Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.4062
Peer-review started: January 20, 2021
First decision: February 24, 2021
Revised: February 28, 2021
Accepted: March 25, 2021
Article in press: March 25, 2021
Published online: June 6, 2021
Processing time: 112 Days and 2 Hours
Muscle growth promoters are being developed for the treatment of disease-induced loss of muscle mass. Ligandrol and ostarine are selective androgen receptor modulators (SARMs) with a non-steroidal structure and a presumably more favorable side effect profile. In recent years, these substances with or without “post-cycle therapy” (PCT) are often misused by amateur athletes aiming to promote muscle growth. At the same time, reports on their toxic effects on organ systems are emerging.
We report two cases of liver injury in young men who used ligandrol and/or ostarine for a few weeks followed by the use of substances for PCT. Acute liver injury occurred in both cases after stopping SARMs while on PCT. The clinical picture was dominated by jaundice and fatigue. The biochemical pattern showed a mixed type of injury with normal alkaline phosphatase and high concentrations of bilirubin and serum bile acids. Histological evidence showed predominantly cholestatic injury with canalicular bile plugs, ductopenia, and mild hepatocellular damage without significant fibrosis. The patients recovered from the condition after 3 mo. The off target effects of SARMs were likely idiosyncratic, but our report highlights the yet unrecognized effects of other toxic substances used for PCT, supra-therapeutic doses, and the complete absence of monitoring for adverse effects.
Among muscle-building amateur athletes, SARMs (ligandrol or ostarine) and/or substances in PCT may cause cholestatic liver injury with prolonged recovery.
Core Tip: Ligandrol and ostarine are selective androgen receptor modulators with a presumably more favorable side effect profile. They are often misused by amateur body builders aiming to promote muscle growth. Reports on the toxic effects on organ systems are emerging. We report the liver injury in two young men who used ligandrol and/or ostarine in addition to other muscle-promoting substances known as post-cycle therapy. They showed jaundice and fatigue, a mixed type of injury, normal alkaline phosphatase, and high levels of bilirubin and bile acids. Histological evidence showed cholestatic injury, canalicular bile plugs, and ductopenia. The patients recovered after only 3 mo.
- Citation: Koller T, Vrbova P, Meciarova I, Molcan P, Smitka M, Adamcova Selcanova S, Skladany L. Liver injury associated with the use of selective androgen receptor modulators and post-cycle therapy: Two case reports and literature review . World J Clin Cases 2021; 9(16): 4062-4071
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/4062.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.4062
Muscle growth promoters and anabolic substances have been developed for the treatment of disease-induced muscle loss and impaired functional status[1,2]. They include a variety of anabolic substances such as testosterone, anabolic androgenic steroids (AAS), selective androgen receptor modulators (SARMs), and growth hormone. Their use in clinical practice is currently limited, but their benefit in treating sarcopenia and frailty could be substantial[3]. Unfortunately, these substances have not escaped the attention of muscle-building enthusiasts[4]. Although banned in professional sport, their use is significantly widespread since they are available for online purchase as nutritional supplements[5]. Although they are hormonal prepara
In this report, we present two recently observed cases with distinct biochemical and histological phenotypes.
Case 1: A 19-year-old performance athlete was hospitalized for recent jaundice.
Case 2: A 28-year-old man with an interest in body-building without any significant medical or family history was referred to a liver transplantation center for jaundice.
Case 1: To build muscle mass, his friend recommended ligandrol, which he purchased online. He took one capsule daily for 4 wk following the package recommendation, but he did not know the exact amount of the active substance. After 4 wk, he started using the so-called “post-cycle therapy“ (PCT), again, not recalling the exact names of the substances. He stopped taking the PCT after 3 wk after noticing dark urine, yellow sclera, and thinner light-colored stools.
Case 2: At 3 mo before admission, his liver tests were normal. The patient was exercising several times weekly while taking protein supplements, amino acids, and fat burners for a long period of time. Over the course of 3 mo, he took a combination of ligandrol and ostarine for an unknown period. At 3 wk after stopping their intake, he started using the PCT preparation sold under the commercial name of “Spartan” (Warrior Labs, Fernley, NV, United States). After the 4th dose, he started complaining of nausea and fatigue. Overall, the patient took 6 tablets.
Case 1: The patient did not report any significant medical or family history, alcohol, or illicit drug use.
Case 2: No significant medical history.
Case 1: At the initial clinical examination, he did not report weight loss, abdominal pain, or nausea nor did he present any signs of chronic liver disease or portal hypertension. His body mass index (BMI) was 21 kg/m2.
Case 2: On transfer from a secondary care hospital, his BMI was 27.5 kg/m2
Case 1: The total serum bilirubin concentration was 238 µmol/L (normal range 3.4-17.1), conjugated bilirubin 197.5 µmol/L (0-5.0), alanine aminotransferase (ALT) 2.2 µkat/L (0.2-0.80), gamma-glutamyl transferase 0.41 µkat/L (0.18- 1.02), alkaline phosphatase (ALP) 1.54 µkat/L (0.67-2.15), serum bile acids 299 µmol/L (2-10), total cholesterol 4.77 mmol/L (3.1-5.0), and high-density lipoprotein (HDL) cholesterol 0.21 (1.0-2.7). Testing for serum antibodies ruled out viral hepatitis A, B, C, E, and the patient did not present any clinical signs of Epstein–Barr virus (EBV), cytomegalovirus (CMV), or herpes simplex virus (HSV) infections. Abdominal ultrasound showed normal liver and spleen size, with no apparent bile duct dilation. The characteristics of drug-induced liver injury expressed in the R score (r = 3.9) were consistent with a mixed type of injury. The causality assessment of ligandrol or PCT as being the cause of the condition assessed by the Roussel Uclaf Causality Assessment Method (RUCAM) score (6 points) suggested a probable association[16].
Case 2: The total serum bilirubin was 401 µmol/L, conjugated bilirubin 256 µmol/L, ALT 2.4 µkat/L, ALP 1.54 µkat/L, and HDL cholesterol 0.53 mmol/L. Viral hepatitis A, B, C, E as well as HSV, EBV, and CMV infections were excluded. The R score was 3.3 confirming a mixed type of injury, while the RUCAM score suggested a role of ligandrol/ostarine and PCT in the hepatotoxicity.
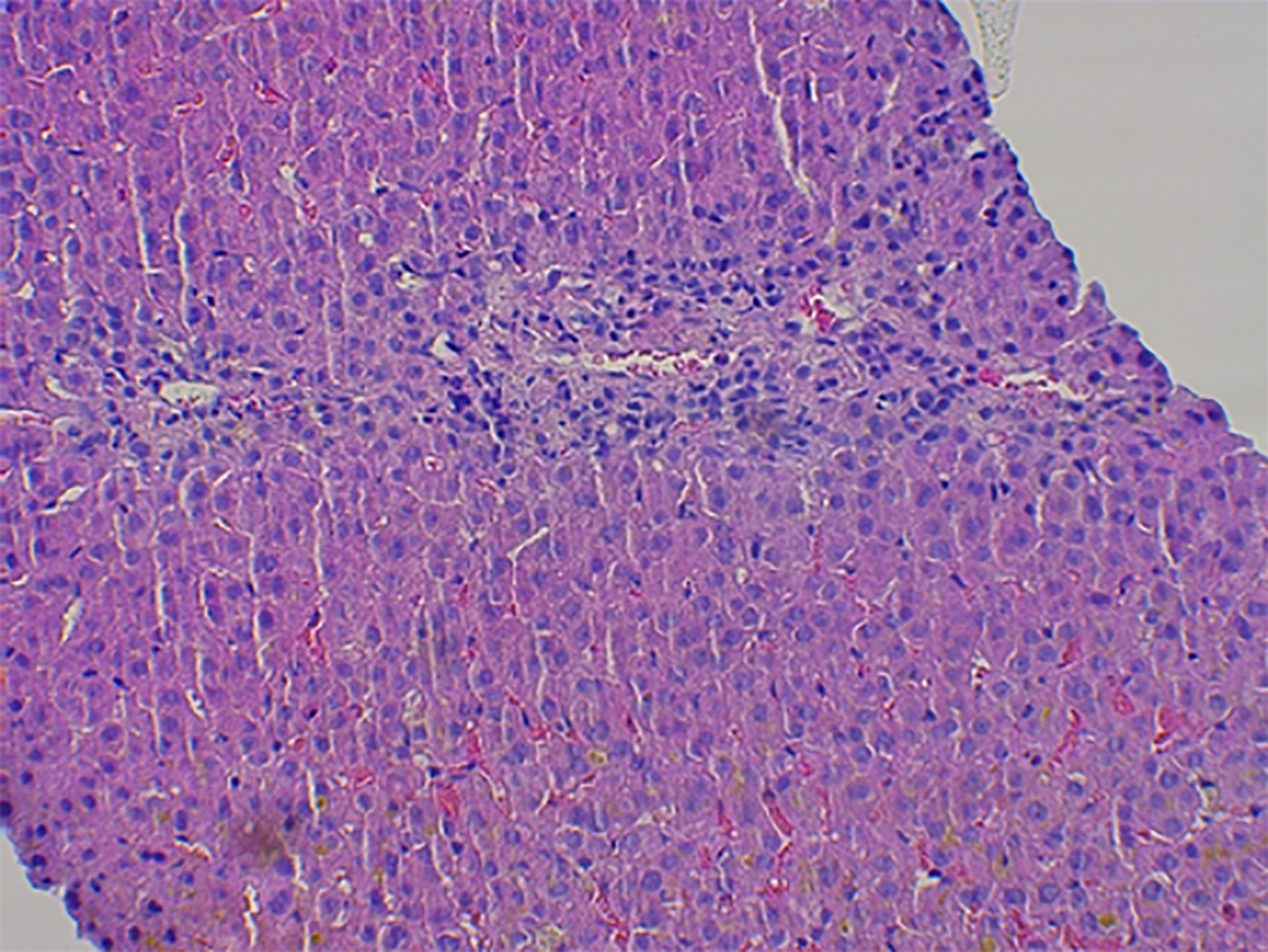
Case 1: Abdominal ultrasound showed normal liver and spleen size, with no apparent bile duct dilation. A transcutaneous liver biopsy was performed and histological examination of the tissue showed mild septal fibrosis, canalicular cholestasis in the hepatocytes with numerous biliary plugs in the ducts, between hepatocytes, and in the Kupffer cells. The cholestasis was accompanied by few necrotic hepatocytes, centrilobular mostly lymphocytic infiltrate, and ductopenia with almost complete loss of bile ducts in the portal spaces (Figure 1).
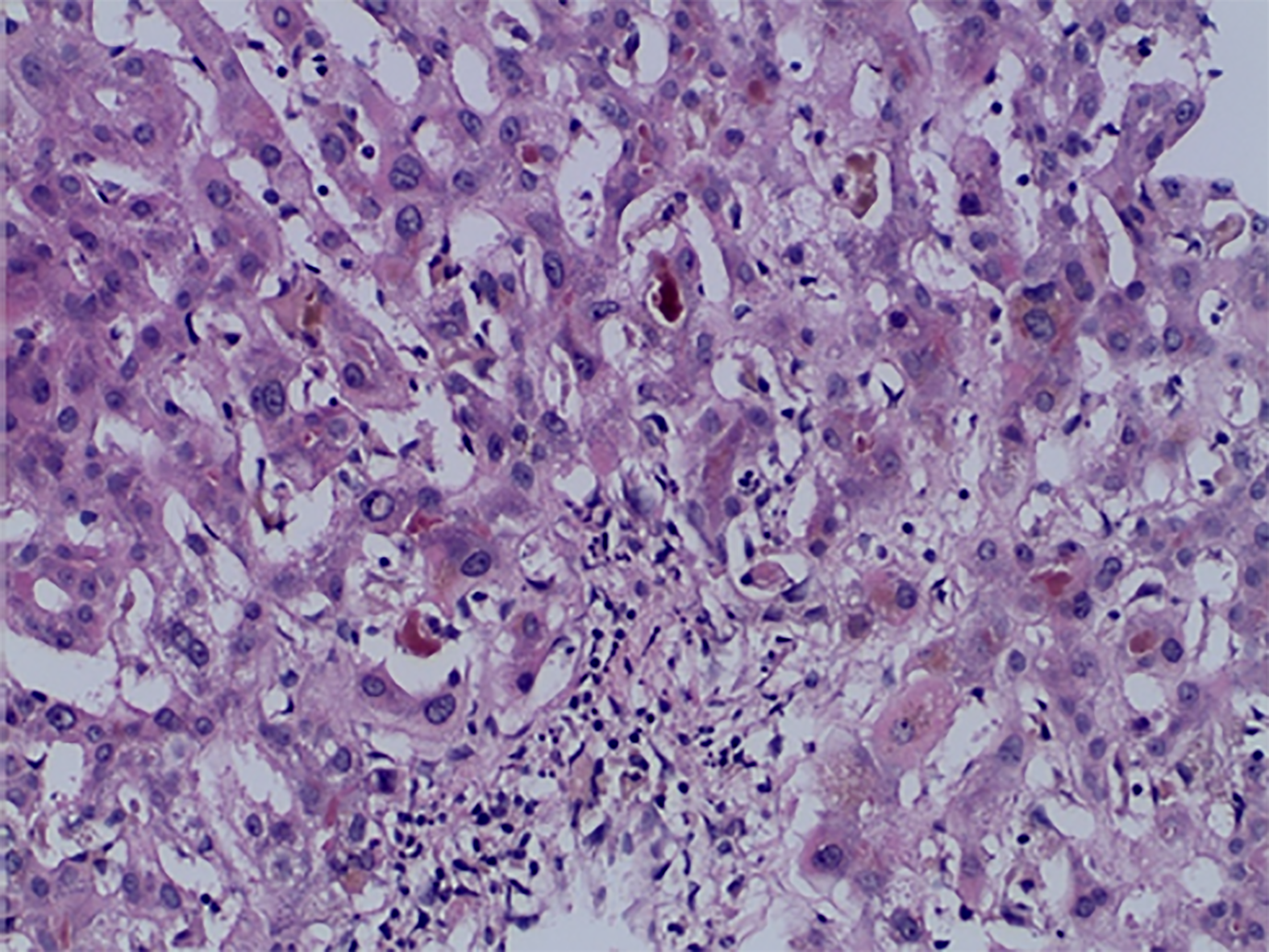
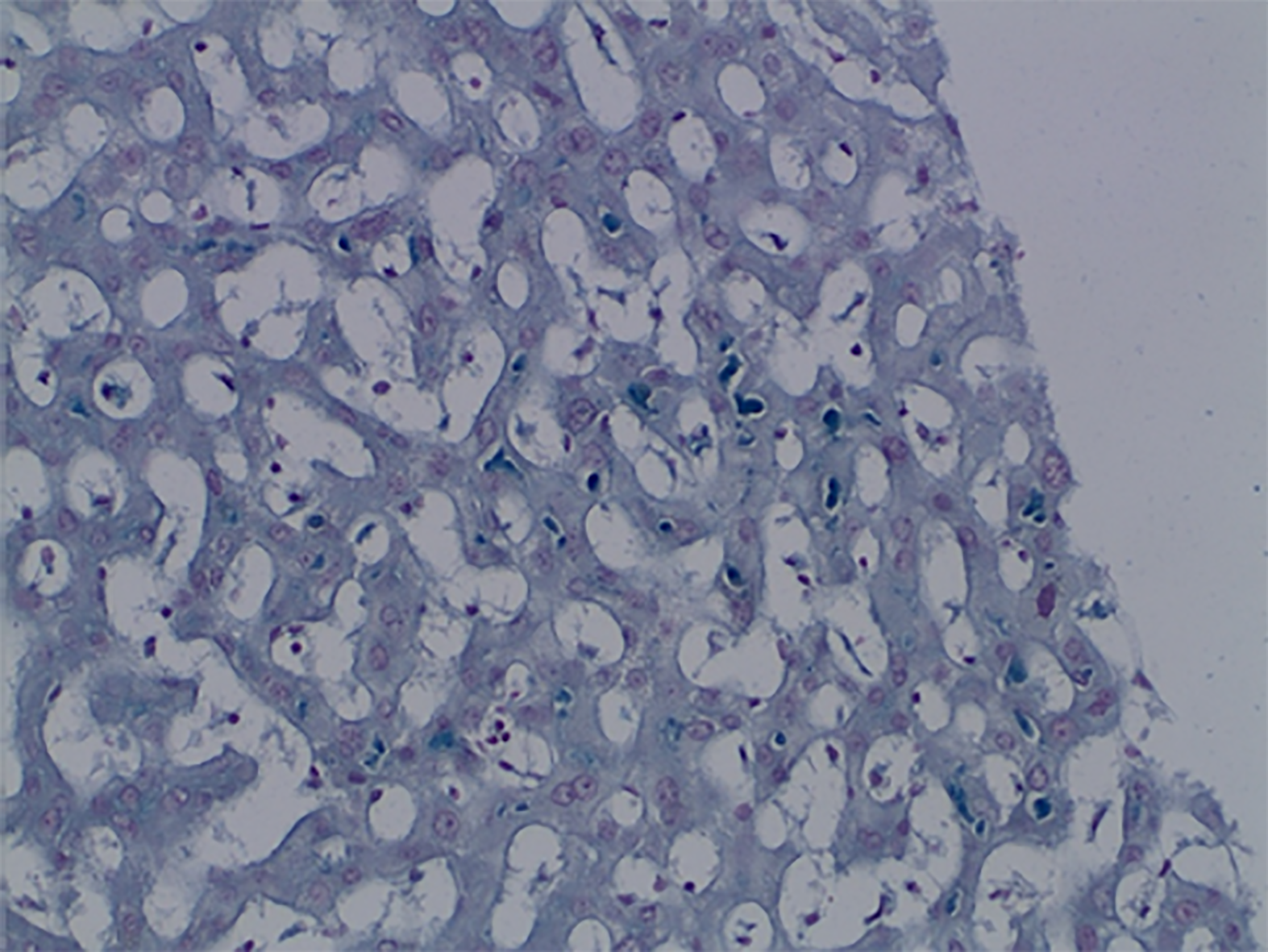
Case 2: Magnetic resonance imaging showed hepatomegaly without biliary pathology. A transjugular liver biopsy was performed and histological examination of the tissue showed mild bridging fibrosis, destruction of bile ducts, centrilobular canalicular cholestasis with numerous bile plugs in the canaliculi, phagocytosis of the plugs in the Kupffer cells, hepatocellular apoptosis, and perivenular necrosis (Figures 2 and 3).
Mixed type of drug-induced liver injury (cholestasis and hepatitis) with ductopenia and mild fibrosis.
Mixed type of drug-induced liver injury (cholestasis and hepatitis) with bile duct destruction and mild fibrosis
1000 mg ursodeoxycholic acid (UDCA) daily for 2 mo.
The initial treatment included 300 mg intravenous N-acetyl cysteine 4 times daily, 1000 mg oral UDCA daily, and 450 mg silymarin daily.
We observed a decrease in serum bilirubin levels below entry levels after 7 d. The clinical course remained favorable and the patient was discharged after 11 d. Without further intervention, the serum parameters normalized within 3 mo. The patient did not report any adverse events related to the treatment. The laboratory findings are summarized in Table 1.
| Normal range | On admission | 7 d | 3 mo | |
| Case 1, man, 19 yr | ||||
| Total bilirubin, µmol/L | 3.4-17.1 | 238 | 221 | 12 |
| Conjugated bilirubin, µmol/L | 0-5 | 197 | 184 | |
| Alanine aminotransferase, µkat/L | 0.2-0.8 | 2.21 | 1.57 | 0.38 |
| Alkaline phosphatase, µkat/L | 0.67-2.15 | 1.54 | 1.37 | 1.2 |
| Total bile acids, µmol/L | 2-10 | 299 | 226 | 8.6 |
| Serum creatinine, µmol/L | 62-106 | 113.8 | 113.3 | |
| INR | 0.8-1.2 | 0.98 | 0.98 | |
| Case 2, man, 28 yr | ||||
| Total bilirubin, µmol/L | 5.1-19 | 401 | 394 | 25.1 |
| Conjugated bilirubin, µmol/L | 0.8-5.1 | 244 | 238 | 4.6 |
| Alanine aminotransferase, µkat/L | 0.0-0.8 | 2.42 | 1.74 | 1.6 |
| Alkaline phosphatase, µkat/L | 0.5-2.0 | 1.54 | 2.19 | 1.56 |
| INR | 0.8-1.2 | 1.07 | 0.93 | 0.96 |
| Serum creatinine, µmol/L | 64-104 | 90 | 87 | 80 |
The patient remained in the hospital for 13 d and did not report any adverse events related to the treatment. After 3 mo, he was seen at the clinic reporting a good clinical condition with a total serum bilirubin 25 µmol/L, ALT 1.6 µmol/L, and ALP 1.56 µmol/L. The summary of laboratory findings is displayed in Table 1.
We report two cases of SARMs and PCT toxicity, which showed some unique observations. We provide evidence of bile duct loss or destruction that has not been previously reported in this context. Also, despite high bilirubin, histological evidence of cholestasis, and significantly elevated serum bile acids, we consistently observed normal or mildly elevated alkaline phosphatase. In both cases, patients did not develop symptoms or clinical signs while using ligandrol or ostarine, and those signs only appeared after stopping SARMs and taking PCT for 4 d or 3 wk.
Liver injury induced by the use of anabolic substances has been reported in the Spanish registry, where they were implicated in 8% of all drug-induced liver injury (DILI) between years 2010-2013[9]. In most cases, they led to hospitalization and in some cases, a molecular adsorbent recirculating system therapy was required. The content of nutritional supplements available online has been investigated and confirmed presence of the active substance[15,17]. The effects of 1.0 or 3.0 mg of ostarine (enobosarm) on lean body mass has been studied in a randomized, controlled phase 2 trial[12]. The study did not report any particular liver-related adverse events. The effects of 0.1 to 1.0 mg of ligandrol daily (LGD-4033 or VK5211) on muscle growth have been studied in a randomized, double-blind, placebo-controlled phase II study[18]. Likewise, the study did not report any significant difference in liver-related adverse events in patients receiving ligandrol compared to the placebo. Two cases of liver-related adverse effects of ligandrol have been reported in the context of its misuse. Flores et al[14] described a 24-year-old man presenting with jaundice, anorexia, and weight loss. He took 1 capsule of ligandrol as a dietary supplement for 9 wk. The patient also reported binge drinking once a week. His complaints appeared 1 wk after stopping ligandrol, while the use of PCT was not reported. The R-ratio was 8.2 indicating a hepatocellular injury. Histological examination of the liver tissue was not reported. Liver tests normalized within 4 mo. The authors also analyzed the composition of the capsules and have confirmed that they contained ligandrol a no anabolic steroids. The biochemical pattern of liver injury was distinct from our two patients. However, the time delay between stopping ligandrol and onset of symptoms and the recovery time was similar. Barbara et al[15] reported a case of a 32-year-old man without previous chronic diseases who was admitted for jaundice and severe weight loss. Weight loss, fatigue, and pruritus started approximately 50 d before presentation. For muscle building, he reported taking 10 mg daily of a liquid preparation of ligandrol for 2 wk. The use of PCT nor other substances was not reported. Laboratory parameters showed hyperbilirubinemia at 38 times the upper limit of normal, and later in the course, an elevated alkaline phosphatase (ALP 425 U/L). A transjugular liver biopsy revealed liver parenchyma containing canalicular bile plugs with preserved ductal structure and a chronic, mild, predominantly lymphocytic infiltrate. Although the initial biochemical signature showed normal alkaline phosphatase, it later evolved into a typical cholestatic pattern. Histological findings were very similar to our two cases but there was no evidence of ductopenia. During follow-up, they reported a gradual decrease in bilirubin and alkaline phosphatase, but the parameters did not normalize even after 84 d.
All of the reported cases of SARMSs toxicity had in common the clinical presentation of jaundice, and in three out of four cases, a predominantly cholestatic histological pattern with or without bile duct destruction. Duration of SARMs intake varied from 2 to 12 wk, the time delay between stopping of SARMs and the appearance of symptoms varied from 0 to 3 wk. The evidence is therefore very similar to the pattern of DILI which was reported among users of AAS. However, in contrast to the previous reports, our two patients reported the use of PCT. This usually means a sequential self-administration of one or more substances after a cycle or SARMs or steroids[19]. PCT aims to prevent anabolic users from the rebound effect while enabling the return of spontaneous testosterone secretion. Substances taken as PCT may include inhibitors of aromatase converting testosterone to estrogens, selective estrogen receptor modulators (tamoxifen, clomiphene), human chorionic gonado
The precise mechanisms of liver injury caused by SARMs are yet to be deciphered. However, several possible mechanisms involved need to be highlighted. Analogically to the effect of anabolic steroids, idiosyncrasy likely plays a major role[24]. The idiosyncratic immune response is suggested by the relative rarity of the reported cases relative to the extent of misuse, a predominantly lymphocytic infiltrate in the liver tissue and no association between the length of use and the severity of the liver injury. The biochemical pattern of mixed-type injury and histological findings in all reported cases suggest that hepatocytes and cholangiocytes were targeted by the immune response. In vitro and in vivo studies have shown that ligandrol is extensively metabolized by hydroxylation combined with keto formation or cleavage of the pyrrolidine ring, dihydroxylation, and hydroxylation combined with methy
Cholangiocytes express androgenic receptors in animal models, which modulate cholangiocyte proliferation and secretion[30]. In the absence of androgenic signaling after rat castration, cholangiocyte proliferation has been impaired. However, data from human studies on the effects of androgen receptor signaling in cholangiocytes are lacking and should be investigated in the future[31].
Ligandrol metabolism may be affected by genetic or drug-induced alterations in the mechanisms of xenobiotic biotransformation (CYP450) or the activity of hepatocyte surface membrane proteins. Functional polymorphisms of transport proteins have been reported[32] in two patients with androgen-induced cholestatic liver injury in whom the activity of canalicular ectoenzymes ATP8B1/ABCB11 was decreased. In humans, the spontaneous expression of biliary transporters varies greatly even among healthy individuals with up to 300-fold differences[33].
Substances used in PCT have also been implicated in causing DILI. Apart from AAS[9,24], a recent study reported that after in vitro hepatocyte androgen receptor stimulation with epistane, there is a significant increase in the intracellular concentration of primary conjugated bile acids[33]. The increase was attributed to upregulated bile acid synthesis by a mechanism of increased expression of CYP8B1. Also, low HDL cholesterol levels in both reported cases likely indicate the shift of cholesterol transport towards bile acid synthesis, thus contributing to increased intracellular content of toxic bile acids.
Monitoring for possible side effects is completely absent when such substances are being used by body builders. Analogically to the therapeutic use of hormonal preparations elsewhere, persisting or progressive liver test abnormalities might have identified patients with SARM induced toxicity. Early drug discontinuation could have prevented further injury.
Due to the significant time delay from the intake of incriminated medication to the delivery of specialist medical care, drug packages and specimens were not available for exact chemical analysis. In the first patient, the time delay might suggest the effect of PCT rather than ligandrol, but the exact substances were not identified. In the second patient, PCT substances were known to cause hepatotoxicity, but they were only taken for 4 d, suggesting a less likely role in causing DILI compared with SARMs.
The unique character of liver injury in the presented cases is beyond the traditional definition of DILI or liver-related adverse events in the common terminology criteria for adverse events (CTCAE)[34]. Both require ALT elevation superior to three-times the upper limit of normal (ULN) and ALP elevation > 2.5 × ULN, which were not observed. However, both our patients had serum bilirubin levels increased more than ten-times the ULN, which according to the CTCAE v 5.0 corresponds to Grade 4 injury. Besides, due to normal alkaline phosphatase and low ALT values (< 3 × ULN), the type of liver injury according to R-value should be interpreted with caution. The biochemical pattern corresponded to a mixed-type injury (hepatocellular and cholestatic). However, even though the histological findings confirmed a mixed-type of injury, it was dominantly cholestatic with hepatocellular injury being much less significant.
Ligandrol and ostarine are SARMs with hepatotoxic potential. To date, all the reported cases of drug-induced liver injury were the consequence of their misuse in the form of nutritional supplements. In our case series, we are highlighting that its use is often followed by the use of various substances in the post-cycle therapy, whose role in causing liver injury cannot be separated from the effect of SARMs. We provide unique histological evidence for a predominantly cholestatic liver injury with consistently normal alkaline phosphatase. The histological features of bile duct injury are similar to the pattern described previously for anabolic steroids. In addition to an innate predisposition to idiosyncratic reactions, high-doses, and the absence of monitoring for adverse effects likely contributed to the injury. It is up to regulators and public health authorities to remedy these aspects and to prevent the general public from having unrestricted access to these potentially dangerous substances through stricter regulation. These measures would likely prevent the adverse events and provide an opportunity for identifying the possible clinical benefit of SARMs in patients with frailty and/or sarcopenia.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Slovak Gastroenterological Society; Slovak Hepatological Society; and Societe Francaise d'Hepatologie - AFEF, No. 50688868.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Slovakia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Stephens C S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Xing YX
| 1. | Tournadre A, Vial G, Capel F, Soubrier M, Boirie Y. Sarcopenia. Joint Bone Spine. 2019;86:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 2. | Davis MP, Panikkar R. Sarcopenia associated with chemotherapy and targeted agents for cancer therapy. Ann Palliat Med. 2019;8:86-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2); and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7793] [Article Influence: 1298.8] [Reference Citation Analysis (1)] |
| 4. | Wood RI, Stanton SJ. Testosterone and sport: current perspectives. Horm Behav. 2012;61:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Tsarouhas K, Kioukia-Fougia N, Papalexis P, Tsatsakis A, Kouretas D, Bacopoulou F, Tsitsimpikou C. Use of nutritional supplements contaminated with banned doping substances by recreational adolescent athletes in Athens, Greece. Food Chem Toxicol. 2018;115:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Harvey O, Keen S, Parrish M, van Teijlingen E. Support for people who use Anabolic Androgenic Steroids: A Systematic Scoping Review into what they want and what they access. BMC Public Health. 2019;19:1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 7. | Auchus RJ, Brower KJ. The Public Health Consequences of Performance-Enhancing Substances: Who Bears Responsibility? JAMA. 2017;318:1983-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Piacentino D, Kotzalidis GD, Del Casale A, Aromatario MR, Pomara C, Girardi P, Sani G. Anabolic-androgenic steroid use and psychopathology in athletes. A systematic review. Curr Neuropharmacol. 2015;13:101-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 9. | Robles-Diaz M, Gonzalez-Jimenez A, Medina-Caliz I, Stephens C, García-Cortes M, García-Muñoz B, Ortega-Alonso A, Blanco-Reina E, Gonzalez-Grande R, Jimenez-Perez M, Rendón P, Navarro JM, Gines P, Prieto M, Garcia-Eliz M, Bessone F, Brahm JR, Paraná R, Lucena MI, Andrade RJ; Spanish DILI Registry; SLatinDILI Network. Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment Pharmacol Ther. 2015;41:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Narayanan R, Coss CC, Dalton JT. Development of selective androgen receptor modulators (SARMs). Mol Cell Endocrinol. 2018;465:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Solomon ZJ, Mirabal JR, Mazur DJ, Kohn TP, Lipshultz LI, Pastuszak AW. Selective Androgen Receptor Modulators: Current Knowledge and Clinical Applications. Sex Med Rev. 2019;7:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML, Johnston MA, Steiner MS. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 267] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 13. | US Food and Drug Administration. FDA In Brief: FDA warns against using SARMs in body-building products. 2017. [cited 20 January 2021]. Available from: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-against-using-sarms-body-building-products. |
| 14. | Flores JE, Chitturi S, Walker S. Drug-Induced Liver Injury by Selective Androgenic Receptor Modulators. Hepatol Commun. 2020;4:450-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Barbara M, Dhingra S, Mindikoglu AL. Ligandrol (LGD-4033)-Induced Liver Injury. ACG Case Rep J. 2020;7:e00370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1069] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 17. | Fragkaki AG, Sakellariou P, Kiousi P, Kioukia-Fougia N, Tsivou M, Petrou M, Angelis Y. Human in vivo metabolism study of LGD-4033. Drug Test Anal. 2018;10:1635-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Basaria S, Collins L, Dillon EL, Orwoll K, Storer TW, Miciek R, Ulloor J, Zhang A, Eder R, Zientek H, Gordon G, Kazmi S, Sheffield-Moore M, Bhasin S. The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A Biol Sci Med Sci. 2013;68:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (2)] |
| 19. | Griffiths S, Henshaw R, Mckay FH, Dunn M. Post-cycle therapy for performance and image enhancing drug users: A qualitative investigation. Perform Enhanc Heal. 2017;5:103-107. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Chaillet P. Hepatox version 1.8.5. 2020. [cited 20 January 2021]. Available from: https://itunes.apple.com/us/app/hepatox/id48973847. |
| 21. | Livertox. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. [cited 15 September 2020]. Available from: https://LiverTox.nih.gov. |
| 22. | Martinez Brito D, de la Torre X, Botrè F. Detection of urinary metabolites of arimistane in humans by gas chromatography coupled to high-accuracy mass spectrometry for antidoping analyses. Rapid Commun Mass Spectrom. 2019;33:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Úradu Verejného Zdravotníctva Slovenskej Republiky. Informácia o výskyte škodlivého výrobku – výživový doplnok obsahujúci anabolické steroidy. 2020. [cited 20 January 2021]. Available from: https://www.uvzsr.sk/index.php?option=com_content&view=article&id=4248:informacia-o-vyskyte-kodliveho-vyrobku-vyivovy-doplnok-obsahujuci-anabolicke-steroidy&catid=95:informacie-pre-spotrebiteov. |
| 24. | Kolarić TO, Ninčević V, Smolić R, Smolić M, Wu GY. Mechanisms of Hepatic Cholestatic Drug Injury. J Clin Transl Hepatol. 2019;7:86-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 25. | Geldof L, Pozo OJ, Lootens L, Morthier W, Van Eenoo P, Deventer K. In vitro metabolism study of a black market product containing SARM LGD-4033. Drug Test Anal. 2017;9:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 26. |
Holderbaum A.
Emerging Anabolic Drugs - Investigation of the |
| 27. | Lammert C, Einarsson S, Saha C, Niklasson A, Bjornsson E, Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 283] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 28. | Visentin M, Lenggenhager D, Gai Z, Kullak-Ublick GA. Drug-induced bile duct injury. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1498-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Herkel J, Jagemann B, Wiegard C, Lazaro JF, Lueth S, Kanzler S, Blessing M, Schmitt E, Lohse AW. MHC class II-expressing hepatocytes function as antigen-presenting cells and activate specific CD4 T lymphocyutes. Hepatology. 2003;37:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Franchitto A, Onori P, Renzi A, Carpino G, Mancinelli R, Alvaro D, Gaudio E. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann Transl Med. 2013;1:27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 31. | Kur P, Kolasa-Wołosiuk A, Misiakiewicz-Has K, Wiszniewska B. Sex Hormone-Dependent Physiology and Diseases of Liver. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 32. | El Sherrif Y, Potts JR, Howard MR, Barnardo A, Cairns S, Knisely AS, Verma S. Hepatotoxicity from anabolic androgenic steroids marketed as dietary supplements: contribution from ATP8B1/ABCB11 mutations? Liver Int. 2013;33:1266-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Petrov PD, Fernández-Murga L, Conde I, Martínez-Sena T, Guzmán C, Castell JV, Jover R. Epistane, an anabolic steroid used for recreational purposes, causes cholestasis with elevated levels of cholic acid conjugates, by upregulating bile acid synthesis (CYP8B1) and cross-talking with nuclear receptors in human hepatocytes. Arch Toxicol. 2020;94:589-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [Internet]. 2017. [cited 20 January 2021]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. |