125 |
| III/I | 8.093 (4.825-13.574) | 0.001b | 4.751 (2.753-8.200) | 0.001b | 4.968 (2.876-8.582) | 0.001b |
| Laparoscopic surgery | ||||||
| Yes/No | 0.463 (0.306-0.700) | 0.001b | ||||
| Type of resection | ||||||
| Total/Subtotal | 1.864 (1.400-2.481) | 0.001b | ||||
| Anastomosis method | ||||||
| B2/B1 | 2.571 (1.672-3.954) | 0.001b | 1.414 (0.904-2.210) | 0.129 | 1.423 (0.911-2.223) | 0.122 |
| RouY/B1 | 2.747 (1.920-3.390) | 0.001b | 1.614 (1.109-2.348) | 0.012a | 1.600 (1.100-2.328) | 0.014a |
| Lymph node dissection | ||||||
| D2/D1 | 1.036 (0.693-1.548) | 0.864 | ||||
| Histologic type | ||||||
| Undifferentiated/differentiated | 2.756 (1.855-4.095) | 0.001b | 1.899 (1.263-2.856) | 0.002b | 1.972 (1.309-2.970) | 0.001b |
| Total complications | 1.678 (1.221-2.307) | 0.001b | ||||
| Pneumonia | 2.574 (1.601-4.137) | 0.001b | 1.990 (1.222-3.241) | 0.006b | 1.935 (1.184-3.163) | 0.008b |
| Leakage | 3.325 (1.757-6.292) | 0.001b | 3.018 (1.557-5.853) | 0.001b | 3.339 (1.722-6.476) | 0.001b |
| Wound infection | 1.171 (0.435-3.153) | 0.755 | ||||
| Intestinal obstruction | 2.159 (0.888-5.252) | 0.090 | ||||
| Gastrointestinal dysfunction | 0.982 (0.484-1.994) | 0.960 | ||||
| Intra-abdominal infection | 1.410 (0.767-2.594) | 0.269 | ||||
| Intra-abdominal hemorrhage | 0.888 (0.365-2.160) | 0.794 | ||||
| Venous thrombosis | 0.997 (0.410-2.425) | 0.995 | ||||
| First time to Flatus | 1.101 (1.050-1.154) | 0.001b | ||||
| Hospital stay | 1.026 (1.017-1.035) | 0.001b | ||||
| Readmission within 30 d | 1.601 (0.929-2.759) | 0.090 |
The C-index for AWGS2019-sarcopenia model was 0.773 (0.742-0.804), the AIC was 2193.7, and the time-dependent AUC was 0.812 (0.756-0.867), 0.815 (0.778-0.852), and 0.809 (0.759-0.859), respectively, for 1-year, 2-year, and 5-year OS. For EWGSOP2-sarcopenia model, the C-index was 0.762 (0.729-0.795), the AIC was 2215.2, and the time-dependent AUC was 0.797 (0.741-0.854), 0.804 (0.767-0.842), and 0.799 (0.748-0.850), respectively, for 1-year, 3-year, and 5-year OS. The higher C-index (P < 0.001), the lower AIC, and the higher time-dependent AUC of AWGS2019-sarcopenia model reflected a more accurate model in predicting the long-term OS than EWGSOP2-sarcopenia model (Figure 3).
In recent years, the quantity of cancer patients has been rising, especially in the rapidly aging population[13]. The morbidity of gastric cancer patients ranks third in China and seems to be much higher than the world average, while advanced gastric cancer accounts for a considerable proportion. Hypermetabolism (decreased muscle strength and muscle depletion) inevitably occurs in tumor patients. In such case, substantial cancer patients are accompanied by an age-associated muscle disease, namely, sarcopenia[14]. Multiple cancer studies indicated that sarcopenia was independently interrelated to poor long-term prognosis, such as gastric, hepatic, colorectal, gynecologic, and pancreatic carcinomas[15-18]. As our results exhibited, sarcopenia was a significant predictor of the long-term OS, thus preoperative detection of sarcopenia may help implement clinical interventions for improving the long-term OS. Despite racial and physical similarities, the wide-ranging prevalence of sarcopenia in Asian studies was caused by different diagnostic algorithms. The present observational study was the first to compare the effect of sarcopenia diagnosed by AWGS2019 and EWGSOP2 on the long-term OS of Chinese gastric cancer patients after radical gastrectomy.
In comparison with EWGSOP1, the most remarkable change of EWGSOP2 was that low muscle strength was used to replace low muscle mass, because low muscle strength was recognized to be a more powerful predictor of increased functional limitations, longer hospital stays, poorer health-related quality of life, and death[2,19]. Several studies indicated that hand-grip strength correlated moderately with overall muscle strength, physical fitness, and health status[20-25]. According to these findings, muscle strength seemed to be the prerequisite of physical functioning and should be deemed as the primary diagnostic feature for sarcopenia. In our clinical center, hand-grip strength is routinely measured. Considering its quick, simple, and highly reproducible measure, hand-grip strength could serve as a reliable surrogate instead of more complicated measures of arm or leg strength[26].
Unlike radical change of EWGSOP2 from EWGSOP1, AWGS2019 was slightly revised from AWGS2014. The sarcopenia diagnosis algorithm remains the same. Diagnosing sarcopenia requires low muscle quality and quantity, while diagnosing severe sarcopenia requires patients with low muscle mass, low muscle strength, and low physical performance simultaneously. The discrepancy between the new version and the old one was the cut-offs of muscle strength and physical performance. Numerous published and unpublished data of hand-grip strength from East and Southeast Asia communities have been available since AWGS2014 was proposed. AWGS2019 suggested that low muscle strength diagnostic cutoffs were < 18.0 kg for women and < 28.0 kg for men[27]. Gait speed, as the most frequently used assessment of the physical performance, was intensely correlated with the onset of disability, severe mobility limitation, and mortality[28-32]. Gait speed < 1 m/s could independently predict cognitive decline, dementia risk, frailty, and functional disability[33,34]. Several relevant Asian sarcopenia data exhibited a similar lowest quintile for gait speed (< 1 m/s). Therefore, AWGS2019 increased the cut-off of low gait speed from 0.8 m/s to < 1.0 m/s.
Cross-sectional abdominal CT scans were routinely applied for assessing gastric tumors before surgery. CT is currently considered the non-invasive gold standard for muscle quantity/mass assessment[35]. In particular, CT images of a lumbar vertebra (L3) correlated significantly with whole-body muscle mass[14,36]. Along with the deep-going research, skeletal muscle mass was no more sufficient to represent the unique parameter for diagnosing sarcopenia. And some researchers pointed out that when coming to defining sarcopenia in cancer surgery research, the investigators should focus on muscle function and physical performance as well as muscle mass[37]. Therefore, muscle strength and physical performance were important components of sarcopenia according to EWGSOP2[2,4]. Martin et al[38] and Prado et al[39] had proposed their own cut-off values for muscle mass which were now widely used, and especially, these values were based on Canadian patients. Compared with Eastern populations, Western populations generally have a higher BMI and larger physique. These factors rendered that these values were unsuitable for direct application in Asian populations and should be modified based on ethnicity and tumor type[15,40].
The latest version EWGSOP2 and AWGS2019 criteria have some substantial differences, for example, muscle strength is the precondition of sarcopenia diagnosis in EWGSOP2, while muscle mass occupies the same status in AWGS2019. And the cut-offs of each component are slightly different. Moreover, comparing the two versions of sarcopenia criteria, the model with AWGS2019-sarcopenia seemed to have better predictive power for survival than the model with EWGSOP2-sarcopenia [C-index 0.773 (0.742-0.804) vs 0.762 (0.729-0.795)]. The prevalence of sarcopenia with AWGS2019 was 20.5%, 9.3% higher than that of EWGSOP2. AWGS2019-sarcopenia also led to a greater HR in the multivariate model than EWGSOP2-sarcopenia [HR 2.150 (1.547-2.988) vs 1.599 (1.092-2.339)]. These results suggested that the updated definition and diagnosis of sarcopenia by AWGS2019 are more suitable for Chinese gastric cancer patients and could improve the predictive ability of the long-term OS.
This observational study of the data revealed that sarcopenia diagnosis with any version of criteria had a negative effect on the long-term OS in gastric cancer cases. Our results highlighted the requirement of proper sarcopenia assessment for particular individuals as it had potential to predict the long-term OS and affected future clinical decision making. What’s more, the model with sarcopenia defined by AWGS2019 had better predictive power on the long-term OS than the model with EWGSOP2 in Chinese patients with gastric cancer after radical gastrectomy. In the future, the clinical interventions according to these criteria require further evaluation in prospective clinical trials.
The present study inevitably has some limitations. First of all, our conclusions were drawn from single-center patients with gastric cancer. The prognostic significance of sarcopenia established here needs further validation in other centers. Second, although the 3 years following surgery is a period with high mortality risk for gastric cancer patients, the applied follow-up period in the study (median follow-up time: 1630 d) seems not long enough and the longer-term OS with sarcopenia requires further verification. Moreover, surgeons often treated sarcopenia patients with high protein treatment prior to surgery due to poor nutritional status in sarcopenia patients. These individual nutritional interventions may diminish the effect of sarcopenia on the long-term OS.
In conclusion, sarcopenia based on both criteria is an independent risk factor for the long-term OS in Chinese gastric cancer patients undergoing radical gastrectomy. The prediction model with AWGS2019-sarcopenia has better predictive power and model fits than the prediction model with EWGSOP2-sarcopenia. AWGS2019 may be more appropriate for diagnosing sarcopenia in these Chinese patients than EWGSOP2. Surgeons should be aware of the importance of sarcopenia and pay close attention to the nutrition of these patients. Corresponding clinical interventions to ameliorate the effect of sarcopenia on the long-term OS need further evaluation in prospective clinical trials.
Sarcopenia is a nutrition-related disease and has a profound effect on the long-term overall survival (OS) of patients with gastric cancer. Previous studies reported widely differing sarcopenia prevalence due to different criteria. AWGS2019 and EWGSOP2 are two latest and extensively adopted criteria.
To compare the effects of these two criteria on the long-term OS of Chinese gastric cancer patient after radical gastrectomy.
To determine which method for diagnosing sarcopenia is more appropriate for Chinese gastric cancer patient after radical gastrectomy.
An observational study was conducted from July 2014 to January 2017. Multivariate cox regression analysis was performed to determine the association of sarcopenia according to these two criteria separately with the long-term OS of these patients. The predictive performance of the models with AWGS2019 and EWGSOP2 was evaluated using the concordance index and area under the time-dependent receiver operating characteristic curve. The Akaike information criterion was used to compare model fits.
Sarcopenia was an independent risk factor for the long-term OS no matter based on AWGS2019 or EWGSOP2, but AWGS2019-sarcopenia in multivariate model had a higher hazard ratio than EWGSOP2-sarcopenia. Meanwhile, the model with AWGS2019-sarcopenia had better predictive power and model fits than the model with EWGSOP2-sarcopenia.
Sarcopenia based on both criteria is an independent risk factor for the long-term OS in Chinese patients undergoing radical gastrectomy for gastric cancer. The prediction model with AWGS2019-sarcopenia has better predictive power and model fits than the prediction model with EWGSOP2-sarcopenia. AWGS2019 may be more appropriate for diagnosing sarcopenia in these Chinese patients than EWGSOP2.
Surgeons should be aware of the importance of sarcopenia and pay close attention to the nutrition of these patients. Corresponding clinical interventions to ameliorate the effect of sarcopenia on the long-term OS need further evaluation in prospective clinical trials.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Keikha M, Wang X, Watanabe D S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 2. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2); and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 599] [Cited by in RCA: 1502] [Article Influence: 250.3] [Reference Citation Analysis (0)] |
| 3. | O'Brien S, Twomey M, Moloney F, Kavanagh RG, Carey BW, Power D, Maher MM, O'Connor OJ, Ó'Súilleabháin C. Sarcopenia and Post-Operative Morbidity and Mortality in Patients with Gastric Cancer. J Gastric Cancer. 2018;18:242-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8472] [Article Influence: 564.8] [Reference Citation Analysis (0)] |
| 5. | Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2330] [Cited by in RCA: 2968] [Article Influence: 269.8] [Reference Citation Analysis (0)] |
| 6. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 7. | van der Werf A, Langius JAE, de van der Schueren MAE, Nurmohamed SA, van der Pant KAMI, Blauwhoff-Buskermolen S, Wierdsma NJ. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr. 2018;72:288-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 8. | Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, Ma LL, Yu Z, Shen X. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine (Baltimore). 2016;95:e3164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 9. | Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020; 21: 300-307. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2739] [Cited by in RCA: 3797] [Article Influence: 759.4] [Reference Citation Analysis (0)] |
| 10. | Pérez-Morales J, Tunali I, Stringfield O, Eschrich SA, Balagurunathan Y, Gillies RJ, Schabath MB. Peritumoral and intratumoral radiomic features predict survival outcomes among patients diagnosed in lung cancer screening. Sci Rep. 2020;10:10528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Weiss A, Chavez-MacGregor M, Lichtensztajn DY, Yi M, Tadros A, Hortobagyi GN, Giordano SH, Hunt KK, Mittendorf EA. Validation Study of the American Joint Committee on Cancer Eighth Edition Prognostic Stage Compared With the Anatomic Stage in Breast Cancer. JAMA Oncol. 2018;4:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 12. | Liu J, Huang X, Yang W, Li C, Li Z, Zhang C, Chen S, Wu G, Xie W, Wei C, Tian C, Huang L, Jeen F, Mo X, Tang W. Nomogram for predicting overall survival in stage II-III colorectal cancer. Cancer Med. 2020;9:2363-2371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Katanoda K, Yako-Suketomo H. Comparison of time trends in stomach cancer incidence (1973-2002) in Asia, from Cancer Incidence in Five Continents, Vols IV-IX. Jpn J Clin Oncol. 2009;39:71-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 3842] [Article Influence: 274.4] [Reference Citation Analysis (0)] |
| 15. | Wu CH, Chang MC, Lyadov VK, Liang PC, Chen CM, Shih TT, Chang YT. Comparing Western and Eastern criteria for sarcopenia and their association with survival in patients with pancreatic cancer. Clin Nutr. 2019;38:862-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Bronger H, Hederich P, Hapfelmeier A, Metz S, Noël PB, Kiechle M, Schmalfeldt B. Sarcopenia in Advanced Serous Ovarian Cancer. Int J Gynecol Cancer. 2017;27:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Yoshida M, Watanabe M, Baba H. Sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann Surg Oncol. 2015;22:2663-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 278] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 18. | Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y, Nishie A, Yamanaka T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 397] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 19. | Schaap LA, van Schoor NM, Lips P, Visser M. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci. 2018;73:1199-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 20. | McGrath RP, Kraemer WJ, Snih SA, Peterson MD. Handgrip Strength and Health in Aging Adults. Sports Med. 2018;48:1993-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, Iliodromiti S, Sillars A, Graham N, Mackay DF, Pell JP, Gill JMR, Sattar N, Gray SR. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 325] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 22. | Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 1094] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 23. | Close JC, Lord SR, Antonova EJ, Martin M, Lensberg B, Taylor M, Hallen J, Kelly A. Older people presenting to the emergency department after a fall: a population with substantial recurrent healthcare use. Emerg Med J. 2012;29:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37:1849-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 25. | Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 975] [Cited by in RCA: 1019] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 26. | Kuh D, Bassey EJ, Butterworth S, Hardy R, Wadsworth ME; Musculoskeletal Study Team. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. J Gerontol A Biol Sci Med Sci. 2005;60:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Auyeung TW, Arai H, Chen LK, Woo J. Letter to the editor: Normative data of handgrip strength in 26344 older adults - a pooled dataset from eight cohorts in Asia. J Nutr Health Aging. 2020;24:125-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Matsumoto H, Tanimura C, Tanishima S, Hagino H. Association between speed of sound of calcaneal bone assessed by quantitative ultrasound and sarcopenia in a general older adult population: A cross-sectional study. J Orthop Sci. 2019;24:906-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Yang M, Hu X, Xie L, Zhang L, Zhou J, Lin J, Wang Y, Li Y, Han Z, Zhang D, Zuo Y. Comparing Mini Sarcopenia Risk Assessment With SARC-F for Screening Sarcopenia in Community-Dwelling Older Adults. J Am Med Dir Assoc. 2019;20:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Kim S, Kim M, Won CW. Validation of the Korean Version of the SARC-F Questionnaire to Assess Sarcopenia: Korean Frailty and Aging Cohort Study. J Am Med Dir Assoc 2018; 19: 40-45. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 31. | Kera T, Kawai H, Hirano H, Kojima M, Fujiwara Y, Ihara K, Obuchi S. Differences in body composition and physical function related to pure sarcopenia and sarcopenic obesity: A study of community-dwelling older adults in Japan. Geriatr Gerontol Int. 2017;17:2602-2609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Tanaka S, Kamiya K, Hamazaki N, Matsuzawa R, Nozaki K, Maekawa E, Noda C, Yamaoka-Tojo M, Matsunaga A, Masuda T, Ako J. Utility of SARC-F for Assessing Physical Function in Elderly Patients With Cardiovascular Disease. J Am Med Dir Assoc. 2017;18:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Taniguchi Y, Kitamura A, Seino S, Murayama H, Amano H, Nofuji Y, Nishi M, Yokoyama Y, Shinozaki T, Yokota I, Matsuyama Y, Fujiwara Y, Shinkai S. Gait Performance Trajectories and Incident Disabling Dementia Among Community-Dwelling Older Japanese. J Am Med Dir Assoc 2017; 18: 192.e13-192. e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2197] [Cited by in RCA: 2312] [Article Influence: 165.1] [Reference Citation Analysis (0)] |
| 35. | Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, Bautmans I, Bertière MC, Brandi ML, Al-Daghri NM, Burlet N, Cavalier E, Cerreta F, Cherubini A, Fielding R, Gielen E, Landi F, Petermans J, Reginster JY, Visser M, Kanis J, Cooper C. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 519] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 36. | Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1653] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 37. | Binay Safer V, Safer U, Kaplan M, Terekeci H, Top C. Limitations of the definition of sarcopenia in cancer surgery. J Surg Oncol. 2015;112:116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1512] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 39. | Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1822] [Cited by in RCA: 2380] [Article Influence: 140.0] [Reference Citation Analysis (0)] |
| 40. | Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J Surg Oncol. 2015;112:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |