Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4654
Peer-review started: January 5, 2021
First decision: January 27, 2021
Revised: February 4, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: June 26, 2021
Processing time: 156 Days and 10.4 Hours
Coronavirus disease 2019 (COVID-19) started in Asia, and Iran was one of its first epicenters.
To study the gastrointestinal (GI) symptoms and comorbidities associated with this pandemic in four different regions of Iran.
We analyzed data from severe acute respiratory syndrome coronavirus 2 positive patients evaluated at four hospitals of Iran (n = 91), including South (Shiraz), Southeast (Dezful), Rasht (North), and Northwest (Mashhad) between April and September 2020. Demographics, comorbidities and clinical findings including GI symptoms were collected. Statistical descriptive analysis and correlation analyses of symptoms, comorbidities, and mortality were performed.
The average age of COVID-19 patients was 51.1 years, and 56% were male. Mortality rate was 17%. Cough with 84.6%, shortness of breath with 71.4%, fever with 52.7%, and loss of appetite with 43.9% were the main symptoms. Overall cardiac disease was the most common comorbidity with an average of 28.5% followed by hypertension (28.5%) and diabetes (25.2%). The highest comorbidity in North (Rasht) was diabetes (30%) and in South (Dezful) hypertension (37%). Shiraz leads cardiac disease with 43.4%. The most reported GI symptoms included nausea, diarrhea, vomiting, and abdominal pain, with 42.8%, 31.8%, 26.8%, and 12% prevalence, respectively. In addition, albumin, alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase were elevated in 26.3%.
Our results show hypertension and diabetes as the most common comorbidities, but their distribution was different in COVID-19 patients in the four studied regions of Iran. Nausea, diarrhea, and elevated liver enzymes were the most common GI symptoms. There was also a high mortality rate that was associated with high infection rates in Iran at the beginning of the pandemic.
Core Tip: The location of the places the coronavirus disease 2019 (COVID-19) impacted in the world varies based on many social and economic factors. We chose and studied four different sites from Iran and determined that hypertension and diabetes as the most common comorbidities, but their distribution was different in COVID-19 patients in the four studied regions of Iran. Nausea, diarrhea, and elevated liver enzymes were the most common gastrointestinal symptoms.
- Citation: Mokarram P, Dalivand MM, Pizuorno A, Aligolighasemabadi F, Sadeghdoust M, Sadeghdoust E, Aduli F, Oskrochi G, Brim H, Ashktorab H. Clinical characteristics, gastrointestinal manifestations and outcomes of COVID-19 patients in Iran; does the location matters? World J Clin Cases 2021; 9(18): 4654-4667
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4654.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4654
Over the last two decades, the emergence of diseases, such as severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) (2002 in China) with a case fatality rate of over 15%[1] and Middle East Respiratory Syndrome (June 2012 in Saudi Arabia) with 43% case fatality rate[2], and were considered public health challenges[2]. The coronavirus disease 2019 (COVID-19), which is the result of the infection by SARS-CoV-2, was first reported in Wuhan in December 2019. It achieved a pandemic status since the WHO declared that the virus was present in more countries with more than 70 million cases and 16 million deaths by December 15, 2020[3,4]. On February 7, 2020, a 75-year old man presented to a Hospital Emergency Department in Qom Iran[5] with a new onset of fever and non-productive cough with no recent travel. This patient was the first COVID-19 case diagnosed in Iran. Iran, with more than 80 million inhabitants, is one of the most populous countries in the Middle East with a high prevalence of chronic and metabolic diseases[5]. It is expected to be one of the most affected countries in the region during the pandemic. According to the evolving data compiled by Johns Hopkins University[3,4], Iran has reported more than 16 million diagnosed cases and more than 56000 deaths (accessed December 18, 2020). The local government has imposed safety regulations such as mandatory mask in public places, social distancing, restaurants and sport centers closure, limitations on religious gathering places, and traffic restriction after 6 pm.
COVID-19 was represented primarily as a respiratory tract infection, but a different array of symptoms have been described along with the evolution of the pandemic. The disease symptoms can range from mild to severe. Severe symptoms associate with critical illness resulting in respiratory failure or multiorgan dysfunction and/or death[6]. Fever and cough remain the most prevalent symptoms in adults. However, gastrointestinal (GI) symptoms are also encountered[7]. The underlying causes of the variability of COVID-19-related symptoms and their potential association to different outcomes have not been defined yet. Several studies demonstrated that the variability in incidence and manifestations of COVID-19 in different populations could be attributed to the human genome differences. Studies have been conducted in Asian and European populations. However, differences associated to specific populations present issues with regard to generalizing findings.
The development of COVID-19 symptoms and the behavior of chronic comor
We collected the de-identified data from the medical records of 91 COVID-19 hospitalized patients between March and September 2020 in four different locations, including south (Shiraz from single Hospital, Ali Asghar Hospital), Northeast [Mashhad from (Bahman Hospital), Southeast (Dezful from Ganjavian Hospital), and North (Rasht from Razi Hospital)] of Iran. Local IRB approval was obtained from the health authority in each hospital for the chart review. All patients had COVID-19 confirmed by positive SARS-CoV-2 polymerase chain reaction (PCR) test. The outcome was defined as either discharge from hospital or death.
The following inclusion criteria were considered to select the patients: Confirmed detection of virus by positive PCR test and chest computed tomography scan, no distinction on number of adult (≥ 18 years) patients, no distinction on sex, treatment, manifestations, and morbidities or outcome.
Patients who were not confirmed by PCR test, age < 18 and > 85 years old, and patients with incomplete medical reports were excluded from this study.
From the de-identified data, tables were generated for each cohort on Microsoft Excel that included the following information: Location, confirmed cases, deaths, median age, symptoms (cough, fever, loss of appetite, nausea, fatigue, myalgia, diarrhea, vomiting, dysphagia, loss of taste), comorbidities (cardiac disease, hypertension, diabetes, luminal gastrointestinal disease, immunocompromised status), smoking, alcohol intake, history of disease [liver disease, inflammatory bowel disease (IBD), acquired immunodeficiency syndrome, pancreatitis, cancer, and laboratory paraclinical values blood type, alkaline phosphatase (ALP), albumin, international normalized ratio (INR), platelet count, white blood cell count, interleukin-6 (IL-6), total and direct bilirubin, creatinine, creatine phosphokinase (CPK), C-reactive protein (CRP), lactate dehydrogenase (LDH), and liver function tests], and also other parameters such as body mass index (BMI), oxygen saturation, transfer to intensive care unit, length of stay, and treatment (hydroxychloroquine, tocilizumab, remdesivir, convalescent plasma, intubation, and mechanical ventilation).
Patient demographics, symptoms, underlying comorbidities, treatment, and outcomes were compared in infected patients from Iran. The common symptoms and comorbidities were analyzed by weighted analysis methods where applicable. Correlation coefficients were calculated together with regression analysis to establish associations between comorbidities and death as an outcome. The effect of symptoms was reported using weighted analysis where weights were related to the size of each of the local cohorts (Shiraz, Mashhad, Dezful, and Rasht). SPSS (SPSS Inc., Chicago, IL, United States) was used for this analysis.
There were 91 inpatients (hospitalized) confirmed cases in our study from four different cities of Iran (Shiraz, Mashhad, Dezful, Rasht; Table 1). The largest cohort is Dezful with 35 patients, followed by Shiraz with 23 patients, Rasht with 20 patients and Mashhad with 13 patients. The overall number of deaths was 13 patients. Symptoms and comorbidities of our patients is summarized in Table 1. The mean duration of symptoms before going to the emergency room was 2.8 d overall with an average length of stay at the hospital of 6.8 d (the most Mashhad with 12.3 d in average). For patients that reported GI manifestations, these symptoms started 1.2 d after the typical initial COVID-19 symptoms. The most prescribed treatment was hydroxychloroquine/chloroquine on 86.8% of the patients, followed by vasopressor and glucocorticoids with 9% (for each).
| Overall, n = 91 | Shiraz, n = 23 | Mashhad, n = 13 | Dezful, n = 35 | Rasht, n = 20 | |
| Sex | |||||
| Male | 51 (56) | 13 (56) | 2 (15) | 21 (60) | 15 (75) |
| Female | 40 (44) | 10 (44) | 11 (85) | 14 (40) | 5 (25) |
| Average age | 51 | 51 | 52 | 53 | 48 |
| BMI > 25 kg/m2, % (n) | 26.3 (78) | 26 (20) | 25.9 (12) | 26.6 (29) | 26.4 (17) |
| Pneumonia % (n) | 100 (91) | 100 (23) | 100 (13) | 100 (35) | 100 (20) |
| Cough | 84.6 (77) | 82.6 (19) | 69.2 (9) | 88.5 (31) | 90 (18) |
| Shortness of breath | 71.4 (65) | 69.5 (16) | 53.8 (7) | 80 (28) | 70 (14) |
| Fever | 52.7 (48) | 73.9 (17) | 30.7 (4) | 42.8 (15) | 60 (12) |
| Loss of appetite | 43.9 (40) | 47.8 (11) | 38.4 (5) | 48.5 (17) | 35 (7) |
| Nausea | 42.8 (39) | 56.5 (13) | 23 (3) | 42.8 (15) | 40 (8) |
| Fatigue | 41.7 (38) | 39.1 (9) | 30.7 (4) | 60 (21) | 20 (4) |
| Myalgia | 32.9 (30) | 21.7 (5) | 23 (3) | 51.4 (18) | 20 (4) |
| Diarrhea | 31.8 (29) | 34.7 (8) | 38.4 (5) | 28.5 (10) | 30 (6) |
| Vomiting | 26.3 (24) | 47.8 (11) | 15.3 (2) | 22.8 (8) | 15 (3) |
| Abdominal pain | 12 (11) | 8.7 (2) | 2 (3) | 11.4 (4) | 10 (2) |
| GI bleed | 5.4 (5) | 0 | 0 | 8.5 (3) | 10 (2) |
| Cholecystitis | 3.3 (3) | 4.3 (1) | 0 | 2.8 (1) | 5 (1) |
| Hepatomegaly | 3.3 (3) | 4.3 (1) | 0 | 5.7 (2) | 0 |
| Alcohol | 2.2 (2) | 0 | 0 | 5.7 (2) | 0 |
| Cardiac disease | 28.5 (26) | 43.4 (10) | 7.6 (1) | 31.4 (11) | 20 (4) |
| Hypertension | 28.5 (26) | 13 (3) | 30.7 (4) | 37.1 (13) | 30 (6) |
| Diabetes | 25.2 (23) | 34.7 (8) | 7.6 (1) | 22.8 (8) | 30 (6) |
| Luminal GI disease | 23 (21) | 26 (6) | 23 (3) | 17.1 (6) | 30 (6) |
| Immunocompromised | 20.8 (19) | 17.3 (4) | 46.1 (6) | 20 (7) | 10 (2) |
| Smoking | 19 (17) | 10 (2) | 15.3 (2) | 28.1 (10) | 15.7 (3) |
| H/O liver disease | 10.9 (10) | 8.7 (2) | 15.3 (2) | 11.4 (4) | 10 (2) |
| H/O GERD/PUD | 8.7 (8) | 13 (3) | 0 | 5.7 (2) | 15 (3) |
| H/O alcohol abuse | 1.1 (1) | 0 | 0 | 2.8 (1) | 0 |
| H/O IBD | 1.1 (1) | 0 | 0 | 2.8 (1) | 0 |
| Abnormal CT chest | 91.8 (79) | 85.7 (19) | 84.6 (11) | 96.9 (32) | 94.7 (19) |
| Abnormal O2 sat in admission | 64.8 (59) | 43.4 (10) | 61.5 (8) | 77.1 (27) | 70 (14) |
| Abnormal O2 sat at 24 h day 1 | 42.8 (39) | 30.4 (7) | 38.4 (5) | 51.4 (18) | 45 (9) |
| Abnormal O2 sat at 48 h day 2 | 39 (34) | 17.3 (4) | 40 (4) | 50 (17) | 45 (9) |
| Abnormal O2 sat at 72 h day 3 | 37.9 (33) | 26 (6) | 30 (3) | 47 (16) | 40 (8) |
| Abnormal ALT | 27.4 (25) | 30.4 (7) | 15.3 (2) | 28.5 (10) | 30.4 (6) |
| Abnormal AST | 23 (21) | 26 (6) | 15.3 (2) | 22.8 (8) | 25 (5) |
| Abnormal albumin | 22 (20) | 13 (3) | 15.3 (2) | 31.4 (11) | 20 (4) |
| Abnormal creatinine | 43(36) | 41(9) | 42(5) | 43(15) | 47(7) |
| Hydroxychloroquine/chloroquine | 86.8 (79) | 91.3 (21) | 69.2 (9) | 94.2 (33) | 80 (16) |
| Mechanical ventilation | 18.6 (17) | 17.3 (4) | 38.4 (5) | 11.4 (4) | 20 (4) |
| ICU transfer | 15.3 (14) | 17.3 (4) | 15.3 (2) | 17.1 (6) | 10 (2) |
| Admitted to floor or ICU | 14.2 (13) | 13 (3) | 15.3 (2) | 17.1 (6) | 10 (2) |
| Vasopressor support | 9.8 (9) | 8.7 (2) | 0 | 17.1 (6) | 5 (1) |
| Glucocorticoids | 9.8 (9) | 8.7 (2) | 23 (3) | 11.4 (4) | 0 |
| Nursing home patient | 4.4 (4) | 4.3 (1) | 7.6 (1) | 2.8 (1) | 5 (1) |
| Health worker | 2.2 (2) | 4.3 (1) | 0 | 2.8 (1) | 0 |
| Known exposure | 10.9 (10) | 13 (3) | 15.3 (2) | 11.4 (4) | 5 (1) |
| Death | 14.2 (13) | 13 (3) | 15.3 (2) | 17.1 (6) | 10 (2) |
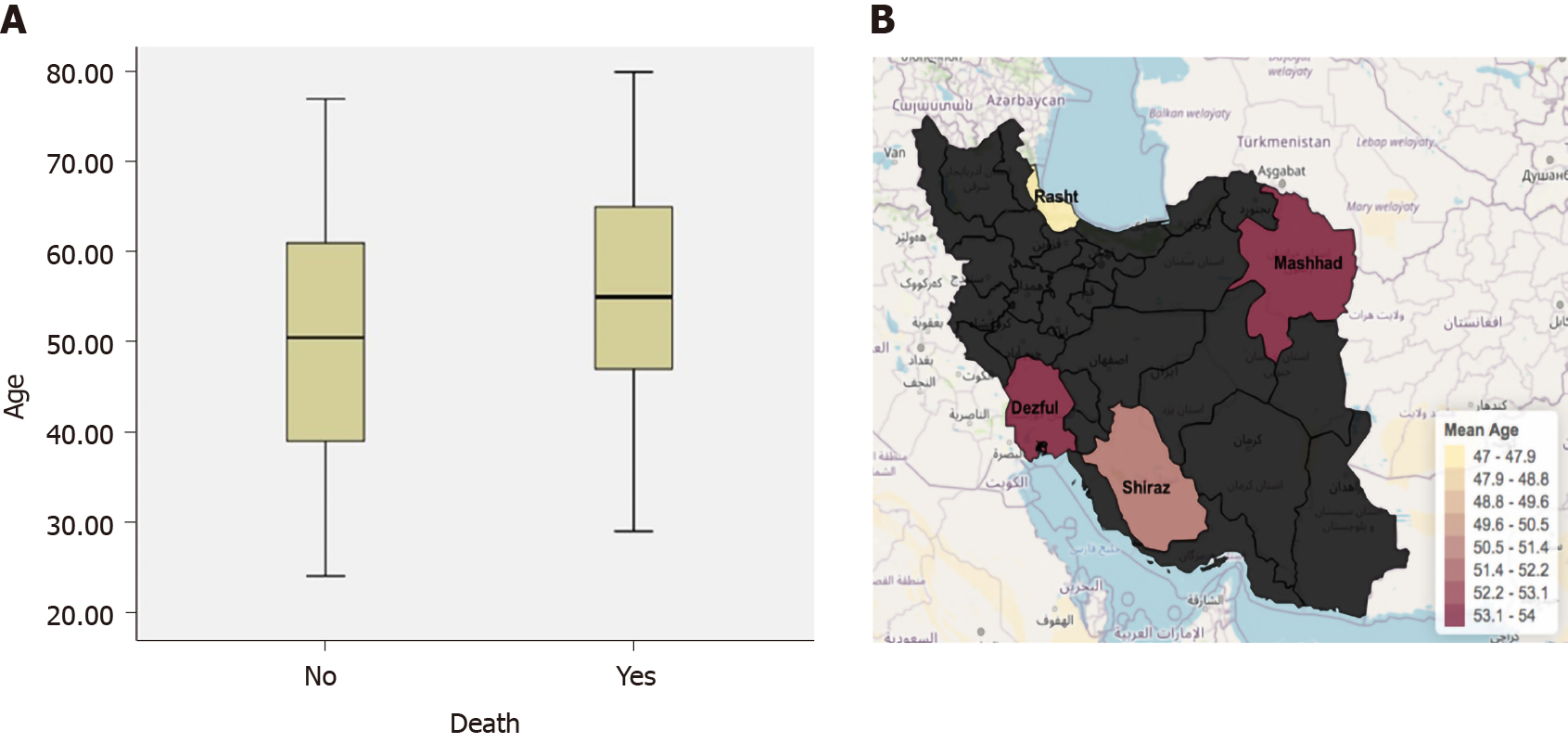
The average age for this Iran COVID-19 cohort was 51.1 years old (Table 1). Age ranged from 24-80 years old. There were age differences in this cohort of COVID-19 patients in 4 different cities (Figure 1). The cohort from Rasht was the youngest with an average age of 47 years old. Dezful and Mashhad both had the older age average with 53.2 years and Shiraz with a mean of 50.6 years old.
There was no association between age and mortality (P = 0.26). The mean age of those that passed away due to COVID-19 in this cohort was 55.2 years old, while those that survived were almost five years younger with mean age of 50.5 years old.
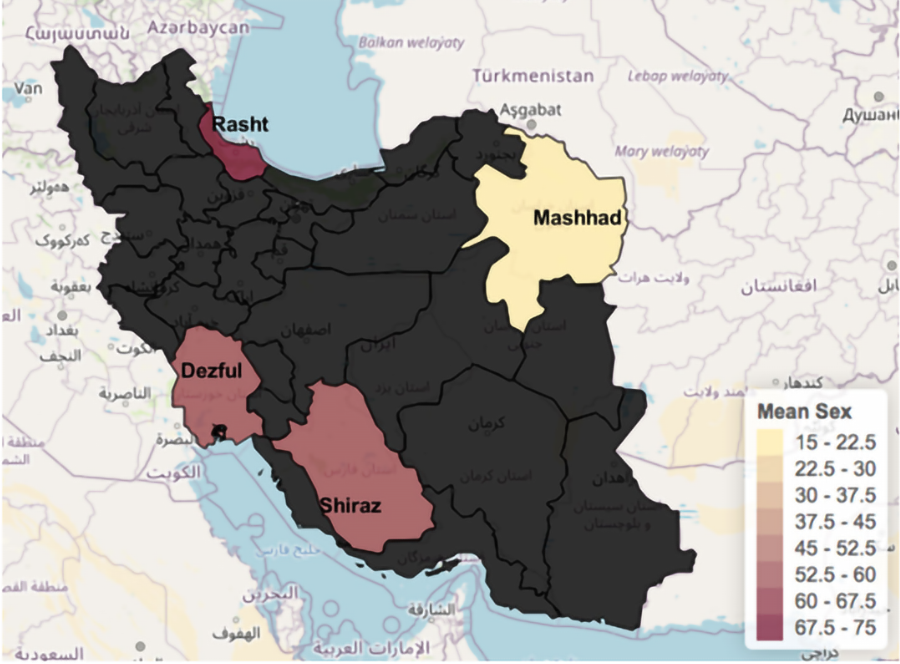
The distribution between males and females was 56% (n = 51) vs 44% (n = 40), respectively. Males’ infection rate was highest (56.8%) in Rasht (75%) and Dezful (60%), while Mashhad reported the lowest rate (15.4%, Figure 2). However, the four cohorts had comparable overall sex distributions. There was no significant association between sex and mortality (P = 0.66; Table 1). Overall, in our cohort, more males (8 patients) than females (5 patients) died due to COVID-19.
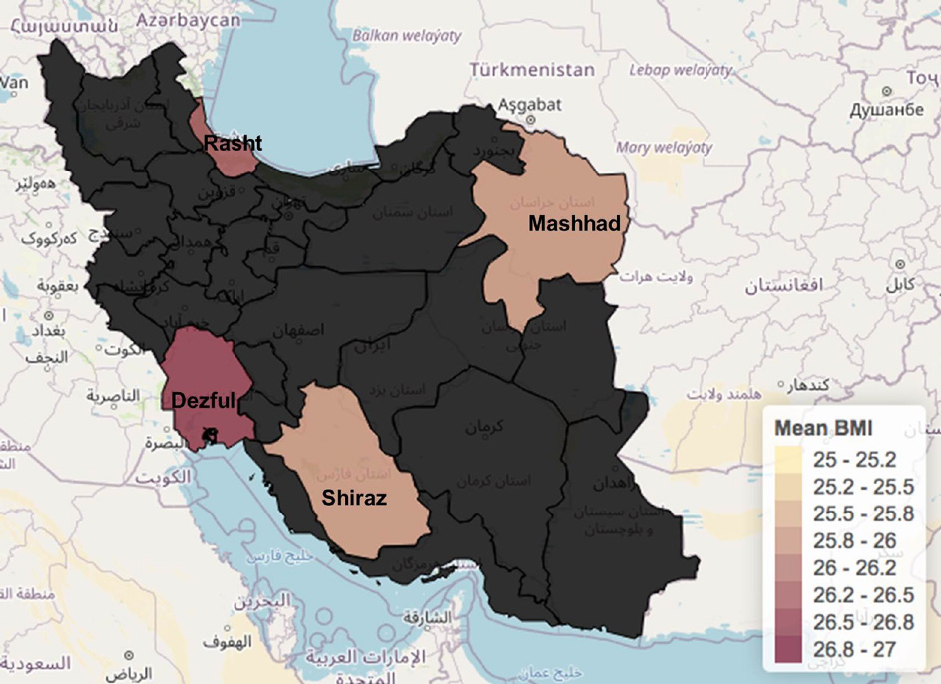
The mean BMI for the population of the study was 26.3. Overall, 53.8% (n = 78) of the cohort was overweight 25 < BMI < 30), 30.8% (n = 22) had a normal BMI (< 25) and 15.4% of the cohort were obese (BMI > 30). Regarding the distribution of the population according to BMI status, Rasht had the highest overweight rate with 58.8% (n = 10), and 11.8% (2/17) with obesity (mean BMI is 26.4). Dezful had 55.2% (16/29) overweight and the most obese population of the study with 17.2% (5/12) obese (mean BMI is 26.6 kg/m2). Mashhad had 41.7% overweight and (5/12) 16.7% obese (mean BMI is 25.9) and Shiraz had 55% (11/20) overweight and 20% (4/20) obese patients (mean BMI is 26 kg/m2). The Pearson correlation showed that obesity is not significantly associated with mortality (P = 0.13) (Figure 3).
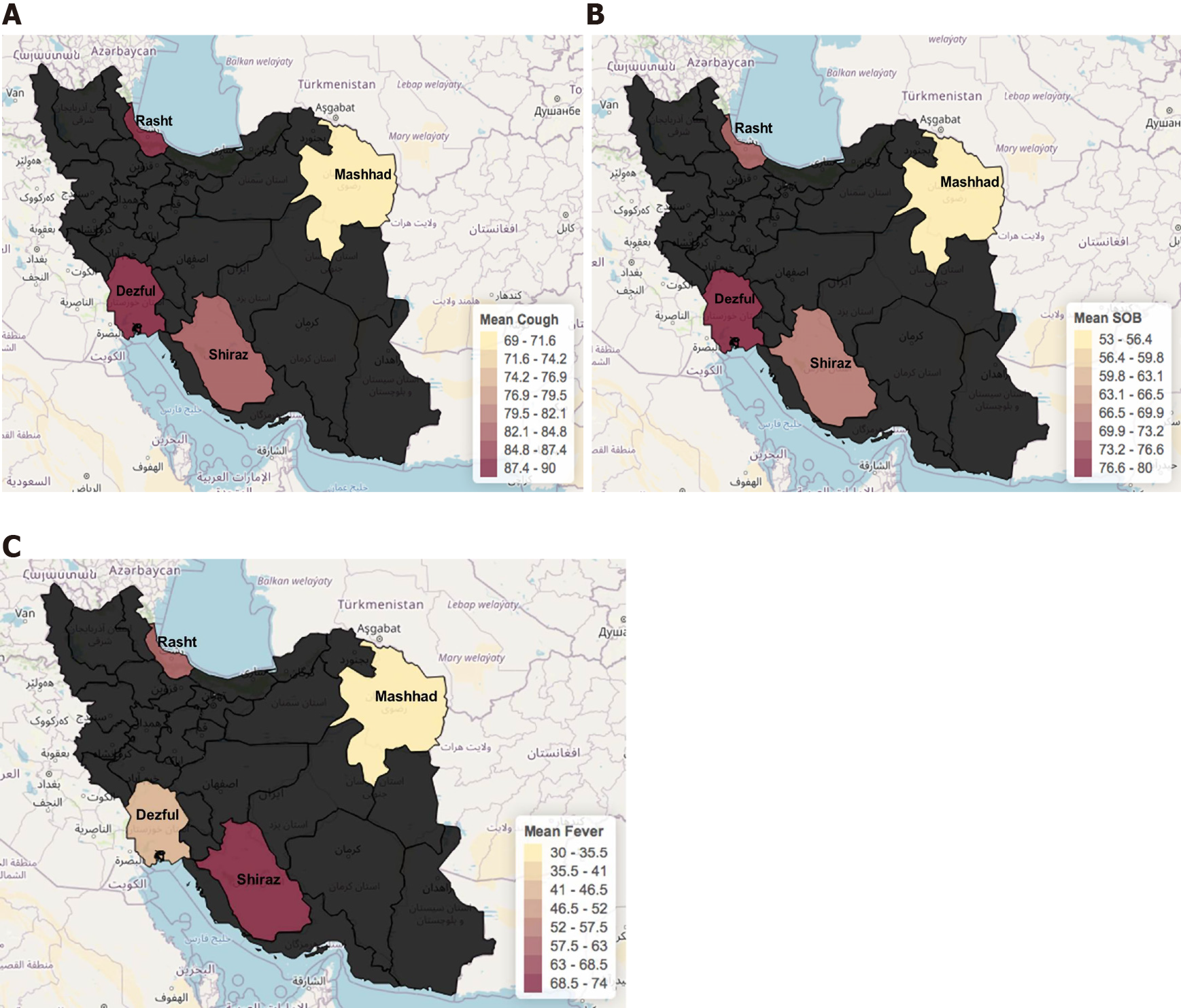
According to the combined overall weighted averages, the most common symptoms reported in the four Iranian cities for positive cases of SARS-CoV-2 infection were cough with 84.6% (n = 77), shortness of breath with 71.4% (n = 65), fever with 52.7% (n = 48), and loss of appetite with 43.9% (n = 40). Rasht was reported to have the highest positivity for cough (Figure 4) 90% (n = 18); at least 2% more than Dezful in second place. Mashhad reported the lowest prevalence of cough in infected patients with 69.2%. Dezful (n = 28) reported the highest prevalence for shortness of breath followed by Rasht with 80% (n = 14) and 70%, respectively.
Fever was the third prevalent symptom in Iranian COVID-19 patients. While fever is probably one of the most common symptoms for COVID-19, that was not the case for the population we studied in Iran. Fever was reported in 52.7%, while the loss of appetite was reported in 43.9%. Nausea and fatigue were also significant symptoms with 42.8% and 41.7%, respectively.
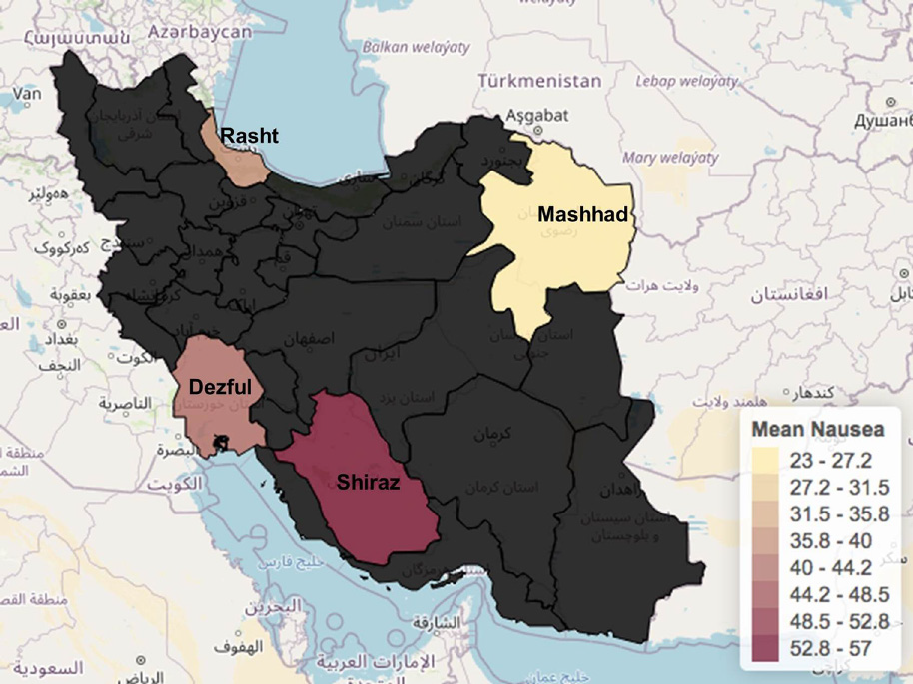
Our cohort of patients reported a different array of gastrointestinal symptoms, including diarrhea, abdominal pain, nausea, and vomiting. Nausea was the most reported symptom being present in 42.8% of patients. Shiraz was the city with the highest prevalence 56.5% (n = 13), followed by Dezful with 42.8% (n = 15). Nausea was followed by diarrhea in 31.8% (n = 29) of patients, vomiting with 26.8% (n = 13), and lastly abdominal pain in 12% in the whole group analysis. As for gastrointestinal diseases and comorbidities, the prevalence of luminal gastrointestinal symptoms was 23% in positive cases followed by preexisting or history of liver disease in 10% of the cohort (Figure 5).
There is a different array of findings when comparing the liver characteristics on the cohort. Regarding liver enzymes, 26.3% of the infected patients had abnormal levels of either albumin, ALP, AST, or ALT (24 patients). With regard to history of liver disease, 10.9% of the patients reported it. Overall, 8.7% had liver steatosis diagnosed by abdominal ultrasound, 3.3% with hepatomegaly, 1.1% with liver nodularity, 1.1% with liver fibrosis, and none with hepatitis B and C positive serology.
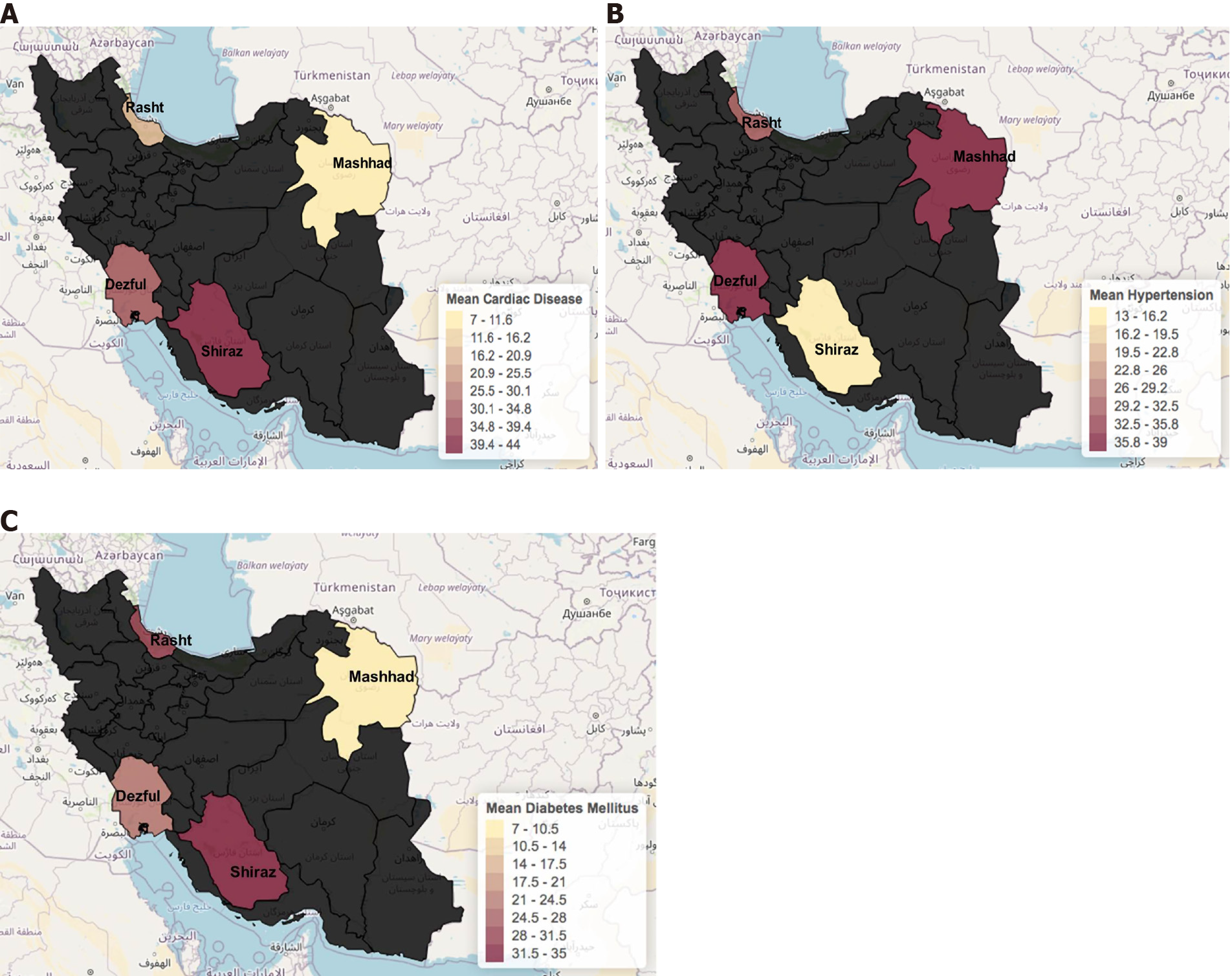
Cardiac disease and hypertension were the most common comorbidities with a prevalence of 28.5% (n = 26) followed by diabetes with 25.2% (n = 23) (Table 1). The least common comorbidities were history of IBD in 1.1% of patients and history of cholecystitis in 3.3%. Overall, 28.5% of the cohort had at least one comorbidity. Differences for comorbidities were present among the different cities. Regarding the distribution for the comorbidities by city, we found: In Rasht, Dezful, and Mashhad hypertension was the most common comorbidity with 39%, 37.1%, and 30.7 % of patients, respectively. In Shiraz, 43.4% of patients had a history of cardiac disease (Figure 6).
Trends in the laboratory results were prominent for certain tests. The majority of the patients reported a normal platelet count and white blood cell count (84.6% and 86.8% respectively). Just a few patients reported a decreased platelets and white blood cell count (14.3% and 7.7%, respectively). With respect to inflammatory markers, IL-6 was elevated in 94.6% of the infected patients. CRP was elevated in 71.4% while CPK only in 35.7%, LDH was found to be normal in 60.9% of the cohort. Regarding the metabolic panel, we found that 63.7% of the cohort had a normal ALP while 28.6% had a decreased value for this test. A normal range for albumin was found in the majority of the patients (59.2% or 29 patients), while 38.8% (19 patients) had a decreased albumin. In respect of creatinine, 60.7% (51 patients) of the cohort had a normal result and 35.7% reported elevation (30 patients). It seems elevated creatinine is highly associated (P < 0.001) with mortality.
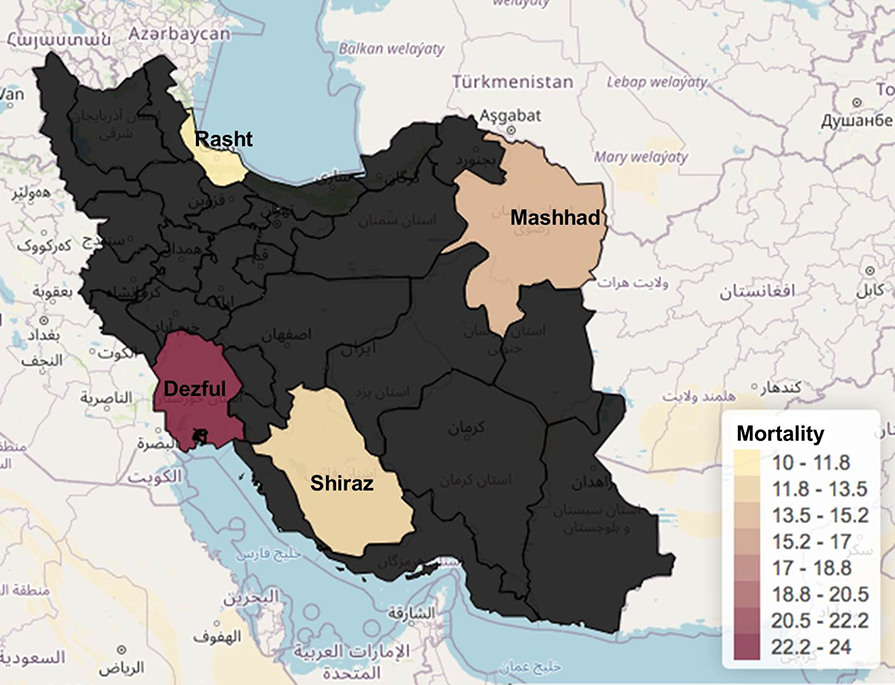
We analyzed death rates for the four cities, and there are some important differences between them. The overall weighted average for the total population of our study showed that the death rate was 14.2%. In relation to the stratification per city (Figure 7), there were mixed results, with Dezful reporting 24% deaths, followed by Mashhad with 15% and then Shiraz with 13%. Rasht reported the lowest death rate with 10%.
We explored the association of death rate with several variables such as oxygen saturation at admission, oxygen saturation 72 h post-admission, hypertension, obesity, hypertension, and diabetes. Each of these analyses were statistically significant except for hypertension, diabetes, and obesity. This analysis shows that cardiac disease and O2 saturation, especially on day three, are associated with mortality in COVID-19 patients in Iran.
We explored the relationship of death rate with several variables of the GI symptoms (abdominal pain, nausea, vomiting, diarrhea, and dysphagia). None of the symptoms individually or collectively was associated with death as an outcome in Iran using Pearson correlation.
In respect of the correlation between death and other laboratory parameters (such as AST, ALT, ALP, albumin, platelet count, white blood cell count, IL-6, total and direct bilirubin, creatinine, CPK, CRP, and LDH), the only variable that was significantly (P < 0.05) associated with death was the creatinine of our patients. While other variables had a trend that was not significant by Pearson correlation analysis.
The overall length of stay for our cohort was 6.8 d. Mashhad was the region in which the hospitalized patients remained longer under supervision, with 12.3 d on average. Rasht was the region in which patients had the shortest hospital stay with only 2.3 d. Pearson correlation analysis showed that the length of stay was not associated (not significant) with a negative outcome.
COVID-19 pandemic has so far caused more than 1700000 deaths worldwide, and unfortunately, there is still a long way to go before the pandemic will be under control and the effects of the vaccines will be felt. Therefore, identifying risk factors or variables relevant to the disease will help allocate the appropriate hospital and governmental resources to the population in order to mitigate the impact of the pandemic on its population. While there are general shared features of COVID-19 worldwide, the continuous spread and the nature of the different affected populations displayed different courses of disease evolution that makes population-specific studies of relevance for surgical (local) preventive and interventional strategies[10].
In this study, we investigated the demographic, clinical characteristics, and comorbidities affecting COVID-19 patients’ outcomes in four cities of Iran: Shiraz, Dezful, Mashhad and Rasht. Iran is one of the most populated countries of the Middle East, with more than 80 million inhabitants with intra-region differences[11]. Thus, it’s pertinent to know the behavior of the infection in order to forecast and plan strategies to modulate its effects. Our results show hypertension and diabetes are the most common comorbidities in our cohort; however, the rates are varying in COVID-19 patients in the four regions of Iran. The mean age of patients with COVID-19 was 51.1 years. This finding is similar to Nikpouraghdam et al[12] and Mehraeen et al[13] studies, where the mean ages of COVID-19 patients were 55.5 and 54.1 years old[12,13]. The median age of the Iranian population is 32 years old, so differences in the infected pool vs the actual population of Iran in respect of age exist. With respect to the gender of the 91 patients belonging to the four Iranian cities, we found that 56% were male. This is similar to findings from different parts of the world where men were more prevalent in the pool of infected, hospitalized, and dead COVID-19 patients as a likely reflection of men’s higher exposure when compared to women[14]. Other studies like Dana et al[15] and Suba[16] have also reported that women might be more protected. Thanks to chromosome X associated immune functions, women were shown to have a lower level of viral load and less inflammation compared to men. Estrogen hormones are known to enhance immune responses and provide a faster clearance of pathogens[15,16]. One can expect that since men are widely more affected by COVID-19 in Iran, mortality would go upwards in a considerable gap, but this did not happen.
The mean length of stay in hospitals was 6.8 d, there were differences from one region to another. While Mashhad was the region with the longest hospital stay averaging 12.3 d, Rasht was the city in which patients spent less time at the hospital averaging 5.4 d. Correlating this with mortality, Mashhad had a higher death rate with 15.3% vs 10% for Rasht. This is in contrast to other studies from Iran like Zali et al[17], in which the mean stay in hospital was 1 d for survivors and 5 d for non-survivors[17]. Whether this is because of better resources in this hospital or the level of severity of patients remains to be determined. In addition, the difference in the quality of healthcare in Rasht compared to Mashhad hospital could be another factor. We found that 86.4% of our entire cohort received Hydroxychloroquine or Chloroquine, and few patients were treated with Glucocorticoids. These treatments were widely used in the early months of the pandemic and constituted the only adopted treatment protocol at the time. In addition, we showed that the mortality is associated with O2 saturation in general, but more associated with O2 saturation on day three as expected.
Many studies have investigated and described laboratory test findings in COVID-19 patients that are associated with disease severity and outcome. Patients with COVID-19 disease tend to have abnormal blood counts, changes in the coagulation profile, and also in liver and kidney function markers[18,19]. The presence of elevated values for inflammatory markers such as D-Dimer, LDH, creatinine kinase, CRP, and procalcitonin was also reported. In our study, we found that INR, IL-6, and CRP were frequently elevated, but the only one which we can correlate with the severity/ mortality of the disease was creatinine. High serum creatinine on admission may indicate the initial stages of kidney damage. Close to 30% of COVID-19 patients had evidence of kidney disease on admission, with elevated serum creatinine, and this was associated with greater in-hospital mortality[20]. Nephropathic patients are mainly affected by hypertension and cardiovascular disease per se, and this can lead to a higher risk of COVID-19 infection when compared with the general population or with patients without kidney disease[21]. Renal and cardiovascular disease are currently considered as risk factors for COVID-19 infection and are associated with poor prognosis[21]. However, an increased risk of death, about 3–8 times, was found in patients infected with other viruses such as influenza A flu virus and who developed kidney injury during infection compared to those who had not[22]. In addition, patients with increased baseline serum creatinine levels show an alteration of leukocyte count with an increase in the absolute number of leukocytes and a decrease in lymphocyte and platelet counts. Coagulation pathway abnormalities, which include prolonged activated partial thromboplastin time and higher D-Dimer, are more frequent in patients with increased baseline serum levels of creatinine[23]. This is not in agreement with the study by Kermali et al[24] that found elevated CRP, increased IL-6, increased D-Dimer, and decreased platelet count had a strong correlation with severe COVID-19[24]. It has been widely reported that inflammatory markers or acute phase reactant responses particularly IL-6 which increase vascular permeability and result in redistribution of albumin in the interstitial space, induction of apoptosis in lymphocytes leading to lymphopenia in sick patients failed to show significance and correlation with outcome[25].
Regarding the clinical aspect of COVID-19 patients, it is expected that aging populations have an upward trend of comorbidities or chronic conditions such as hypertension, diabetes mellitus, chronic kidney disease[26]. Iran does not escape from this situation, and there is a high prevalence of hypertension, diabetes mellitus (85%), chronic kidney disease, and obesity (20%)[27]. It has been reported by Liu et al[28] that diabetes mellitus, hypertension, coronary artery disease, or chronic pulmonary disease play a major role in determining the severity of COVID-19, although they did not find any relationship with mortality. In our study, we found that cardiac disease was the most chronic condition related to COVID-19. Its presence leads to a negative impact on the evolution of the disease[28]. Our study also shows a trend (but not significant) relation to the other chronic conditions, as what has been shown in other studies from Iran where patients with chronic respiratory disease, hypertension, and chronic kidney disease were the most susceptible to have higher rate of case-fatality[12].
One of the main focuses of this study is the GI symptoms that present in a notable number of hospitalized patients. It has been well reported that SARS-CoV-2 enters via the angiotensin-converting enzyme II receptors expressed in many cells in the kidney (most likely the cause why we reported the impact of creatinine in mortality in our cohort), lungs, blood vessels, and in the GI tract[29]. We also have neuropilin as a COVID-19 receptor that increases GI symptoms such as diarrhea. Interestingly, our cohort manifested an elevated frequency of nausea and other GI symptoms compared with other meta-analyses from different countries where diarrhea was the predominant symptom[30]. Although this finding stood out in our report, none of the GI manifestations, comorbidities, or laboratory values such as an elevation in liver enzymes, ALP, AST, ALT was impactful in the evolution/outcome of patients from Shiraz, Dezful, Rasht, and Mashhad. GI symptoms reported in our study were related to the admission and early hospitalization.
The mortality of hospitalized patients included in the current study was 14.2%, which is far more than the national mortality rate in Iran that has been reported to be 5.4% in COVID-19 patients[3]. In contrast to other countries in the region like Iran, China efforts to stop the pandemic were successful, most likely due to previous experience in China and other countries from the region for past outbreaks like the SARS in 2002-2003[31]. It is highly suspected that inadequate awareness towards the disease at early stages, public adherence to the protective measures, protective equipment (mask), the high infection rate of the virus, and lack of treatment measures in Iran has led to a rapid increase in the number of patients and mortality rates. Our investigation has its own limitations, including some missing data for certain patients and the limited number of patients. Given the fact that most patients with mild symptoms were not hospitalized and were not included in the study, further community-based studies with a larger population from each city are needed.
Our results show that hypertension and diabetes are the most common comorbidities, but their rate varied in COVID-19 patients in four regions of Iran. Nausea, diarrhea, and elevated liver enzyme are the most common GI symptoms. The high infection rate may be the reason of the high rate of mortality in our cohort. Of all the tested markers, creatinine alteration is the only one that was significantly associated with mortality.
Coronavirus disease 2019 (COVID-19) and associated gastrointestinal symptoms (GI) in patients from different regions of Iran have not been reported, and this has major implications for GI related health and comorbidities.
The key issues are whether the outcome of COVID-19 and GI manifestations is different across regions of Iran.
To obtain a full understanding of GI manifestations in COVID-19 patients.
We analyzed data from severe acute respiratory syndrome coronavirus 2 positive patients admitted at four hospitals in Iran (n = 91), including South, Southeast, North, and Northwest, between April and September 2020. Demographics, comorbidities, and clinical findings including GI symptoms were collected.
The average age of COVID-19 patients was 51.1 years, and 56% were male. Mortality rate was 17%. Cough with 84.6%, shortness of breath with 71.4%, fever with 52.7%, and loss of appetite with 43.9% were the main symptoms. Overall, cardiac disease was the most common comorbidity with an average of 28.5%, followed by hypertension (28.5%) and diabetes (25.2%). The highest comorbidity in North (Rasht) was diabetes (30%) and in South was (Dezful) hypertension (37%). Shiraz leads cardiac disease with 43.4%. The most reported GI symptoms included nausea, diarrhea, vomiting, and abdominal pain, with 42.8%, 31.8%, 26.8%, and 12% prevalence, respectively. In addition, albumin, alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase were elevated in 26.3%.
Our results show hypertension and diabetes as the most common comorbidities, but their distribution was different in COVID-19 patients in the four studied regions of Iran. Nausea, diarrhea, and elevated liver enzymes were the most common GI findings. There was also a high mortality rate that was associated with high infection rates in Iran at the beginning of the pandemic. GI manifestations and liver function markers should be monitored in COVID-19 patients.
Larger long-term prospective studies and predictive factors for duration and follow-up of GI symptoms in COVID-19 should be performed in future studies.
The authors are indebted to doctors, and staff at Division of Gastroenterology and Hepatology, for COVID-19-related clinical manifestations, and to other physicians at the 4 different location in Iran hospitals involved in the diagnosis and management of reported COVID-19 patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu T S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | Anderson RM, Fraser C, Ghani AC, Donnelly CA, Riley S, Ferguson NM, Leung GM, Lam TH, Hedley AJ. Epidemiology, transmission dynamics and control of SARS: the 2002-2003 epidemic. Philos Trans R Soc Lond B Biol Sci. 2004;359:1091-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 326] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 2. | Hajjar SA, Memish ZA, McIntosh K. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): a perpetual challenge. Ann Saudi Med. 2013;33:427-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU), 2020. [cited 10 December 2020]. Available from: URL: https://coronavirus.jhu.edu/map.html. |
| 4. | World Health Organization. COVID-19. [cited 15 December 2020]. Available from: URL: https://www.who.int/publications/m/item/weekly-epidemiological-update---15-december-2020. |
| 5. | Ghadir MR, Ebrazeh A, Khodadadi J, Zamanlu M, Shams S, Nasiri M, Koohpaei A, Abbasinia M, Sharifipour E, Golzari SE. The COVID-19 Outbreak in Iran; The First Patient with a Definite Diagnosis. Arch Iran Med. 2020;23:503-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J Mol Histol. 2020;51:613-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 291] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 7. | Mandal A, Konala VM, Adapa S, Naramala S, Gayam V. Gastrointestinal Manifestations in COVID-19 Infection and Its Practical Applications. Cureus. 2020;12:e8750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Mehrjoo Z, Fattahi Z, Beheshtian M, Mohseni M, Poustchi H, Ardalani F, Jalalvand K, Arzhangi S, Mohammadi Z, Khoshbakht S, Najafi F, Nikuei P, Haddadi M, Zohrehvand E, Oladnabi M, Mohammadzadeh A, Jafari MH, Akhtarkhavari T, Gooshki ES, Haghdoost A, Najafipour R, Niestroj LM, Helwing B, Gossmann Y, Toliat MR, Malekzadeh R, Nürnberg P, Kahrizi K, Najmabadi H, Nothnagel M. Distinct genetic variation and heterogeneity of the Iranian population. PLoS Genet. 2019;15:e1008385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Banihashemi K. Iranian human genome project: Overview of a research process among Iranian ethnicities. Indian J Hum Genet. 2009;15:88-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Yi Y, Lagniton PNP, Ye S, Li E, Xu RH. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753-1766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 421] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 11. | Devi S. COVID-19 resurgence in Iran. Lancet. 2020;395:1896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Nikpouraghdam M, Jalali Farahani A, Alishiri G, Heydari S, Ebrahimnia M, Samadinia H, Sepandi M, Jafari NJ, Izadi M, Qazvini A, Dorostkar R, Tat M, Shahriary A, Farnoosh G, Hosseini Zijoud SR, Taghdir M, Alimohamadi Y, Abbaszadeh S, Gouvarchin Ghaleh HE, Bagheri M. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: A single center study. J Clin Virol. 2020;127:104378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 230] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 13. | Mehraeen E, Karimi A, Barzegary A, Vahedi F, Afsahi AM, Dadras O, Moradmand-Badie B, Seyed Alinaghi SA, Jahanfar S. Predictors of mortality in patients with COVID-19-a systematic review. Eur J Integr Med. 2020;40:101226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 14. | Shahriarirad R, Khodamoradi Z, Erfani A, Hosseinpour H, Ranjbar K, Emami Y, Mirahmadizadeh A, Lotfi M, Shirazi Yeganeh B, Dorrani Nejad A, Hemmati A, Ebrahimi M, Moghadami M. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect Dis. 2020;20:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 15. | Dana PM, Sadoughi F, Hallajzadeh J, Asemi Z, Mansournia MA, Yousefi B, Momen-Heravi M. An Insight into the Sex Differences in COVID-19 Patients: What are the Possible Causes? Prehosp Disaster Med. 2020;35:438-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Suba Z. Prevention and therapy of COVID-19 via exogenous estrogen treatment for both male and female patients. J Pharm Pharm Sci. 2020;23:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Zali A, Gholamzadeh S, Mohammadi G, Azizmohammad Looha M, Akrami F, Zarean E, Vafaee R, Maher A, Khodadoost M. Baseline Characteristics and Associated Factors of Mortality in COVID-19 Patients; an Analysis of 16000 Cases in Tehran, Iran. Arch Acad Emerg Med. 2020;8:e70. [PubMed] |
| 18. | Vinayagam S, Sattu K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. 2020;260:118431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 19. | Lazzaroni MG, Piantoni S, Masneri S, Garrafa E, Martini G, Tincani A, Andreoli L, Franceschini F. Coagulation dysfunction in COVID-19: The interplay between inflammation, viral infection and the coagulation system. Blood Rev. 2021;46:100745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 20. | Uribarri A, Núñez-Gil IJ, Aparisi A, Becerra-Muñoz VM, Feltes G, Trabattoni D, Fernández-Rozas I, Viana-Llamas MC, Pepe M, Cerrato E, Capel-Astrua T, Romero R, Castro-Mejía AF, El-Battrawy I, López-País J, D'Ascenzo F, Fabregat-Andres O, Bardají A, Raposeiras-Roubin S, Marín F, Fernández-Ortiz A, Macaya C, Estrada V; HOPE COVID-19 Investigators. Impact of renal function on admission in COVID-19 patients: an analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) Registry. J Nephrol. 2020;33:737-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 21. | Ielapi N, Licastro N, Provenzano M, Andreucci M, Franciscis S, Serra R. Cardiovascular disease as a biomarker for an increased risk of COVID-19 infection and related poor prognosis. Biomark Med. 2020;14:713-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Jung JY, Park BH, Hong SB, Koh Y, Suh GY, Jeon K, Koh SO, Kim JY, Cho JH, Choi HS, Park YB, Kim HC, Kim YS, Lim CY, Park MS. Acute kidney injury in critically ill patients with pandemic influenza A pneumonia 2009 in Korea: a multicenter study. J Crit Care. 2011;26:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Gagliardi I, Patella G, Michael A, Serra R, Provenzano M, Andreucci M. COVID-19 and the Kidney: From Epidemiology to Clinical Practice. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19 - A systematic review. Life Sci. 2020;254:117788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 420] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 25. | Cao W, Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30:367-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 26. | Dexter PR, Miller DK, Clark DO, Weiner M, Harris LE, Livin L, Myers I, Shaw D, Blue LA, Kunzer J, Overhage JM. Preparing for an aging population and improving chronic disease management. AMIA Annu Symp Proc. 2010;2010:162-166. [PubMed] |
| 27. | Esteghamati A, Larijani B, Aghajani MH, Ghaemi F, Kermanchi J, Shahrami A, Saadat M, Esfahani EN, Ganji M, Noshad S, Khajeh E, Ghajar A, Heidari B, Afarideh M, Mechanick JI, Ismail-Beigi F. Diabetes in Iran: Prospective Analysis from First Nationwide Diabetes Report of National Program for Prevention and Control of Diabetes (NPPCD-2016). Sci Rep. 2017;7:13461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 28. | Liu H, Chen S, Liu M, Nie H, Lu H. Comorbid Chronic Diseases are Strongly Correlated with Disease Severity among COVID-19 Patients: A Systematic Review and Meta-Analysis. Aging Dis. 2020;11:668-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 177] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 29. | Vuille-Dit-Bille RN, Liechty KW, Verrey F, Guglielmetti LC. SARS-CoV-2 receptor ACE2 gene expression in small intestine correlates with age. Amino Acids. 2020;52:1063-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Rokkas T. Gastrointestinal involvement in COVID-19: a systematic review and meta-analysis. Ann Gastroenterol. 2020;33:355-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Zhong N, Zeng G. What we have learnt from SARS epidemics in China. BMJ. 2006;333:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |