Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4617
Peer-review started: January 26, 2021
First decision: February 25, 2021
Revised: March 11, 2021
Accepted: April 12, 2021
Article in press: April 12, 2021
Published online: June 26, 2021
Processing time: 135 Days and 18.4 Hours
Histological transformation is one of the numerous mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs). Given its rarity, the underlying transformational mechanisms, clinical features, and therapeutic prognoses are only studied through limited case reports.
To analyze the clinical characteristics and underlying mechanisms in non-small cell lung cancer (SCLC) patients with histological transformation after treatment with EGFR-TKIs.
We retrospectively investigated nine patients diagnosed with non-SCLC transforming to SCLC, large-cell neuroendocrine carcinoma (LCNEC), or squamous cell carcinoma on re-biopsy after first- or third-generation EGFR-TKIs.
The median age of nine patients was 60 years. Among them, six patients had the EGFR 19del mutation, one had the L858R mutation, and one had wild-type EGFR. The level of plasma NSE was measured in six patients with SCLC or LCNEC transformation when transformation occurred, and five patients had elevated plasma NSE levels. All patients received standard chemotherapy after transformation with the exception of one patient who received chemotherapy and anlotinib.
Tumor re-biopsy should be performed routinely when EGFR-TKI therapy fails in lung cancer patients to avoid ignoring histological transformation and to select a subsequent therapeutic strategy. The transformed tumor retained the original EGFR mutation, indicating that histological transformation represents an evolution from the initial tumor.
Core Tip: We retrospectively diagnosed nine cases of non-small cell lung cancer (SCLC) transforming to SCLC, large-cell neuroendocrine carcinoma, or squamous cell carcinoma on re-biopsy after epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs). Tumor re-biopsy should be performed routinely when EGFR-TKI therapy fails in lung cancer patients to avoid ignoring histological transformation and to select a subsequent therapeutic strategy. The plasma pro-gastrin-releasing peptide and NSE levels could be valuable and significant biomarkers to predict histological transformation. The transformed tumor retained the original EGFR mutation, which indicates that histological transformation represents an evolution from the initial tumor.
- Citation: Jin CB, Yang L. Histological transformation of non-small cell lung cancer: Clinical analysis of nine cases. World J Clin Cases 2021; 9(18): 4617-4626
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4617.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4617
Lung cancer is the leading cause of cancer-related death worldwide and is histologically classified into non-small cell lung cancer (NSCLC) and SCLC. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are the standard strategy of first-line therapy for EGFR-sensitive mutant patients with advanced or metastatic NSCLC. In a randomized phase III FLAURA study, the median progression-free survival (PFS) and overall survival (OS) of patients administered third-generation TKIs were significantly prolonged compared with those taking first-generation TKIs (18.9 mo vs 10.2 mo for PFS and 38.6 mo vs 31.8 mo for OS). Currently, the third-generation TKI osimertinib has become the preferred recommended first-line therapeutic strategy.
Although EGFR-TKIs have a favorable and lasting effect, most patients will eventually develop disease progression within 19 mo. Several mechanisms of acquired resistance to EGFR-TKIs have been reported. For first- and second-generation EGFR-TKIs, the most common mechanism is the acquisition of the T790M mutation. The other mechanisms involve alternative pathway activation, such as human epidermal growth factor receptor 2 (HER2) or methionine (MET) amplification, v-raf murine sarcoma viral oncogene homolog B mutation, and histological and phenotypic transformation, such as SCLC transformation and epithelial-mesenchymal transition[1]. For third-generation EGFR-TKIs, in a study in which plasma samples of 38 patients after progression in first-line treatment with osimertinib were collected for next-generation sequencing analysis, the genetic resistance mutations included the following: MET, EGFR, and Kirsten rat sarcoma 2 viral oncogene homolog (KRAS) amplification; PI3K3CA and KRAS mutations; and EGFR C797S mutation. Another researcher also collected and analyzed plasma samples at baseline and during the progression of the FLAURA study. No EGFR T790M mutation was detected under first-line osimertinib therapy. The acquired gene alterations included MET amplification and EGFR C797X mutation. These two studies utilized plasma samples rather than biopsied tissue specimens to study the mechanisms of acquired resistance[2].
Among the mechanisms of acquired resistance to EGFR-TKIs, histological transformation is less common. A number of studies based on case reports have investigated the characteristics and potential mechanisms of transformation to SCLC, large-cell neuroendocrine carcinoma (LCNEC), squamous cell carcinoma (SqCC), and sarcoma after EGFR-TKIs[3]. Many reports have shown that tumor samples after transformation still retain the original EGFR mutation. In addition, tumor re-biopsy is often replaced by peripheral blood analysis during disease progression, ignoring the presence of transformation.
Here, we report nine cases of NSCLC developing histological transformation after EGFR-TKI therapy.
Nine patients with histological transformation were hospitalized at the Department of Thoracic Oncology, Hubei Cancer Hospital (Wuhan, China) from 2014 to 2019. The related clinical data were collected through a retrospective analysis, whereas some data could not be obtained.
The re-biopsy specimens of patients were used to detect the EGFR mutation status through the amplification refractory mutation system in the clinical molecular diagnostic center of our hospital. Pathological diagnosis of biopsy tissues through hematoxylin and eosin and immunohistochemical staining was performed normatively in the pathology department.
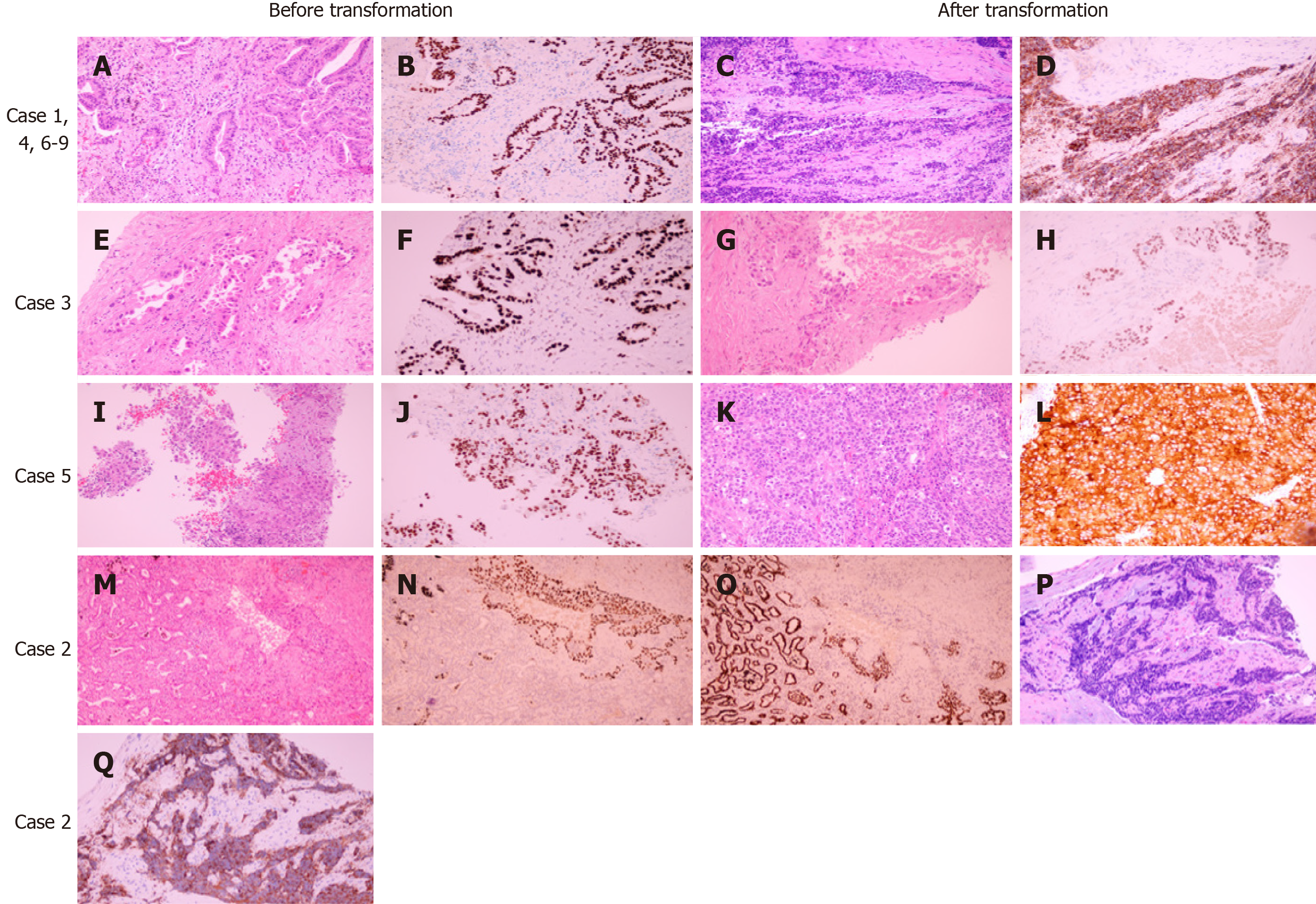
Nine patients, including four females and five males, showed histological transformation. Pathological results before and after transformation are presented in Figure 1. Patient information and pathologic features are summarized in Table 1. The median age was 60 years old. Four patients had smoked for a long time prior to diagnosis. Tissue samples were obtained from eight of nine patients through needle biopsy or cytological specimens from pleural effusion with a diagnosis of adenocarcinoma (ADC). The remaining patient was diagnosed with adenosquamous carcinoma after surgical resection. Of all cases, seven transformed to SCLC, one transformed to LCNEC, and one transformed to SqCC.
| Before transformation onset | Post transformation onset | ||||||||||||||||
| Case No. | Gender, age | Smoking status | Time of initial diagnosis | Clinical stage | Tumor histology | Sample type, acquisition site | Initial treatment | EGFR mutational status | TKI | TKI line | TKI treatment time (m) | Tunor histology | NSE level | Sample type, acquisition site | EGFR mutational status | Treatment | Response and PFS (mo) |
| 1 | M, 66 | Former | September, 2018 | T4N3M1 | ADC | Biopsy, bone | No | 19del | Iconitib | I | 11 | SCLC | ND | Biopsy, lung | 19del | CTx (etoposide/cisplatin) | 1 |
| 2 | F, 62 | Never | 2014 | 1 | ASC | Resection, lung | Surgery, CTx | 1 | Iconitib | I | 14 | SCLC | Elevated | Biopsy, chest wall | ND | CTx (etoposide/lobaplatin) | 1 |
| 3 | M, 60 | Former | January, 2016 | T4N3M1 | ADC | Biopsy, lung | CTx, RT | ND | Gefitinib | II | 15 | SqCC | Normal (SCC) | Biopsy, axillary lymph node | 21 L858R | CTx (docetaxel/cisplatin) | PD, 2 |
| 4 | M, 66 | Former | January, 2014 | T1N0M1 | ADC | Cytology, pleural effusion | No | 19del | Erlotinib | I | 21 | SCLC | Elevated | Biopsy, lung | 19del | CTx (etoposide) | 1 |
| 5 | M, 57 | Never | February, 2017 | T4N2M1 | ADC | Biopsy, lung | CTx | 19del | Iconitib | II | 12 | LCNEC | Elevated | Biopsy, lung | 19del | CTx (etoposide/cisplatin) | SD, 4 |
| 62 | M, 60 | Former | July, 2016 | TxN0M1 | ADC | Biopsy, lung | CTx, Apatinib | Wide type | Gefitinib, Osimertinib | VI | 6 + 3 | SCLC | Normal | Biopsy, lung | Wide type | CTx (etoposide/cisplatin) | SD, 3 |
| 7 | F, 42 | Never | December, 2018 | TxN0M1 | ADC | Biopsy, lung | No | 19del → 19del, T790M | Iconitib → Osimertinib | I → II | 3 + 8 | SCLC | ND | Excision biopsy, supraclavicular lymph nodes | ND | CTx (etoposide/cisplatin) | 1 |
| 8 | F, 65 | Never | May, 2019 | T2N3M1 | ADC | Biopsy, lung | No | 19del | Gefitinib | I | 16 | SCLC | Elevated | Biopsy, lung | ND | CTx (etoposide/cisplatin) | SD |
| 9 | F, 31 | Never | April, 2018 | T4N0M1 | ADC | Biopsy, lung | No | 19del → 19del, T790M | Iconitib → Osimertinib | I → II | 6 + 15 | SCLC | Elevated | Biopsy, supraclavicular lymph node | ND | Docetaxel/anlotinib | SD |
Initial treatment before TKIs included resection and adjuvant chemotherapy for case 2, chemotherapy and radiotherapy for case 3, palliative chemotherapy for case 5, and multiline chemotherapy and apatinib for case 6.
Of the nine patients in the first biopsy, six (cases 1, 4, 5, 7, 8, and 9) harbored EGFR exon 19 deletion mutations. The EGFR status of cases 2 and 3 was unknown or undetected. In addition, case 6 had wild-type EGFR. Five patients (cases 1, 2, 5, 7, and 9) were treated with icotinib. Three patients (cases 3, 6, and 8) were treated with gefitinib. Case 4 was treated with erlotinib. In addition, case 6 proceeded with osimertinib therapy after progression. Patients 7 and 9 also received osimertinib treatment after the second biopsy as the disease progressed, indicating EGFR exon 20 T790M and exon 19 deletion mutations. The time of TKI treatment, including the first- and third-generation agents, ranged from 11 to 21 mo.
After confirmation of histological transformation, three patients (cases 1, 4, and 5) still had the original EGFR mutation. The EGFR exon 21 L858R mutation was detected in the re-biopsy sample of case 2, whereas case 6 showed no EGFR mutation as previously noted. Unfortunately, cases 2, 7, 8 and 9 did not undergo genetic testing of EGFR.
When SCLC or LCNEC transformation occurred, the serum NSE levels of five patients (cases 2, 4, 5, 8, and 9) were elevated significantly compared with normal baseline levels. Case 6 had normal NSE levels. In addition, NSE levels were not detected in cases 1 and 7.
After transformation to SCLC or LCNEC, cases 5, 6 and 8 were treated with etoposide and cisplatin and showed a stable disease response. Case 9 achieved stable disease with docetaxel and anlotinib treatment. In addition, after the transformation to SqCC, case 3 had disease progression after two cycles of chemotherapy with docetaxel and cisplatin. The follow-up data of the remaining patients after switching to chemotherapy were not obtained.
Approximately 80%-85% of lung cancers are NSCLC, among which approximately 50% of Asian patients and 16% of Caucasian patients have EGFR mutations[4,5]. Although considerable improvement has been obtained by EGFR-TKIs for EGFR-sensitive mutations, most patients will inevitably develop acquired resistance. Various mechanisms have been validated, including the EGFR T790M mutation, which accounts for 60% of first- and second-generation EGFR-TKI resistance[6]. Less common mechanisms include MET amplification, HER2 amplification, PIK3CA mutation, histological or phenotypical transformation, and others[7-10]. The most common histological transformation is ADC to SCLC, occurring in 3%-15% of patients[11]. Acquired resistance mechanisms to third-generation TKIs administered as first- or second-line therapy can also be grouped into EGFR-dependent mechanisms, including EGFR C797S and G796 mutations, and EGFR-independent mechanisms, including MET and HER2 amplification and rare histological transformation[12].
In our reported cases, cases 1, 4, 7, 8, and 9 transformed from NSCLC to SCLC after TKI therapy with EGFR mutations, and case 2 with unknown EGFR status also experienced SCLC transformation after EGFR-TKI treatment for 14 mo. Considering that the median progression-free survival of first-generation EGFR-TKIs is approximately 11 mo in treating EGFR-sensitive NSCLC, we hypothesize that case 2 may initially have had EGFR mutations. The potential mechanisms underlying SCLC transformation after TKI treatment remain controversial. On the one hand, some researchers believe that SCLC transformation occurs in NSCLC after EGFR-TKI therapy. It is impossible that SCLC could not progress during EGFR-TKI treatment for 1 year. Previously reported cases revealed that secondary biopsy specimens have the same type of EGFR mutation as the original specimens. In our study, cases 1 and 4 shared the EGFR 19del before and after transformation onset. In addition, SCLC has a lower rate of EGFR mutation than NSCLC[13]. Furthermore, alveolar type II cells may be the common progenitor of ADC and SCLC with EGFR mutations[9]. ADC cells ultimately transdifferentiate into SCLC cells regulated by genetic alterations, such as RB1 and TP53 mutations, under the selective pressure of EGFR-TKIs. On the other hand, some studies have also considered that the primary tumor consists of both ADC and SCLC, which is referred to as mixed SCLC. SCLC cells become dominant when ADC cells are eliminated by EGFR-TKIs[3]. Due to the small biopsy specimens used to confirm the diagnosis, some cases of mixed SCLC cannot be accurately identified during the initial assessment; the incidence of mixed SCLC is 12%-28% in surgical samples[14].
In the present case, case 3 with unknown EGFR status progressed after gefitinib treatment for 15 mo. Gene detection in the re-biopsy tissue revealed the EGFR L858R mutation. We deduce that this patient may have had the same EGFR mutation at diagnosis. Perez-Moreno et al[15] summarized the progression of 17 patients with a documented occurrence of SqCC transformation after EGFR-TKI therapy. All these patients and our present case maintained the EGFR mutation detected on ADC at diagnosis. Less than 5% of SqCC patients have EGFR mutations. Thus, it is more likely that SqCC cells arise from ADC cells. Han et al[16] constructed a mouse model of transdifferentiation into SqCC from Lkb-1-deficient ADCs. This study also supports the assumption of SqCC transformation under the pressure of EGFR-TKIs. However, we could not completely exclude the hypothesis that adenosquamous carcinoma exists initially and that both components harbor the same EGFR mutation. However, it is a relatively rare subtype accounting for 0.4%-4% of lung carcinomas[17].
LCNEC transformation is extremely rare. Wang et al[18] reported that only seven cases have transformed from ADC to LCNEC after EGFR-TKI therapy. Among them, four cases occurred after first-generation TKI treatment, the duration of which was from 10 to 96 mo[19-21]. Six of seven cases maintained the original EGFR mutation after transformation. The other three cases had a transformation after consecutive first- and third-generation TKIs[22]. The times from the beginning of first-generation TKIs to the end of third-generation TKIs were 19 mo, 21 mo, and 49 mo. In our case 5, the ADC patient with the EGFR 19del mutation transformed to LCNEC after 12 mo of iconitib treatment. The lung re-biopsy showed the same mutation. LCNEC is a high-grade neuroendocrine carcinoma with low EGFR mutation rates, the prognosis of which is worse than that of ADC. On the basis of these cases, all the cases had a long duration of TKI treatment, and most cases had the same mutation after acquired resistance. We believe that LCNEC probably transforms from ADC under the effect of EGFR-TKIs.
In our case 6, the patient with EGFR wild-type ADC also transformed to SCC after multiline therapy with gefitinib and osimertinib. This finding indicates that histologic transformation is not unique to tumors harboring EGFR mutations. Only two studies by Ahn et al[23] and Gu et al[24] reported three cases of EGFR wild-type ADC bearing SCC transformation[23,24]. One case in Ahn et al[23]’s study with stage I ADC was diagnosed as SCLC at the time of recurrence after surgery. It is difficult to exclude the possibility of a second primary tumor. Another case in Ahn et al[23]’s study experienced a transformation after gefitinib therapy of only 2 mo, indicating primary resistance. This case also suggested that SCC transformation may not exclusively result from TKI treatment. However, in the present case 6, EGFR-TKI therapy was effective, indicating acquired resistance. The transformation of our case is closely related to EGFR-TKIs.
Previous clinical studies have mostly focused on exploring the mechanisms of acquired resistance to osimertinib after first- or second-generation TKIs. In a multicentric respective French study of osimertinib in second-line or greater treatment, 9% of patients developed histological transformation in 56 cases with available re-biopsy samples[25]. A recent study showed that histological transformation, mainly SqCC, was identified in 15% of 27 cases with first-line osimertinib therapy[26]. In cases 6, 7 and 9, SCLC transformation occurred after osimertinib as a second-line TKI treatment. The ongoing MELROSE phase 2 trial will evaluate the resistance mechanisms in tumor tissue and liquid biopsy in EGFR mutant NSCLC patients receiving osimertinib as first-line therapy[22].
In general, chemotherapy, such as etoposide plus cisplatin, becomes the standard strategy for patients with SCLC or LCNEC transformation. Roca et al[27] performed a systematic analysis of 39 patients who had SCLC transformation. The survival outcome of 16 patients was available, and the median survival after SCLC diagnosis was 6 mo. Another study also retrospectively analyzed the clinical outcomes of 58 patients with EGFR mutant NSCLC undergoing SCLC transformation[28]. The median overall survival since the time of SCLC transformation was 10.9 mo. The median PFS of patients treated with platinum-etoposide or taxanes was 3.4 mo and 2.7 mo, respectively. In previous research, the median PFS of cisplatin-based therapy for first-line treatment of both limited-stage SCLC and extensive-stage SCLC was approximately 5.5 mo, and the median OS was 9.6 mo[29]. In cases 5 and 6, the PFS from SCC transformation was just 4 and 3 mo, respectively. Therefore, the prognosis after transformation is poor.
When EGFR-mutant NSCLC progresses due to acquired resistance to TKI therapy, it is crucial for re-biopsy to further confirm the mechanisms of resistance to decide subsequent therapeutic schedules. However, in real clinical work in China, the rate of re-biopsy is especially low for various reasons, such as the convenience of peripheral blood detection and rejection of patients. In a Japanese retrospective study, the success rate of re-biopsy was 79.5%[30]. Thus, it is significant to identify and utilize biomarkers to predict which patients will experience histological transformation. Kato et al[31] found that plasma pro-gastrin-releasing peptide (ProGRP) levels increased before re-biopsy-confirmed SCLC transformation. Gu et al[24] also reported that serum ProGRP and NSE levels were markedly elevated compared with normal levels when SCLC transformation occurred. In addition, many other literature reports have shown a significant increase in NSE levels, indicating SCLC or LCNEC transformation[11,18,32,33]. In our cases, NSE levels were detected in six cases at the time of SCLC transformation. Serum NSE levels of five patients (cases 2, 4, 5, 8, and 9) were elevated, whereas that of case 6 was normal. Therefore, it is meaningful and recommended to monitor plasma ProGRP and NSE levels before invasive re-biopsies for the early prediction of SCLC or LCNEC transformation.
In conclusion, we report nine cases of histological transformation after first- or third-generation EGFR-TKIs, which included SCLC, LCNEC, and SqCC transformation. Histological transformation could occur in patients with either wild-type or mutant EGFR. The detected cases in the present report also retained the original EGFR mutation after transformation. This finding indicates that it is a great possibility that tumor cells post transformation transdifferentiate from original tumor cells before transformation. All patients received standard chemotherapy after transformation with a poor prognosis. Plasma ProGRP and NSE levels could represent valuable and significant biomarkers to predict histological transformation. In addition, tumor re-biopsy should be performed routinely when the disease progresses under EGFR-TKI treatment.
Histological transformation is one of the numerous mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs). Given its rarity, the underlying transformational mechanisms, clinical features, and therapeutic prognosis are only studied through limited case reports.
The study of non-small cell lung cancer (SCLC) patients with histological transformation after treatment with EGFR-TKIs can improve our understanding of the clinical features and underlying mechanisms in this type of patient. This finding can remind us of the importance of re-biopsy when targeted drug resistance occurs.
The main objective of this study was to analyze the clinical characteristics and underlying mechanisms in non-SCLC (NSCLC) patients with histological transformation after treatment with EGFR-TKIs.
We retrospectively investigated nine patients diagnosed with NSCLC transforming to SCLC, large-cell neuroendocrine carcinoma (LCNEC), or squamous cell carcinoma on re-biopsy after first- or third-generation EGFR-TKIs.
The median age of the nine patients in this study was 60 years old. Among them, six patients had the EGFR 19del mutation, one had the L858R mutation, and one had wild-type EGFR. In six patients with SCLC or LCNEC transformation, plasma NSE levels were detected when transformation occurred, and five patients had elevated plasma NSE levels. All patients received standard chemotherapy after transformation except one patient who received chemotherapy and anlotinib.
Tumor re-biopsy should be performed routinely when EGFR-TKI therapy fails in lung cancer patients to avoid ignoring histological transformation and to select a subsequent therapeutic strategy. The transformed tumor retained the original EGFR mutation, which indicates that histological transformation represents an evolution from the initial tumor. Plasma pro-gastrin-releasing peptide and NSE levels could represent valuable and significant biomarkers to predict histological transformation.
Due to the limited cases in this study, it is difficult to draw reliable conclusions. However, our study suggests that the plasma NSE levels potentially represent a valuable biomarker to predict histological transformation, and re-biopsy should be performed to avoid ignoring the occurrence of histological transformation. Furthermore, to a large extent, histological transformation represents an evolution from the initial tumor.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pan M S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 538] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 2. | Bennouna J, Girard N, Audigier-Valette C, le Thuaut A, Gervais R, Masson P, Marcq M, Molinier O, Cortot A, Debieuvre D, Cadranel J, Lena H, Moro-Sibilot D, Chouaid C, Mennecier B, Urban T, Sagan C, Perrier L, Barlesi F, Denis MG. Phase II Study Evaluating the Mechanisms of Resistance on Tumor Tissue and Liquid Biopsy in Patients With EGFR-mutated Non-pretreated Advanced Lung Cancer Receiving Osimertinib Until and Beyond Radiologic Progression: The MELROSE Trial. Clin Lung Cancer. 2020;21:e10-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Shao Y, Zhong DS. Histological transformation after acquired resistance to epidermal growth factor tyrosine kinase inhibitors. Int J Clin Oncol. 2018;23:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 4. | Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, Petrella F, Spaggiari L, Rosell R. Non-small-cell lung cancer. Nat Rev Dis Primers. 2015;1:15009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 695] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 5. | Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 917] [Cited by in RCA: 1140] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 6. | Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10-i19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 517] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 7. | Liu S, Li S, Hai J, Wang X, Chen T, Quinn MM, Gao P, Zhang Y, Ji H, Cross DAE, Wong KK. Targeting HER2 Aberrations in Non-Small Cell Lung Cancer with Osimertinib. Clin Cancer Res. 2018;24:2594-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Ludovini V, Bianconi F, Pistola L, Chiari R, Minotti V, Colella R, Giuffrida D, Tofanetti FR, Siggillino A, Flacco A, Baldelli E, Iacono D, Mameli MG, Cavaliere A, Crinò L. Phosphoinositide-3-kinase catalytic alpha and KRAS mutations are important predictors of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165-e172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 724] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 10. | Song KA, Niederst MJ, Lochmann TL, Hata AN, Kitai H, Ham J, Floros KV, Hicks MA, Hu H, Mulvey HE, Drier Y, Heisey DAR, Hughes MT, Patel NU, Lockerman EL, Garcia A, Gillepsie S, Archibald HL, Gomez-Caraballo M, Nulton TJ, Windle BE, Piotrowska Z, Sahingur SE, Taylor SM, Dozmorov M, Sequist LV, Bernstein B, Ebi H, Engelman JA, Faber AC. Epithelial-to-Mesenchymal Transition Antagonizes Response to Targeted Therapies in Lung Cancer by Suppressing BIM. Clin Cancer Res. 2018;24:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Xie Z, Gu Y, Lin X, Ouyang M, Qin Y, Zhang J, Liu J, Mai S, Zhou C. Unexpected favorable outcome to etoposide and cisplatin in a small cell lung cancer transformed patient: a case report. Cancer Biol Ther. 2019;20:1172-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 895] [Cited by in RCA: 897] [Article Influence: 149.5] [Reference Citation Analysis (1)] |
| 13. | El Hussein S, Khader SN. Transformation of lung adenocarcinoma to small cell lung carcinoma in the setting of tyrosine kinase inhibitor therapy: Cytological approach of a clinically challenging phenomenon. Diagn Cytopathol. 2019;47:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | de Antonio DG, Alfageme F, Gámez P, Córdoba M, Varela A; Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology and Thoracic Surgery (GCCB-S). Results of surgery in small cell carcinoma of the lung. Lung Cancer. 2006;52:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res. 2012;18:2443-2451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 16. | Han X, Li F, Fang Z, Gao Y, Fang R, Yao S, Sun Y, Li L, Zhang W, Ma H, Xiao Q, Ge G, Fang J, Wang H, Zhang L, Wong KK, Chen H, Hou Y, Ji H. Transdifferentiation of lung adenocarcinoma in mice with Lkb1 deficiency to squamous cell carcinoma. Nat Commun. 2014;5:3261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 17. | Li C, Lu H. Adenosquamous carcinoma of the lung. Onco Targets Ther. 2018;11:4829-4835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Wang H, Zhang L, Shi X, Zhang X, Si X. Successful treatment with osimertinib and its subsequent resistance mechanism in a patient with non-small-cell lung cancer harboring acquired EGFR T790M mutation after recovery from AC0010-induced interstitial lung disease. Onco Targets Ther. 2019;12:5545-5549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kogo M, Shimizu R, Uehara K, Takahashi Y, Kokubo M, Imai Y, Tomii K. Transformation to large cell neuroendocrine carcinoma as acquired resistance mechanism of EGFR tyrosine kinase inhibitor. Lung Cancer. 2015;90:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Lim JU, Woo IS, Jung YH, Byeon JH, Park CK, Kim TJ, Kim HR. Transformation into large-cell neuroendocrine carcinoma associated with acquired resistance to erlotinib in nonsmall cell lung cancer. Korean J Intern Med. 2014;29:830-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Yanagisawa S, Morikawa N, Kimura Y, Nagano Y, Murakami K, Tabata T. Large-cell neuroendocrine carcinoma with epidermal growth factor receptor mutation: possible transformation of lung adenocarcinoma. Respirology. 2012;17:1275-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Baglivo S, Ludovini V, Sidoni A, Metro G, Ricciuti B, Siggillino A, Rebonato A, Messina S, Crinò L, Chiari R. Large Cell Neuroendocrine Carcinoma Transformation and EGFR-T790M Mutation as Coexisting Mechanisms of Acquired Resistance to EGFR-TKIs in Lung Cancer. Mayo Clin Proc. 2017;92:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Ahn S, Hwang SH, Han J, Choi YL, Lee SH, Ahn JS, Park K, Ahn MJ, Park WY. Transformation to Small Cell Lung Cancer of Pulmonary Adenocarcinoma: Clinicopathologic Analysis of Six Cases. J Pathol Transl Med. 2016;50:258-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Gu Y, Zhu X, Cao B, Wu X, Tong X, Shao YW, Liang L. Transformation to small cell lung cancer and activation of KRAS during long-term erlotinib maintenance in a patient with non-small cell lung cancer: A case report. Oncol Lett. 2019;17:5219-5223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Mehlman C, Cadranel J, Rousseau-Bussac G, Lacave R, Pujals A, Girard N, Callens C, Gounant V, Théou-Anton N, Friard S, Trédaniel J, Blons H, Dujon C, Duchemann B, Schischmanoff PO, Chinet T, Giroux Leprieur E. Resistance mechanisms to osimertinib in EGFR-mutated advanced non-small-cell lung cancer: A multicentric retrospective French study. Lung Cancer. 2019;137:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Schoenfeld AJ, Chan JM, Kubota D, Sato H, Rizvi H, Daneshbod Y, Chang JC, Paik PK, Offin M, Arcila ME, Davare MA, Shinde U, Pe'er D, Rekhtman N, Kris MG, Somwar R, Riely GJ, Ladanyi M, Yu HA. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin Cancer Res. 2020;26:2654-2663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 27. | Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: A systematic review and pooled analysis. Cancer Treat Rev. 2017;59:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Marcoux N, Gettinger SN, O'Kane G, Arbour KC, Neal JW, Husain H, Evans TL, Brahmer JR, Muzikansky A, Bonomi PD, Del Prete S, Wurtz A, Farago AF, Dias-Santagata D, Mino-Kenudson M, Reckamp KL, Yu HA, Wakelee HA, Shepherd FA, Piotrowska Z, Sequist LV. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol. 2019;37:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 348] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 29. | Kalemkerian GP. Small Cell Lung Cancer. Semin Respir Crit Care Med. 2016;37:783-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Nosaki K, Satouchi M, Kurata T, Yoshida T, Okamoto I, Katakami N, Imamura F, Tanaka K, Yamane Y, Yamamoto N, Kato T, Kiura K, Saka H, Yoshioka H, Watanabe K, Mizuno K, Seto T. Re-biopsy status among non-small cell lung cancer patients in Japan: A retrospective study. Lung Cancer. 2016;101:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Kato Y, Tanaka Y, Hino M, Gemma A. ProGRP as early predictive marker of non-small-cell lung cancer to small-cell lung cancer transformation after EGFR-TKI treatment. Respir Med Case Rep. 2019;27:100837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Fiore M, Trecca P, Perrone G, Amato M, Righi D, Trodella L, D'Angelillo RM, Ramella S. Histologic transformation to small-cell lung cancer following gefitinib and radiotherapy in a patient with pulmonary adenocarcinoma. Tumori. 2019;105:NP12-NP16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Lu H, Chen B, Qin J, Xie F, Han N, Huang Z. Transformation to small-cell lung cancer following treatment with icotinib in a patient with lung adenocarcinoma. Oncol Lett. 2018;15:5799-5802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |