Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4460
Peer-review started: February 2, 2021
First decision: March 6, 2021
Revised: March 28, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: June 26, 2021
Processing time: 129 Days and 7.9 Hours
The spread of the new coronavirus (COVID-19) infection in 2020 has had a significant impact on the treatment of cancer worldwide. During the COVID-19 pandemic, the biggest challenge for pancreatic surgeons is the difficulty in providing oncological care. In this review article, from the standpoint of surgeons, we explain the concept of triaging of patients with pancreatic tumors under the COVID-19 pandemic, and the actual impact of COVID-19 on the treatment of patients with pancreatic tumors. The most vital points in selecting the best therapeutic approach for patients with pancreatic tumors during this pandemic are (1) Oncologists need to tailor the treatment plan based on the COVID-19 phase, tumor malignant potential, and patients’ comorbidities; and (2) Optimal treatment for pancreatic cancer should be planned according to the condition of each patient and tumor resectability based on national comprehensive cancer network resectability criteria. To choose the best therapeutic approach for patients with pancreatic tumors during this pandemic, we need to tailor the treatment plan based on elective surgery acuity scale (ESAS). Newly established ESAS for pancreatic tumor and flowchart indicating the treatment strategy of pancreatic cancer, are feasible to overcome this situation.
Core Tip: In this review article, we focused on the concept of triaging patients with pancreatic tumors under the new coronavirus (COVID-19) pandemic, and the actual impact of COVID-19 on the treatment of patients with pancreatic tumors. To select the best therapeutic strategy for patients with pancreatic tumors during this pandemic, we need to tailor the treatment plan based on elective surgery acuity scale (ESAS). Moreover, newly established ESAS for pancreatic tumor and flowchart indicating the treatment strategy of pancreatic cancer, are feasible to overcome this situation.
- Citation: Kato H, Asano Y, Arakawa S, Ito M, Kawabe N, Shimura M, Hayashi C, Ochi T, Yasuoka H, Higashiguchi T, Kondo Y, Nagata H, Horiguchi A. Surgery for pancreatic tumors in the midst of COVID-19 pandemic. World J Clin Cases 2021; 9(18): 4460-4466
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4460.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4460
The World Health Organization (WHO) declared the coronavirus disease (COVID-19) a pandemic on March 11, 2020. The spread of the new coronavirus infection in 2020 had a significant impact on cancer treatment worldwide. COVID-19 infection is characterized by the following: (1) Significant ability to spread the disease by asymptomatic carriers; (2) Strong infectivity by aerosolized droplets; and (3) Increased mortality in the elderlies and/or patients with comorbidities such as diabetes mellitus[1,2], obesity[3], and cardiovascular disease[4,5]. Moreover, patients with malignant disease have an increased risk for adverse outcome from COVID-19 infection. However, inability to undergo oncological care, including surgical treatments, chemotherapy, and chemoradiotherapy, might be a larger threat, especially for patients with pancreatic cancer because of its rapid progressive nature.
Among various pancreatic tumors, the prognosis of patients with pancreatic ductal adenocarcinoma (PDAC) is still extremely poor. This devastating tumor is a leading cause of cancer death worldwide. Indeed, multimodal treatment, including surgery, chemotherapy and chemoradiotherapy as a combination, are required to improve survival in PDAC patients. In the midst of the COVID-19 pandemic, more medical resources must be allocated to COVID-19 patients with PDAC. Thus, the number of surgeries has to be restricted and many pancreatic surgeries were stopped or postponed, which had a strong negative impact on PDAC patients. In this review article, from the standpoint of surgeons, we focused on the concept of triaging patients with pancreatic tumors, including PDAC, under the COVID-19 pandemic, and the actual impact of COVID-19 on the treatment of patients with pancreatic tumors.
Ideally, surgical resection is the only curative treatment for patients with PDAC and other pancreatic tumors, and it is desirable that the proposed treatment be performed as planned. However, during the COVID-19 pandemic, surgery or other invasive treatments for the patients with benign or low-malignant tumors, even PDAC, might not be possible. This is due to the lack of human and medical resources such as ICU staff, beds, and ventilators. Moreover, it is well known that viral infections are most likely to affect immunocompromised patients[6,7]. Furthermore, recent studies published in China revealed that cancer patients have a two-fold increased risk of COVID-19 infection[8]. Surgical stress may negatively affect cell-mediated immunity, resulting in impaired resistance to viruses. Indeed, COVID-19 may influence the postoperative course, and increase the mortality of operative patients[9,10]. Aminian et al[10] reported four fetal surgical patients who underwent cholecystectomy, hernia repair, gastric bypass, and hysterectomy; all these patients developed severe postoperative complications associated with COVID-19 infection in February 2020. A recent meta-analysis reported a surprising finding, 28 out of 269 patients with COVID-19 infection died from postoperative complications associated with COVID-19, such as respiratory failure and acute respiratory distress syndrome (ARDS)[11]. A multicenter cohort study in Canada reported that postoperative 30-day mortality in COVID-19 patients was notably high (15.9%)[12]. To date, several researchers have warned of the increased risk of postoperative mortality in patients with COVID-19 infection and have elucidated the characteristics of high-risk patients[9,13-15]. Therefore, the management of cancer definitely requires careful triaging of cases and disease-specific considerations.
Meanwhile, the American College of Surgeons (ACS) proposed Elective Case Triage Guidelines for Surgical Care and categorized the growing severity of the COVID-19 pandemic in a hospital into three phases based on the feasibility of medical resources (Table 1)[16]. According to this Tier system, we proposed the modified pancreatic surgery acuity protocol (Table 2), since there has been no Tier system specifying pancreatic tumors so far, which might help to determine surgical urgency for various pancreatic tumors. For example, in patients with intraductal papillary mucinous neoplasms (IPMNs), surgical treatment should be considered only when there are findings such as high-risk stigmata or main duct type IPMNs according to the international consensus in 2017[17]. The surgery for IPMNs with only worrisome features should be postponed, especially when medical resources are limited. However, in IPMNs with multiple risk factors, surgery decision-making depends on the respective hospitals, but it should be performed in hospitals with low/no COVID-19 census. Most pancreatic neuroendocrine tumors (PNETs) are indolent and slow-growing; however, they have significant malignant potential. Moreover, they constitute the second most common malignancy of the pancreas. According to previous reports[18,19], small PNETs (< 2 cm) were considered to be amenable to observation. Oba et al[20] reported a retrospective study of 145 patients with NET ≤ 2 cm, in which 85 patients were under active surveillance. After one year of radiological follow-up, 82% of the patients under active surveillance did not have any change in the size of the tumor. Moreover, a recent study showed the results of a global survey to reveal the role of pancreatic surgery during the COVID-19 pandemic to optimize patients’ and clinicians’ safety and safeguard health care capacity, more than 90% of surgeons agree with the opinion that in the absence of suspicion for malignancy, surgery for PNETs should be delayed. Thus, observation of small PNETs might be acceptable during the COVID-19 pandemic unless there is lymphadenopathy or clinical symptoms, whereas large PNETs or PNETs with lymphadenopathy should be resected since there is no effective chemotherapy. Solid pseudopapillary neoplasm (SPN) is also recognized as a borderline malignant tumor. Its 5-year survival rate was reported to be 98.8% after resection, but sometimes, a large SPN presents aggressive behaviors such as rupture, abdominal bleeding, and adjacent organ invasion[21]. Thus, a large or symptomatic tumor was recommended for resection, at least in hospitals with no/Low COVID-19 census.
| Phase 0: No COVID-19 patients, hospital works as normal |
| Phase 1: Semi-Urgent Setting. Few COVID-19 patients, hospital resources not exhausted, institution has still enough ICU ventilator capacity and COVID case trajectory not in the rapid escalation phase |
| Phase 2: Urgent Setting. Many COVID-19 Patients, ICU beds and ventilator capacity limited, OR supplies limited or COVID case trajectory within the hospital in rapidly escalating phase |
| Phase 3: Emergent setting. All hospital resources devoted to COVID-19 patients, no ventilator, ICU beds, OR supplies exhausted |
| Tiers/Description | Description | Location | Examples of primary diseases | Action |
| Tier 1A | Low acuity surgery/healthy patient; Not life-threatening illness | Hospital with low/no COVID-19 census | BD-IPMN without worrisome feature, SCN < 40 mm, other asymptomatic benign pancreatic tumors | Postpone surgery |
| Tier 1B | Low acuity surgery/unhealthy patient | Hospital with low/no COVID-19 census | Postpone surgery | |
| Tier 2A | Intermediate acuity surgery/healthy patient; Not life-threatening but potential for future morbidity and mortality; May require in-hospital stay | Hospital with low/no COVID-19 census | BD-IPMN with worrisome features, SCN > 40 mm, Asymptomatic PNET < 20 mm, Asymptomatic small SPN (< 20 mm), Mucinous cyst neoplasm | Phase 1: Postpone surgery if possible and active surveillance; Phase 0: Perform surgery under the maximum preparation for infection control |
| Tier 2B | Intermediate acuity surgery/unhealthy patient | Hospital with low/no COVID-19 census | Phase 1: Postpone surgery if possible and active surveillance; Phase 0: Perform surgery under the maximum preparation for infection control | |
| Tier 3A | High acuity surgery/healthy patient | Hospital with low/no COVID-19 census | PDAC, BD-IPMN with high risk stigmata, Symptomatic PNET, PNET > 20 mm, PNET with lymphadenopathy, symptomatic or large (> 20 mm) SPN | Perform surgery under the maximum infection control |
| Tier 3B | High acuity surgery/unhealthy patient | Hospital with low/no COVID-19 census | Perform under the maximum infection control |
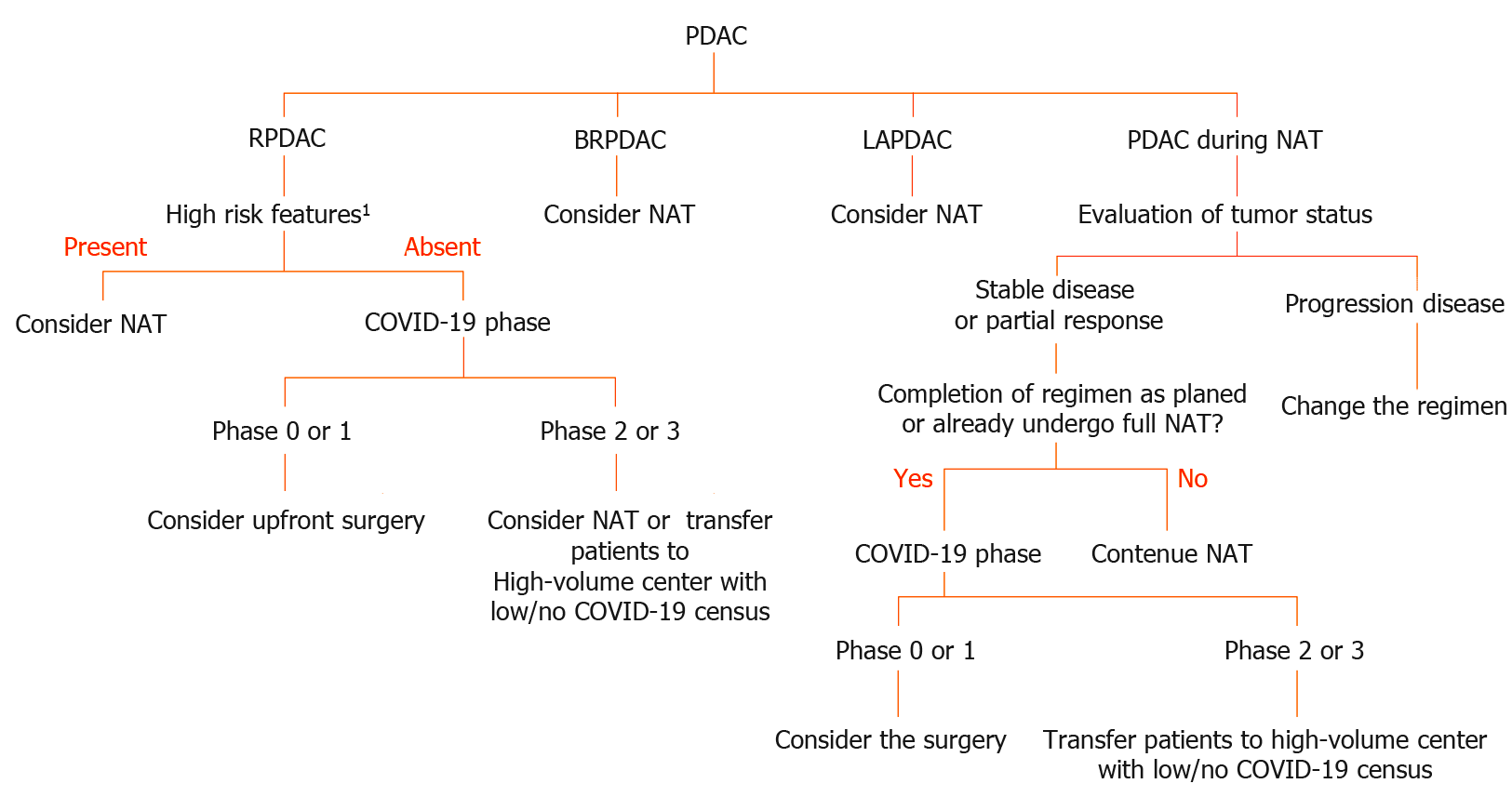
The standard treatment for resectable PDAC (RPDAC) is upfront surgery without neoadjuvant chemotherapy unless there are high-risk features including high CA19-9 Levels, large primary tumors, suspicion of regional lymph node metastasis, excessive weight loss, and extreme pain in accordance with the national comprehensive cancer network (NCCN) guideline 2019[22]. Therefore, surgical resection should be prioritized for patients with PDAC without these high-risk features. However, neoadjuvant chemotherapy might also be an acceptable alternative treatment in such cases, especially when intensive care unit (ICU) beds and ventilator capacity and other medical resources are exhausted.
In contrast to RPDAC, neoadjuvant chemo- or chemoradiotherapy has been regarded as standard induction therapy for patients with borderline resectable (BR) or locally advanced PDAC (LAPDAC), this strategy is adapted for them regardless of the COVID-19 pandemic. Prolongation of neoadjuvant treatment is a rational alternative for patients with BR or LAPDAC if the ICU beds, ventilators and other medical resources are saturated by COVID-19 patients. However, patients with PDAC who have already received full doses of chemotherapy and their tumor local control is favorable, should undergo tumor removal if possible. This type of aggressive pancreatectomy, so-called adjuvant surgery or conversion surgery, might have an increased risk of major postoperative complications and COVID-19 infection compared with that for RPDAC. This is due to the neoadjuvant treatment. Therefore, adequate informed consent (i.e., increased risk of COVID-19 infection, fatal complications associated with COVID-19 infection etc.) are mandatory. Furthermore, prehospital COVID-19 screening using PCR might be useful to prevent further spreading. As shown in Figure 1, we proposed a flowchart diagram providing proper treatment for PDAC patients during the COVID-19 pandemic.
During the COVID-19 pandemic, the importance of neoadjuvant treatment is notably increased, as described above. Therefore, the increased risk of COVID-19 infection in patients receiving chemotherapy should be fully understood before initiating chemotherapy.
In terms of the effect of COVID-19 on patients with chemotherapy, a recent article reported that cytotoxic chemotherapy treatment was not associated with adverse COVID-19 outcomes, but peri–COVID-19 Lymphopenia, or baseline neutropenia, had worse chemotherapy outcomes[23]. Lee et al[24] elucidated that the history of chemotherapy in the past 4 wk had no significant effect on mortality in COVID-19 patients, when compared with cancer patients who had not received recent chemotherapy (OR = 1.18, P = 0.380). They also found no significant effect on mortality in patients with immunotherapy and radiotherapy use within the past 4 wk. According to NCCN guidelines[25], the following regimens are recommended for patients with locally advanced pancreatic cancer (LAPC): (1) FOLFIRINOX (folinic acid, 5-fluorouracil, irinotecan, oxaliplatin) or mFOLFIRINOX ± subsequent chemoradiation; (2) Gemcitabine + albumin-bound paclitaxel ± subsequent chemoradiation; and (3) Only for known BRCA1/2 or PALB2 mutations: FOLFIRINOX or mFOLFIRINOX or gemcitabine + cisplatin (≥2–6 cycles) ± subsequent chemoradiation.
To safely use these chemotherapies for PDAC patients during this pandemic, we need to understand that COVID-19 infection itself induces lymphopenia and neutropenia, causing further weakening of the immune system. Meanwhile, PRODIGE/ACCORD trial with FOLFIRINOX reported that the incidence of grade 3 or 4 neutropenia was seen in 45.7%[26], and MPACT trial reported that grade 3 or higher neutropenia was seen in 38% of patients in nab-paclitaxel plus gemcitabine group[27]. Therefore, to prevent leucopenia caused by COVID-19 infections, modification of the regimen or schedule (i.e., omit 5FU borus administration, switch the schedule from weekly to biweekly) might be acceptable in the setting of phases 1 to 3.
To choose the best therapeutic approach for patients with pancreatic tumors during this pandemic, we need to tailor the treatment plan based on the COVID-19 phase, tumor malignant potential, and patients’ comorbidities. Especially in PDAC, optimal treatment should be carefully individualized according to the condition.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atogebania JW, Palmeri M S-Editor: Ma YJ L-Editor: A P-Editor: Wu YXJ
| 1. | Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 685] [Cited by in RCA: 604] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 2. | Selvin E, Juraschek SP. Diabetes Epidemiology in the COVID-19 Pandemic. Diabetes Care. 2020;43:1690-1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Hussain A, Mahawar K, Xia Z, Yang W, El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020;14:295-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 4. | Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Retraction: Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. DOI: 10.1056/NEJMoa2007621. N Engl J Med. 2020;382:2582. [PubMed] [DOI] [Full Text] |
| 5. | Gori T, Lelieveld J, Münzel T. Perspective: cardiovascular disease and the Covid-19 pandemic. Basic Res Cardiol. 2020;115:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Fishman JA, Grossi PA. Novel Coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant. 2020;20:1765-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 7. | Fung M, Babik JM. COVID-19 in Immunocompromised Hosts: What We Know So Far. Clin Infect Dis. 2021;72:340-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 372] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 8. | Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 Transmission in Patients With Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 2020;6:1108-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 626] [Cited by in RCA: 776] [Article Influence: 155.2] [Reference Citation Analysis (0)] |
| 9. | Egol KA, Konda SR, Bird ML, Dedhia N, Landes EK, Ranson RA, Solasz SJ, Aggarwal VK, Bosco JA 3rd, Furgiuele DL, Ganta A, Gould J, Lyon TR, McLaurin TM, Tejwani NC, Zuckerman JD, Leucht P; NYU COVID Hip Fracture Research Group. Increased Mortality and Major Complications in Hip Fracture Care During the COVID-19 Pandemic: A New York City Perspective. J Orthop Trauma. 2020;34:395-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 10. | Aminian A, Safari S, Razeghian-Jahromi A, Ghorbani M, Delaney CP. COVID-19 Outbreak and Surgical Practice: Unexpected Fatality in Perioperative Period. Ann Surg. 2020;272:e27-e29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 11. | Wang K, Wu C, Xu J, Zhang B, Zhang X, Gao Z, Xia Z. Factors affecting the mortality of patients with COVID-19 undergoing surgery and the safety of medical staff: A systematic review and meta-analysis. EClinicalMedicine. 2020;29:100612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Carrier FM, Amzallag É, Lecluyse V, Côté G, Couture ÉJ, D'Aragon F, Kandelman S, Turgeon AF, Deschamps A, Nitulescu R, Djade CD, Girard M, Beaulieu P, Richebé P. Postoperative outcomes in surgical COVID-19 patients: a multicenter cohort study. BMC Anesthesiol. 2021;21:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Clement ND, Hall AJ, Makaram NS, Robinson PG, Patton RFL, Moran M, Macpherson GJ, Duckworth AD, Jenkins PJ. IMPACT-Restart: the influence of COVID-19 on postoperative mortality and risk factors associated with SARS-CoV-2 infection after orthopaedic and trauma surgery. Bone Joint J. 2020;102-B:1774-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Nahshon C, Bitterman A, Haddad R, Hazzan D, Lavie O. Hazardous Postoperative Outcomes of Unexpected COVID-19 Infected Patients: A Call for Global Consideration of Sampling all Asymptomatic Patients Before Surgical Treatment. World J Surg. 2020;44:2477-2481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 15. | Abate SM, Mantefardo B, Basu B. Postoperative mortality among surgical patients with COVID-19: a systematic review and meta-analysis. Patient Saf Surg. 2020;14:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | (ACS) ACoS. COVID-19: elective case triage guidelines for surgical Care 2020; 2020. Available from: https://www.fac-s.org/covid-19/clinical-guidance/elective-case. |
| 17. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (1)] |
| 18. | Bettini R, Partelli S, Boninsegna L, Capelli P, Crippa S, Pederzoli P, Scarpa A, Falconi M. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Gaujoux S, Partelli S, Maire F, D'Onofrio M, Larroque B, Tamburrino D, Sauvanet A, Falconi M, Ruszniewski P. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98:4784-4789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Oba A, Stoop TF, Löhr M, Hackert T, Zyromski N, Nealon WH, Unno M, Schulick RD, Al-Musawi MH, Wu W, Zhao Y, Satoi S, Wolfgang CL, Abu Hilal M, Besselink MG, Del Chiaro M; Pancreas Club; European Pancreatic Club; Chinese Pancreatic Surgery Association; European Consortium on Minimally Invasive Pancreatic Surgery; Study Group of Preoperative Therapy for Pancreatic Cancer; Study Group of Pancreatic Ductal Adenocarcinoma with Peritoneal Metastasis and International Study Group on Cystic Tumors of the Pancreas. Global Survey on Pancreatic Surgery During the COVID-19 Pandemic. Ann Surg. 2020;272:e87-e93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Hanada K, Kurihara K, Itoi T, Katanuma A, Sasaki T, Hara K, Nakamura M, Kimura W, Suzuki Y, Sugiyama M, Ohike N, Fukushima N, Shimizu M, Ishigami K, Gabata T, Okazaki K. Clinical and Pathological Features of Solid Pseudopapillary Neoplasms of the Pancreas: A Nationwide Multicenter Study in Japan. Pancreas. 2018;47:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Tempero MA, Malafa MP, Chiorean EG, Czito B, Scaife C, Narang AK, Fountzilas C, Wolpin BM, Al-Hawary M, Asbun H, Behrman SW, Benson AB, Binder E, Cardin DB, Cha C, Chung V, Dillhoff M, Dotan E, Ferrone CR, Fisher G, Hardacre J, Hawkins WG, Ko AH, LoConte N, Lowy AM, Moravek C, Nakakura EK, O'Reilly EM, Obando J, Reddy S, Thayer S, Wolff RA, Burns JL, Zuccarino-Catania G. Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw. 2019;17:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (2)] |
| 23. | Jee J, Foote MB, Lumish M, Stonestrom AJ, Wills B, Narendra V, Avutu V, Murciano-Goroff YR, Chan JE, Derkach A, Philip J, Belenkaya R, Kerpelev M, Maloy M, Watson A, Fong C, Janjigian Y, Diaz LA Jr, Bolton KL, Pessin MS. Chemotherapy and COVID-19 Outcomes in Patients With Cancer. J Clin Oncol. 2020;38:3538-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 24. | Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, Chackathayil J, Cheng VW, Curley HM, Fittall MW, Freeman-Mills L, Gennatas S, Goel A, Hartley S, Hughes DJ, Kerr D, Lee AJ, Lee RJ, McGrath SE, Middleton CP, Murugaesu N, Newsom-Davis T, Okines AF, Olsson-Brown AC, Palles C, Pan Y, Pettengell R, Powles T, Protheroe EA, Purshouse K, Sharma-Oates A, Sivakumar S, Smith AJ, Starkey T, Turnbull CD, Várnai C, Yousaf N; UK Coronavirus Monitoring Project Team; Kerr R, Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 839] [Article Influence: 167.8] [Reference Citation Analysis (0)] |
| 25. | Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. J Natl Compr Canc Netw. 2019;17:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 123] [Reference Citation Analysis (0)] |
| 26. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5641] [Article Influence: 402.9] [Reference Citation Analysis (1)] |
| 27. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4890] [Article Influence: 407.5] [Reference Citation Analysis (0)] |