Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.4072
Peer-review started: January 16, 2021
First decision: February 11, 2021
Revised: February 23, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: June 6, 2021
Processing time: 118 Days and 7.2 Hours
Spinal epidural abscess (SEA) is a rare condition that mostly results from infection with either bacteria or tuberculosis. However, coinfection with bacteria and tuberculosis is extremely rare, and it results in delays in diagnosis and antimicrobial treatment causing unfavorable outcomes.
A 75-year-old female visited the hospital with low back pain, and magnetic resonance imaging (MRI) revealed an SEA at the lumbosacral segment. Staphylococcus hominis and methicillin-resistant Staphylococcus epidermidis were identified from preoperative blood culture and intraoperative abscess culture, respectively. Thus, the patient underwent treatment with vancomycin medication for 9 wk after surgical drainage of the SEA. However, the low back pain recurred 2 wk after vancomycin treatment. MRI revealed an aggravated SEA in the same area in addition to erosive destruction of vertebral bodies. Second surgery was performed for SEA removal and spinal instrumentation. The microbiological study and pathological examination confirmed Mycobacterium tuberculosis as the pathogen concurrent with the bacterial SEA. The patient improved completely after 12 mo of antitubercular medication.
We believe that the identification of a certain pathogen in SEAs does not exclude coinfection with other pathogens. Tubercular coinfection should be suspected if an SEA does not improve despite appropriate antibiotics for the identified pathogen.
Core Tip: Spinal epidural abscess (SEA) is a rare condition that mostly results from infection with either bacteria or tuberculosis (TB). However, coinfection with bacteria and TB, as in our case, is extremely rare. Because the blood culture and the surgical specimen results supported bacterial infection, we initially neglected the possibility of TB. Only after SEA recurrence did we suspect coinfection with other organisms. Now, we believe that the identification of a certain pathogen in SEAs does not exclude coinfection with other pathogens. Tubercular coinfection should be suspected if an SEA does not improve despite appropriate antibiotics for the identified pathogen.
- Citation: Kim C, Lee S, Kim J. Spinal epidural abscess due to coinfection of bacteria and tuberculosis: A case report. World J Clin Cases 2021; 9(16): 4072-4080
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/4072.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.4072
Spinal epidural abscess (SEA) is a rare but potentially devastating infection[1-4]. Its incidence was estimated to be 0.2-1.2 cases per 10000 hospital admissions in the mid-1970s[1,3]. However, rates are increasing, having doubled in the past 20 years[1,2]. Several factors are thought to be responsible for this increase: an aging population, a greater number of spinal procedures, drug abuse, and altered immune states[5]. The treatment of choice for SEAs is generally surgical intervention in combination with appropriate antimicrobial treatment[1-3,5].
SEAs mostly result from the infection of either bacteria or tuberculosis (TB). However, coinfection of both bacteria and TB, as in our case, is extremely rare, and few cases have been reported[6-9]. Hematogenous spread is the primary mechanism in both bacterial and tubercular SEAs[1,2,5,8,9]. Bacteremia is an important condition in infectious disease that can sometimes develop into sepsis, although the majority of bacteria are cleared from the bloodstream[10]. Thus, in patients with bacteremia, urgent antibiotic treatment is usually indicated, omitting further studies for the identification of other concomitant pathogens.
In this study, we present a very rare case of an SEA originating from the coinfection of bacteria and TB. Our case is noteworthy in that the bacteremia and pyogenic SEA masked the tubercular SEA, causing delays in diagnosis and antitubercular treatment.
A 75-year-old female was admitted with recently aggravated low back pain.
Patient’s low back pain started 2 mo ago and was aggravated despite epidural block. The pain was continuous and the patient had no trauma history.
The patient had hypertension, unstable angina and congestive heart failure. Although the chest computed tomography (CT) scan found pulmonary edema 2 mo ago, a microbiological study revealed no TB from sputum or bronchoalveolar lavage (BAL) specimens.
The patient had a free personal or family history and denied the history or contact with TB.
The patient was afebrile, and the neurological examination produced normal findings. The patient was free of any pulmonary symptom including dyspnea, hemoptysis or cough.
Routine laboratory examination showed an elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level (59 mm/h and 7.718 mg/dL, respectively) (normal range: 0-20 mm/h and 0-0.75 mg/dL, respectively), although the white blood cell (WBC) count was within the normal range (6700/µL) (normal range: 3800-10000/µL).
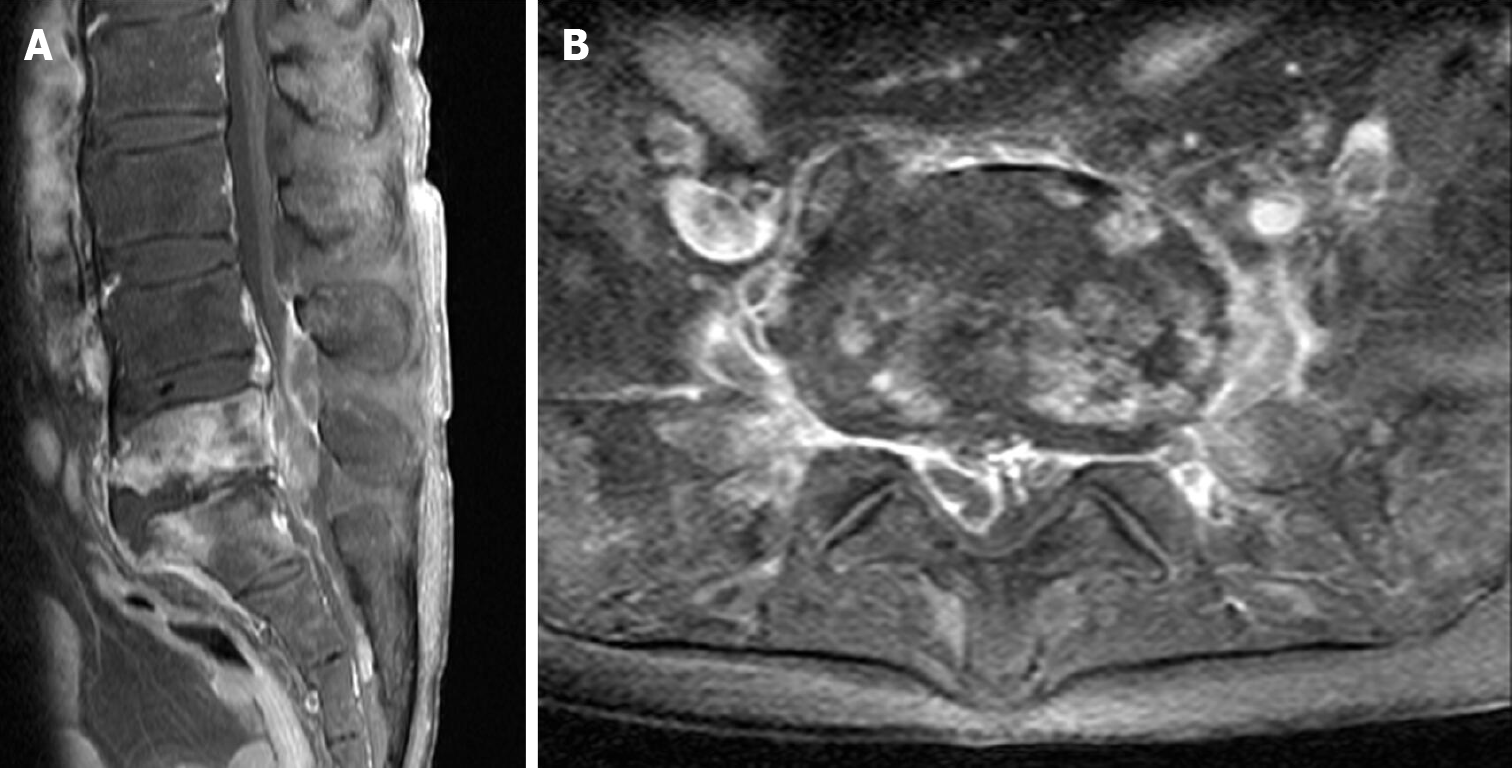
Contrast-enhanced magnetic resonance imaging (MRI) demonstrated spondylitis covering the fifth lumbar and the first sacral (L5-S1) vertebrae and that a significant amount of the SEA involved the spinal canal and psoas muscle (Figure 1).
Peripheral blood culture identified Staphylococcus hominis (S. hominis) preoperatively. They were positive from 2 sets of blood cultures and both were identical in genus and species level (S. hominis genus and hominis species).
The patient underwent L5-S1 laminectomy and SEA drainage via a posterior approach, and methicillin-resistant Staphylococcus epidermidis (S. epidermidis) was identified from intraoperatively acquired abscess culture. Histopathological examination of the surgical specimen showed no evidence of TB.
Thus, vancomycin was administered based on the final microbiological report of drug sensitivity for the identified pathogens. Whereas the low back pain improved, the CRP level did not decrease below 5.0 mg/dL despite 9 wk of postoperative vancomycin treatment.
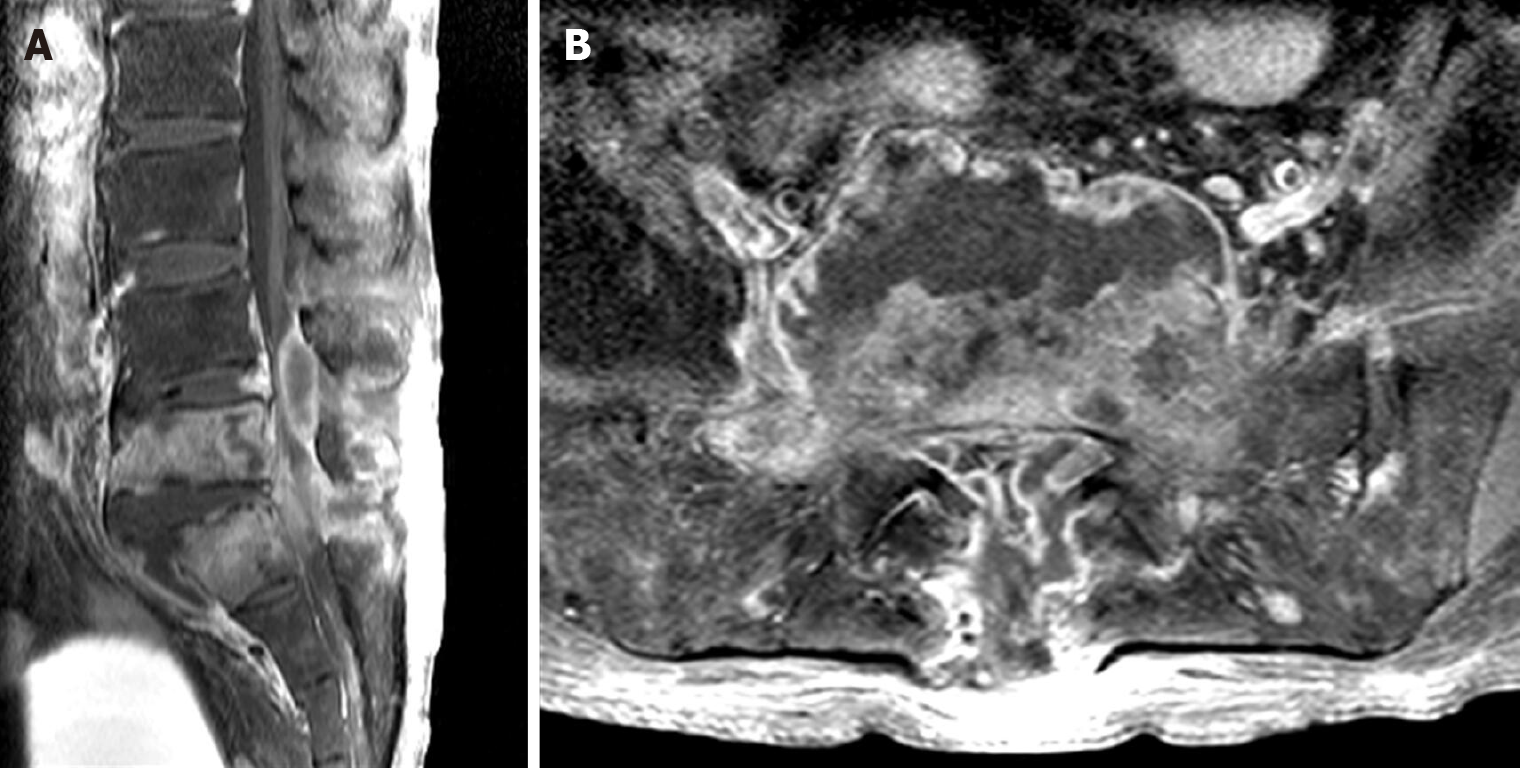
The low back pain deteriorated two weeks after the completion of vancomycin treatment. Despite an intact neurological status, MRI revealed SEA recurrence in the same area (Figure 2). Although the symptoms of infection were vague, the ESR and CRP level were elevated (74 mm/h and 5.525 mg/dL, respectively). The patient underwent second surgery for lumbar corpectomy and SEA removal as well as spinal instrumentation. Corpectomy, debridement, and interbody fusion were performed via a retroperitoneal approach. Then, epidural abscess removal and pedicle screw fixation were performed via a posterior approach.
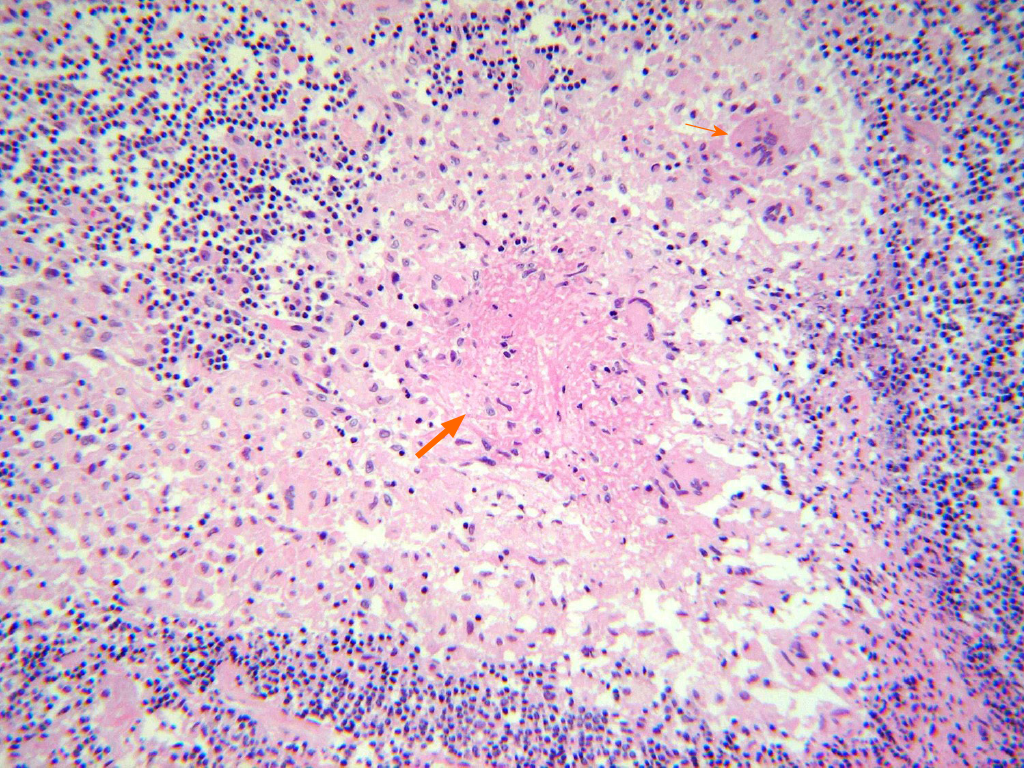
Preoperative peripheral blood culture and intraoperative abscess culture identified bacterial infection. In addition to bacteria, we also identified Mycobacterium tuberculosis (M. tuberculosis) from intraoperatively acquired abscess based on following examinations: (1) Acid-fast bacillus (AFB) staining; (2) AFB culture; (3) Histo-pathological examination (Figure 3); And (4) polymerase chain reaction (PCR). Moreover, AFB staining and culture were also positive in BAL specimens. In conclusion, the coinfection of bacteria and TB has been confirmed. Whereas methicillin-resistant S. epidermidis was identified in first surgery, no bacteria were identified from SEA in second surgery.
The patient had two times of surgeries for abscess drainage and debridement of infected tissue. After first surgery, the patient underwent 9 wk of postoperative vancomycin treatment based on antibiotics susceptibility test for methicillin-resistant S. epidermidis. After second surgery, the patient also had antitubercular medication for 12 mo. We initiated combination therapy using Isoniazid (INH 300 mg qd), Rifampin (RFP 600 mg qd), Ethambutol (EMB 1200 mg qd), and Pyrazinamide (PZA 1500 mg qd). Because the patient experienced urticaria and skin rash in 3 wk, PZA was replaced by Levofloxacin (500 mg qd). Whereas INH and RFP were continued without dose adjustment throughout 1-year of antitubercular treatment, EMB was reduced to 800 mg qd and Levofloxacin was discontinued in 4 mo.
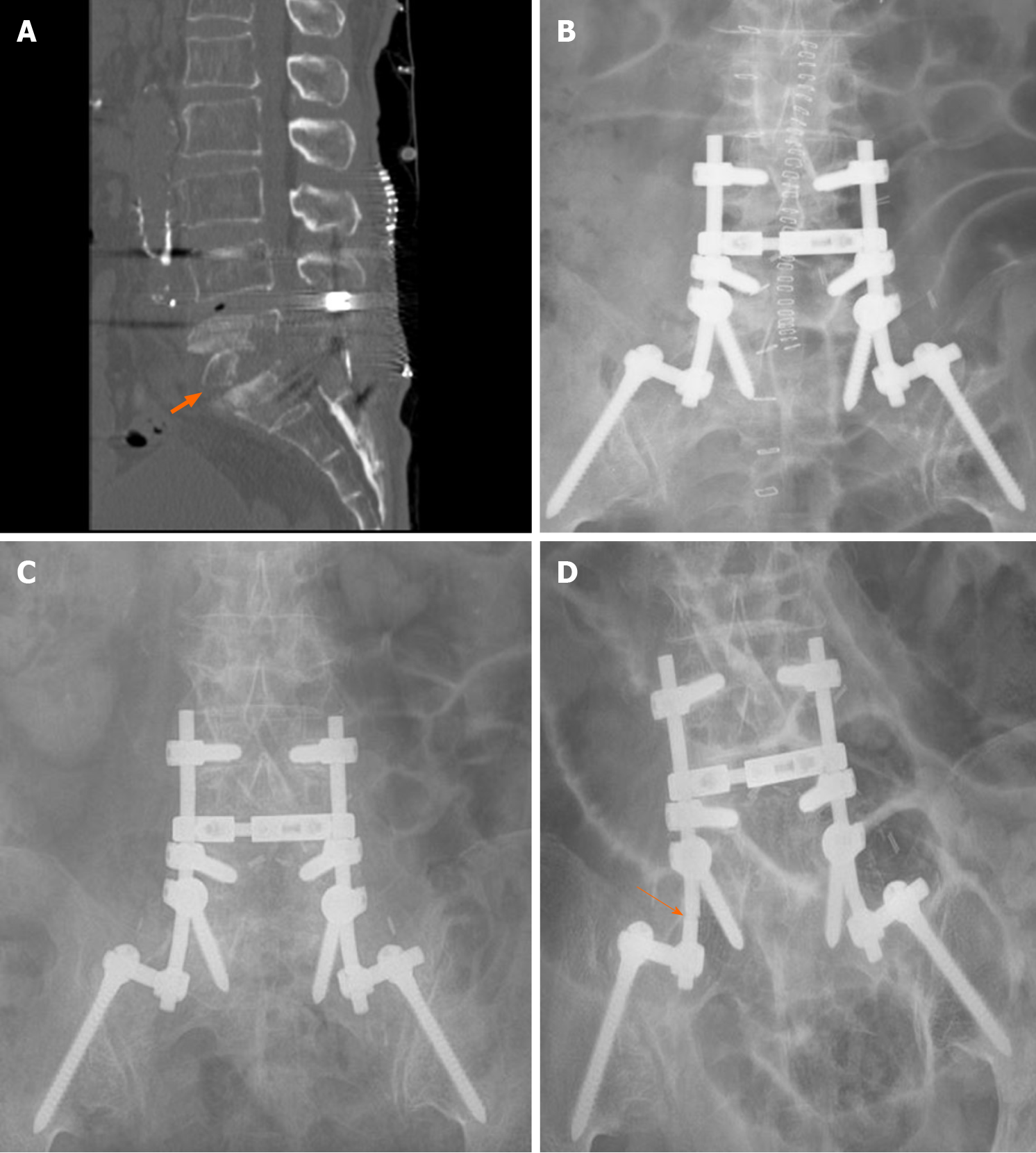
The patient recovered completely without any sequelae. After completion of 1-year of antitubercular medication, the SEA or TB never recurred during the 10-year follow-up period. Serial chest X-rays and PCR test, which was performed 10 years later were free from pulmonary TB. CT scan and serial X-rays showed no change of spinal instrument until follow-up X-ray revealed rod fracture 10 years later (Figure 4). However, low back pain never aggravated and the patient did not have additional medication for pain or spinal intervention after second surgery. ESR has been normalized within 1 wk after second surgery, whereas CRP remained high until completion of antitubercular medication.
Spinal TB accounts for 2% of TB cases and 10%-15% of cases of extrapulmonary TB[8]. Approximately 3%-5% of patients with pulmonary TB develop musculoskeletal lesions[4], and spinal TB is the most common form of skeletal TB, accounting for almost 50% of cases[8]. Spinal TB is usually a result of the hematogenous spread of M. tuberculosis, with primary lesions in either the lungs or genitourinary system[8]. Our patient also had pulmonary TB, which might have been the origin of the tubercular SEA. However, we diagnosed it after spinal TB was confirmed in second surgery because our patient had no previous pulmonary symptoms. Spinal TB is known to be more prevalent in females, and risk factors include diabetes mellitus, immuno-suppression, and prior exposure to TB[6]. There was little evidence to suspect pulmonary or spinal TB in our patient, despite some risk factors.
We have found two cases of spinal abscess originating from coinfection of TB and bacteria[6,9]. The one was related with methicillin-resistant coagulase-negative staphylococcus (CoNS) and M. tuberculosis[6]. Although the patient had a history of pulmonary TB, the diagnosis of spinal TB has been delayed like our case. The other case was related with Nocardia asteroids, Moraxella catarrhalis, and M. tuberculosis[9]. The patient did not have a history of pulmonary TB.
Although 20%-30% of cases of spinal TB have been reported to have constitutional symptoms, including malaise, evening increases in temperature, loss of weight[8], these symptoms were not definite in our patient, and if ever, they would not have been informative because they are nonspecific findings. Such ambiguity of initial symptoms and lack of pathognomonic findings could have caused delayed diagnosis, resulting in neurological complications in our patient. Moreover, the classic triad of back pain, fever and neurological deficits has been reported to be present in only 13% of SEA patients[4]. An elevated ESR and CRP level were helpful for suspecting spinal infection in our patient, although they did not assist with distinguishing tubercular infection from bacterial infection. Approximately 90% of patients with spinal infection exhibit an elevated ESR[11], and an elevated CRP level is more sensitive and more effective in monitoring treatment responses due to its shorter half-life[4]. However, their elevations do not constitute definitive evidence of infection, since these findings are also seen in inflammatory diseases[11]. Although often used as a screening tool, the WBC count is unreliable and may be normal in up to 40% of patients[4]. Some investigators have even reported that compared to pyogenic spinal infections, those due to mycobacteria are associated with milder increases in the ESR, WBC, and CRP level[11].
Positive findings on MRI can be observed just 3-5 d after the onset of infection, with a sensitivity of 96% and a specificity of 93%[11]. Although MRI identified an SEA in our patient, which was considered the cause of low back pain, the causative pathogens had to be identified to select appropriate antimicrobial agents. The mortality rate of spinal infection ranged from 25%-70% before the development and widespread availability of antibiotics and decreased dramatically thereafter[11]. Thus, the selection of appropriate antibiotics is critical, and these agents should be selected based on the consideration of antimicrobial susceptibility and penetration into spinal tissue[11]. Findings on MRI that can help distinguish spinal TB from pyogenic infection include a large, well-defined paraspinal abscess with thin rim enhancement and smooth margins, thoracic spine involvement, subligamentous extension to adjacent vertebrae with preserved disc height, and multilevel involvement with skip lesions[4]. Although our patient’s MRI showed bony erosion and cold abscess, preoperative blood culture result strongly suggested bacterial infection and S. epidermidis was identified from multiple intraoperative abscess specimens. We were more tentative to diagnose the spinal TB, because chest CT scans preformed 2 and 6 mo prior to first surgery failed to detect pulmonary TB and the patient did not have any symptom of it. After recognizing the recurrence of spinal abscess, we carefully suspected coinfection of TB. However, we could not confident of the coinfection of bacteria and TB until second surgery, because it was very rare and few cases had been reported.
Our patient showed nonspecific histopathological results at the first surgery. It is unclear whether spinal TB was absent in our patient initially and occurred later or was already present but missed at the first surgery. We believe that our patient’s spinal TB was already present at the first surgery and that SEA recurrence was the continuation of spinal TB, not a new event, because the elevated CRP level did not decrease throughout vancomycin treatment, and the location of the SEA at the time of second surgery was the same as on initial MRI. If the initial SEA and the recurred SEA were unrelated conditions, they were likely to have involved another spinal segment, showing different radiological features. Considering the short time interval and similar radiological features, we believe that both the initial and recurred SEAs were due to concurrent infection with both bacteria and TB. Finally, our patient had latent pulmonary TB, which was diagnosed after second surgery. Considering the major portal of entry for TB is respiratory and genitourinary system, it is very likely to be the source of SEA. We think it is more reasonable explanation rather than that postoperative TB infection resulted in SEA, which became the source of pulmonary TB. Nevertheless, the bacteremia and positive bacterial culture results from the abscess misled us to neglect tubercular involvement.
The accuracy of blood culture for the identification of pathogens is reported to be 85%[6]. The preoperative bacteremia was a very significant sign in that close observation was indispensable for preventing the development of sepsis and for the selection of optimal antibiotics. Compared with S. aureus, which is the most commonly identified pathogen in spinal infections[4,11], S. epidermidis identified in our patient is considered an organism with low pathogenicity[11]. The positive bacterial culture results in the surgical specimen reinforced the diagnosis of pyogenic infection, neglecting coinfection with M. tuberculosis in our patient. However, our patient’s clinical symptoms and radiological findings deteriorated after brief improvement, despite appropriate antibiotic treatment. The unresponsiveness to vancomycin treatment observed in our patient was an important clue for coinfection with M. tuberculosis. An ESR > 55 mm/h and a CRP level > 2.75 mg/dL after 4 wk of antibiotic treatment is associated with treatment failure (odds ratio 5.15) in the treatment of discitis and vertebral osteomyelitis[4].
Although the most important factor for diagnosing spinal infection is the result of microbiological studies and histopathologic examination, the time required to obtain culture results varies by pathogen[6]. Of 175 mycobacteria species currently recognized, slow-growing mycobacteria are the most suspected pathogens in humans[12]. In contrast, most rapidly growing mycobacteria are thought to be incapable of infecting humans[12]. Thus, the results of tubercular culture may sometimes appear much later than those of other pyogenic bacteria, resulting in missed or delayed diagnosis[6]. The examination of direct smears for AFB is the most rapid method for the detection of mycobacteria[13]. However, a relatively large number of bacteria (> 104-105/mL) must be present in the sample for detection[12-14], and if that number is less than 1000/mL, the chance of finding AFB would be less than 10%[12]. In contrast to the high specificity of 95%-98%, its sensitivity is reported to be only 20%-70%[12].
The culturing of organisms has a specificity that approaches 100% and permits drug susceptibility testing of isolates, but the slow growth of most pathogenic mycobacteria (3-6 wk) results in the delay of a definitive diagnosis[13]. The minimum number of bacilli can be as low as 100 for the diagnosis to become positive[12]. The sensitivity and specificity of culture-based diagnosis are known to be 73%-95% and 98%, respectively[12]. However, these data are primarily obtained from pulmonary TB and depend on the stage of TB and the presence of bacilli in the sample as well as the geographic location[12]. Other studies have documented that the sensitivity of culture can be as low as 50%, with a relatively low detection of TB at extrapulmonary sites[14]. Since it takes several weeks until cultures of mycobacterial species are completed, PCR has been used for rapid identification[11,13]. However, clinicians should be aware that PCR will be positive even during the inactive phase since it can detect dead mycobacteria[11]. Under the best conditions, assuming the test is performed in a modern laboratory with experienced technicians, the sensitivity of PCR is 90% for smear-positive and culture-positive samples and 40%-77% for smear-negative samples[12].
Radical debridement followed by autologous strut bone grafting has been considered the gold-standard surgical treatment for spinal infection since the 1950s[11,15]. We performed interbody fusion and pedicle screw instrumentation following corpectomy for the infected vertebral bodies in second surgery, whereas only laminectomy and SEA drainage without instrumentation were performed in the first surgery. Extensive infected tissue debridement was also likely to be advantageous in the histopathological and microbiological diagnoses, providing a sufficient number of specimens to minimize the possibility of false-negative results. Since biofilm-covered colonies of M. tuberculosis have been reported to be very rare, spinal infection caused by this organism is now widely treated with anterior instrumentation performed concurrently with anterior debridement[11]. This technique was also introduced for pyogenic spinal infection not long ago; therefore, the addition of posterior instrumentation to debridement and strut bone grafting, as in our case, has recently become popular[11].
S. hominis is a coagulase-negative member of the genus Staphylococuss (CoNS). They are commonly encountered blood culture contaminants whose contamination rate is reported to be over 44%[16,17]. On the other hand, they also constitute an important cause of blood stream infection in the ever-expanding population of patients with biomedical devices, broad-spectrum antibiotics and indwelling catheters[16,17]. Thus, the isolation of CoNS from blood cultures remains a clinical dilemma in many cases, and it is difficult to determine with certainty the clinical significance of these isolates[18]. However, our result was derived from 2 sets of blood cultures and both were identical even in species level (S. hominis genus and hominis species). Kirn and Weinstein[16] recommended to identify CoNS to the species level (not just genus level) when more than one set of blood cultures are positive. If the CoNS from multiple blood culture sets are same not only in genus level but also in species level like our case, the odds of contamination decrease[16]. Weinstein et al[18] also described if 2 or more blood cultures grow CoNS and they are identical in biochemical profile and susceptibility, the probability of true infection increases. Therefore, we think our blood culture result is likely to be true infection, although it is difficult to completely exclude the possibility of contamination. Moreover, we believe that the intraoperative abscess culture was not contamination, because the methicillin-resistant S. epidermidis were identified from multiple sites.
Because the causative pathogens were already identified in blood culture, we initially neglected the possibility of TB. Moreover, the surgical specimen results also supported bacterial infection, and our patient had no symptoms or history of TB. Only after SEA recurrence did we suspect concurrent infection with other organisms. However, coinfection with TB was minimally considered because it is extremely rare. Now, we believe a high index of suspicion is mandatory even in such conditions.
We believe that the identification of a certain pathogen in SEA does not rule out coinfection of other pathogens. Thus, coinfection should be suspected if a certain infection does not improve despite appropriate antibiotic treatment for the pathogen. Because TB can be masked by concurrent bacterial infection, a high index of suspicion is mandatory even in patients with bacteremia.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C, C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Chen X, Liang P, Luan SD, Ma X, Mao HJ, Wu HY, Zhang L, Zhao GH S-Editor: Gong ZM L-Editor: A P-Editor: Liu JH
| 1. | Patel AR, Alton TB, Bransford RJ, Lee MJ, Bellabarba CB, Chapman JR. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 2014;14:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Vakili M, Crum-Cianflone NF. Spinal Epidural Abscess: A Series of 101 Cases. Am J Med. 2017;130:1458-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Arko L 4th, Quach E, Nguyen V, Chang D, Sukul V, Kim BS. Medical and surgical management of spinal epidural abscess: a systematic review. Neurosurg Focus. 2014;37:E4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Boody BS, Tarazona DA, Vaccaro AR. Evaluation and Management of Pyogenic and Tubercular Spine Infections. Curr Rev Musculoskelet Med. 2018;11:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Akalan N, Ozgen T. Infection as a cause of spinal cord compression: a review of 36 spinal epidural abscess cases. Acta Neurochir (Wien). 2000;142:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Kim YM, Cha JH. Delayed diagnosis of tuberculous spondylitis masked by concomitant methicillin resistant Staphylococcus aureus infection. J Korean Neurosurg Soc. 2010;47:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Fu WK, Wu WC, Ip FK. Concomitant tuberculosis and pyogenic infection of the cervical spine. A case report. Spine (Phila Pa 1976). 1998;23:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Diyora B, Patil S, Bhende B, Patel M, Dhall G, Nayak N. Concurrent Spinal Epidural Tubercular and Pyogenic Abscess of Cervical Spine without Bony Involvement. J Neurosci Rural Pract. 2019;10:374-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Mousa HA. Concomitant spine infection with mycobacterium tuberculosis and pyogenic bacteria: case report. Spine (Phila Pa 1976). 2003;28:E152-E154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Minasyan H. Sepsis and septic shock: Pathogenesis and treatment perspectives. J Crit Care. 2017;40:229-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Nagashima H, Tanishima S, Tanida A. Diagnosis and management of spinal infections. J Orthop Sci. 2018;23:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Azadi D, Motallebirad T, Ghaffari K, Shojaei H. Mycobacteriosis and Tuberculosis: Laboratory Diagnosis. Open Microbiol J. 2018;12:41-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Abe C, Hirano K, Wada M, Kazumi Y, Takahashi M, Fukasawa Y, Yoshimura T, Miyagi C, Goto S. Detection of Mycobacterium tuberculosis in clinical specimens by polymerase chain reaction and Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test. J Clin Microbiol. 1993;31:3270-3274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 140] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Berk H. Concomitant tuberculosis and pyogenic infection of the cervical spine. Spine (Phila Pa 1976). 1998;23:2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | HODGSON AR, STOCK FE. Anterior spinal fusion a preliminary communication on the radical treatment of Pott's disease and Pott's paraplegia. Br J Surg. 1956;44:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 177] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Kirn TJ, Weinstein MP. Update on blood cultures: how to obtain, process, report, and interpret. Clin Microbiol Infect. 2013;19:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Zeng L, Wang S, Lin M, Chen Y, Deng Q, Zhong H, Guan X, Yao S, Liu H. Evaluation of time to positivity for blood culture combined with immature granulocytes, neutrophil-to-lymphocyte ratio, and CRP in identifying bloodstream coagulase-negative Staphylococci infection in pediatric patients. J Clin Lab Anal. 2020;34:e23473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Weinstein MP, Mirrett S, Van Pelt L, McKinnon M, Zimmer BL, Kloos W, Reller LB. Clinical importance of identifying coagulase-negative staphylococci isolated from blood cultures: evaluation of MicroScan Rapid and Dried Overnight Gram-Positive panels versus a conventional reference method. J Clin Microbiol. 1998;36:2089-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |