Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.4032
Peer-review started: January 12, 2021
First decision: February 11, 2021
Revised: February 25, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: June 6, 2021
Processing time: 121 Days and 11 Hours
We report a case of post-coronavirus disease (COVID) immune hepatitis occurring in a young male with no pre-existing comorbidities.
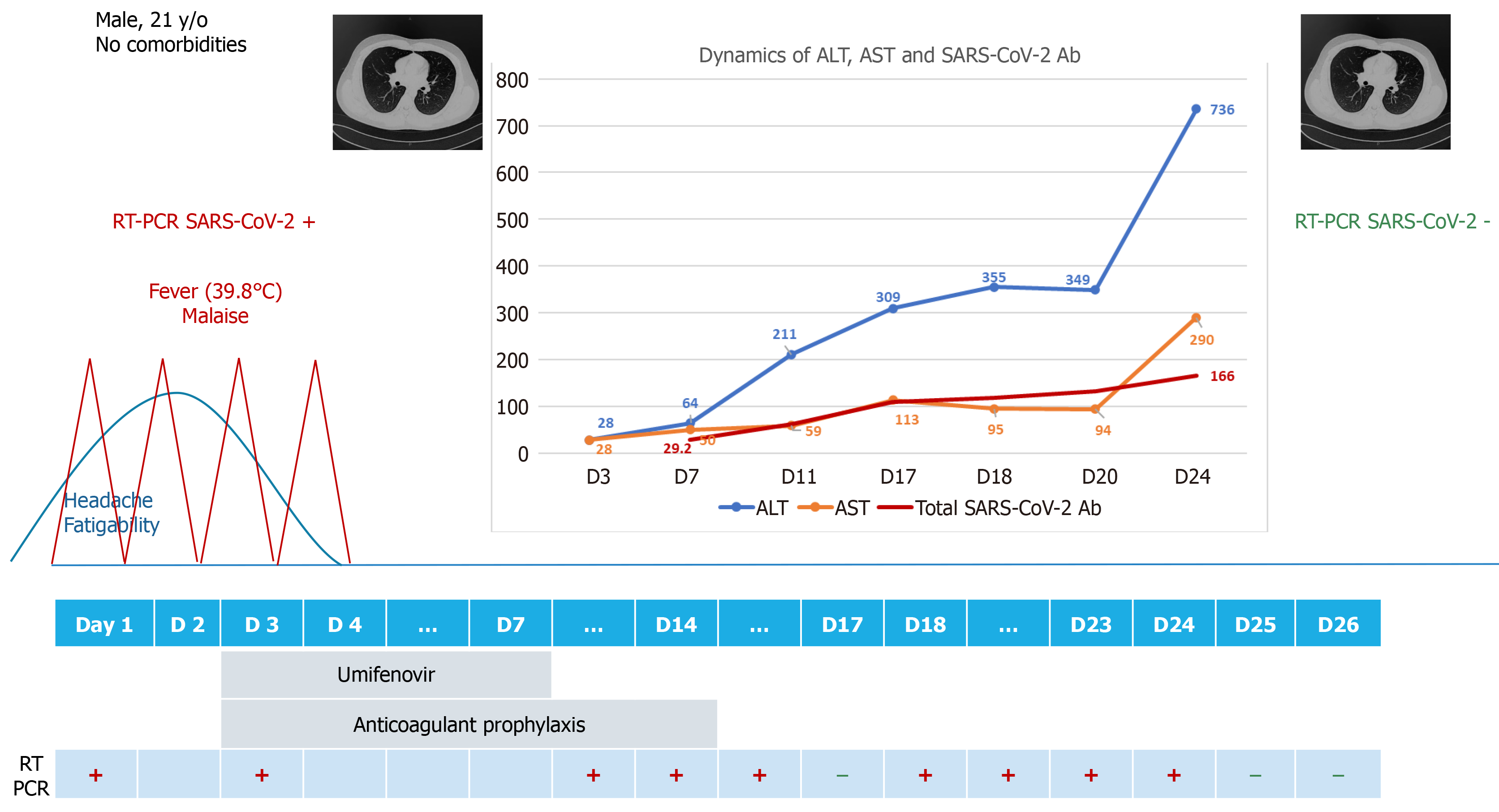
A previously healthy 21-year-old male patient was admitted to our hospital with mild COVID-19. During the course of in-hospital isolation and monitoring, he developed an alanine aminotransferase (ALT) and aspartate aminotransferase (AST) increase, with the enzymes peaking at day 24 (ALT 15 times the upper normal limit), with preserved liver function. The liver enzyme increase occurred 20 d after the complete clinical remission of COVID-19, and ALT dynamics paralleled the increase in total antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The case was interpreted as post-COVID immune hepatitis, with extensive laboratory investigations excluding other potential causes. The hepatocytolysis remitted 20 d after the peak ALT, without further intervention, with complete recovery, but the total anti-SARS-CoV-2 antibodies continued to increase the next 5 mo following the acute infection.
Close attention should also be paid to young patients with mild forms of disease, and a high index of suspicion should be maintained for post-COVID complica
Core Tip: We report an interesting case of post-coronavirus disease immune hepatitis occurring in a young male with no pre-existing comorbidities. The patient acquired the infection from an asymptomatic carrier of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). He developed a mild form of disease, but after apparent clinical remission and real-time reverse transcription polymerase chain reaction negative conversion, an important increase in liver enzymes was recorded in parallel with an increasing SARS-CoV-2 antibody titer.
- Citation: Drăgănescu AC, Săndulescu O, Bilașco A, Kouris C, Streinu-Cercel A, Luminos M, Streinu-Cercel A. Transient immune hepatitis as post-coronavirus disease complication: A case report. World J Clin Cases 2021; 9(16): 4032-4039
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/4032.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.4032
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a novel pathogen has reshaped our understanding of infectious diseases. While immune-mediated post-infectious complications have been previously described for other pathogens, these remain relatively rare occurrences. With the onset of the coronavirus disease 2019 (COVID-19) pandemic, infectious diseases practitioners and colleagues from related specialties need to maintain a double focus–managing the acute disease per se, while also keeping a heightened attention towards post-COVID complications.
We report the case of a previously healthy 21-year-old male patient from Bucharest, Romania, presenting in June 2020, during the first COVID-19 wave in Romania, with a two-day prodrome of fatigability and headache, followed by sudden onset high-grade fever (highest recorded temperature of 39.8 °C) and malaise. Nasopharyngeal and oropharyngeal swabs were collected and the real-time reverse transcription polyme
The patient had been self-isolating at home for the past two months and a half, and had only left the house for one meeting, 4 d prior to symptom onset. After being confirmed with COVID-19 by RT-PCR, epidemiological tracing revealed that the meeting consisted of indoor close contact, without mask wearing on either side, with one person who had no signs or symptoms of infection at the time of the meeting. Upon evaluation of the contact, she was confirmed by RT-PCT to be an asymptomatic carrier of SARS-CoV-2. According to the national regulations at the time, she was also admitted to the hospital, where she remained completely asymptomatic, and reverted to negative RT-PCR on days 16 and 17 from the initial RT-PCR confirmation.
Upon hospital admission, our patient underwent a complete evaluation, which revealed pharyngeal erythema at physical exam, normal hematology and biochemistry laboratory tests (Table 1) and normal computed tomography (CT) of the chest. He received antiviral treatment with umifenovir 200 mg orally every 8 h for 5 d, and thromboprophylaxis with low molecular weight heparin subcutaneously once daily for 14 d. The course of disease was mild, with no occurrence of pneumonia, and all symptoms subsided 4 d after onset; no further symptoms developed throughout the course of the disease.
| Parameter | Day 3 | Day 7 | Day 11 | Day 17 | Day 20 | Day 24 | Day 25 | Day 26 | Day 27 | Day 32 | Day 43 | Day 69 | Day 104 | Day 145 | Day 193 | Day 257 |
| Hematology | ||||||||||||||||
| White blood cells (3.6-9.6) × 103/µL | 4.5 | 3.9 | 5.8 | 7.6 | 6.6 | 6.7 | 5.7 | 5.7 | 4.3 | |||||||
| Neutrophils (1.4-6.5) × 103/µL | 1.8 | 1.7 | 3.0 | 3.8 | 3.4 | 3.5 | 3.1 | 3.3 | 2.0 | |||||||
| Lymphocytes (1.2-3.4) × 103/µL | 1.8 | 1.7 | 2.0 | 2.7 | 2.2 | 2.2 | 1.7 | 1.6 | 1.7 | |||||||
| Hemoglobin (12.1-17.2) g/dL | 15.6 | 15.2 | 15.5 | 15.8 | 15.8 | 15.2 | 15.0 | 15.6 | 14.3 | |||||||
| Platelets (200-400) × 103/µL | 205 | 188 | 266 | 311 | 265 | 254 | 245 | 255 | 215 | |||||||
| Coagulation | ||||||||||||||||
| D-dimers hs (50-230) ng/mL | 68 | 38 | 10 | 18 | 56 | 30 | 23 | 61 | ||||||||
| Activated partial thromboplastin time (24-38) s | 31.6 | 33.4 | 35.4 | 31.9 | 32.3 | 27.2 | ||||||||||
| Prothrombin concentration (80-115) % | 75 | 95 | 73.9 | 89 | 84 | 90 | 89 | 83 | 86 | 83 | 75 | 72.6 | 86.9 | |||
| Biochemistry | ||||||||||||||||
| ALT (4-50) U/L | 28 | 64 | 211 | 309 | 349 | 736 | 627 | 592 | 523 | 270 | 46 | 23 | 19 | 13 | ||
| AST (17-59) U/L | 28 | 50 | 59 | 13 | 94 | 290 | 196 | 148 | 113 | 63 | 28 | 24 | 22 | 18 | ||
| Total bilirubin (0.2-1.3) mg/dL | 0.4 | 0.4 | 0.4 | 0.6 | 0.5 | 0.6 | 0.5 | 0.5 | 0.8 | 0.6 | 0.6 | 0.6 | 0.6 | |||
| Direct bilirubin (0.0–0.4) mg/dL | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | |||
| GGT (15-85) U/L | 29 | 38 | 64 | 81 | 94 | 93 | 92 | 86 | 74 | 46 | 26 | 22 | 14 | |||
| Alkaline phosphatase (38-126) U/L | 58 | 69 | 71 | 65 | 67 | 65 | 69 | |||||||||
| Albumin (3.5-5) g/dL | 4.7 | 4.5 | 4.9 | 4.6 | 4.8 | |||||||||||
| Amylase (30-110) U/L | 59 | 57 | ||||||||||||||
| Lipase (23-300) U/L | 35 | 37 | ||||||||||||||
| Creatine kinase (55-170) U/L | 89 | 59 | 46 | 52 | 56 | 62 | 59 | 58 | 54 | 78 | 117 | |||||
| Creatine kinase-MB (1-16) U/L | 9 | 6 | 6 | 7 | 4 | 7 | 6 | 10 | 7 | 5 | 9 | |||||
| Fasting plasma glucose (74-106) mg/dL | 84 | 102 | 97 | 86 | 88 | 98 | 90 | 92 | 84 | 89 | 87 | 96 | 97 | |||
| Lactate dehydrogenase (120-246) U/L | 189 | 221 | 214 | 252 | 258 | 426 | 378 | 285 | 245 | 260 | 189 | 172 | 162 | 145 | ||
| Total proteins (6.3-8.2) g/dL | 7.9 | 8.0 | 8.2 | 8.2 | 7.9 | 8.7 | 8.2 | 8.5 | 7.8 | 7.9 | 7.8 | |||||
| Cholesterol (50-200) mg/dL | 150 | 196 | 151 | |||||||||||||
| HDL-cholesterol (40-60) mg/dL | 46 | 48 | ||||||||||||||
| Total lipids (400-800) mg/dL | 705 | 482 | ||||||||||||||
| Triglycerides (15-150) mg/dL | 62 | 174 | 52 | |||||||||||||
| Creatinine (0.7-1.3) mg/dL | 0.9 | 0.8 | 0.8 | 0.7 | 0.8 | 0.8 | 0.6 | 0.6 | 0.9 | 0.7 | 0.7 | 0.7 | 0.7 | 0.6 | ||
| Urea (15-45) mg/dL | 31 | 27 | 27 | 27.5 | 20 | 28 | 28 | 29 | 34 | 34 | 41 | 33 | 35 | |||
| Sodium (137-145) mmol/L | 143 | 141 | 142 | 142 | 141 | 139 | 140 | 141 | 141 | 142 | 141 | 143 | ||||
| Potassium (3.6-5.0) mmol/L | 4.3 | 4.4 | 4.6 | 4.4 | 4.4 | 4.7 | 4.7 | 4.8 | 4.6 | 4.5 | 4.3 | 4.7 | ||||
| Calcium (8.4-10.2) mg/dL | 9.3 | 9.4 | 9.9 | 9.7 | 9.7 | 10.0 | 9.9 | 9.9 | 9.9 | |||||||
| Inflammatory markers | ||||||||||||||||
| C-reactive protein (0-3) mg/L | 6.20 | 1.62 | 0.99 | 1.75 | 1.37 | 0.78 | ||||||||||
| Erythrocyte sedimentation rate mm/h | 10 | 16 | 8 | |||||||||||||
| Fibrinogen (200-393) mg/dL | 250 | 243 | 196 | 227 | 221 | 254 | 246 | 244 | 269 | 260 | 221 | |||||
| Ferritin (22-322) ng/mL | 149.7 | 173.8 | 166.3 | |||||||||||||
| IL-6 (0-9.7) pg/mL | 0.453 | 0.420 | ||||||||||||||
| TNF-α (0-1.9) pg/mL | 0.0 | 0.0 | ||||||||||||||
| Procalcitonin (0-0.5) ng/mL | 0.05 | 0.05 | ||||||||||||||
| Cardiac markers | ||||||||||||||||
| Myoglobin (0-99.3) ng/mL | 47.4 | 36.7 | ||||||||||||||
| NT-proBNP (0-125) ng/L | 5 | 5 | ||||||||||||||
| Troponin I (0-0.16) ng/mL | 0.03 | 0.03 | ||||||||||||||
| SARS-CoV-2 antibodies | ||||||||||||||||
| Total SARS-CoV-2 Ab (0.00-0.99) S/CO | 29.2 | 62 | 110 | 132 | 166 | 173 | 180 | 188 | 225 | 305 | 498 | 721 | 865 | 654 | 85.1 | |
While still in the hospital for isolation and monitoring purposes, an increase in liver enzymes was noted, with alanine aminotransferase (ALT) and aspartate aminotrans
For the differential diagnosis of the ALT increase, multiple plausible causes of hepatic cytolysis were ruled out (Table 2): drug-induced liver injury was excluded based on the fact that no drugs had been recently administered. Systemic replication of SARS-CoV-2 was ruled out through negative RT-PCR from peripheral blood, which paralleled the negative RT-PCR from nasopharyngeal and oropharyngeal swabs. Another potential hypothesis considered was a COVID-19 cytokine storm that could also affect the liver. However, the timing of the ALT increase was not superposable to a cytokine storm, as the peak ALT occurred on day 24. We also checked the values of inflammatory markers at that time point and the results were within the normal range, and we repeated the chest CT, which was normal, confirming that no pulmonary lesions had been constituted throughout the course of the disease. We also ruled out infections with other hepatotropic viruses, through negative serological tests for hepatitis viruses A, B, C, E, adenovirus, coxsackie virus, echo virus, herpes simplex viruses, Epstein-Barr virus and cytomegalovirus.
| Hypothesis | Investigation and result |
| Drug-induced liver injury | No drugs recently administered |
| Systemic replication of SARS-CoV-2 | Negative SARS-CoV-2 RT-PCR from blood and nasopharyngeal + oropharyngeal swabs |
| COVID-19 cytokine storm | Timing–day 24 |
| Normal inflammatory markers | |
| Normal chest CT at baseline and on day 24 | |
| Infections with other hepatotropic viruses | Negative: HAV IgM, HBsAg, HCV Abs, HEV IgM and HEV-RNA, adenovirus IgM, coxsackie virus IgM, echo virus IgM, HSV1+2 IgM, EBV IgM, CMV IgM. |
| Post-COVID-19 immune hepatitis | Rapidly increasing SARS-CoV-2 antibodies (chemiluminescence) |
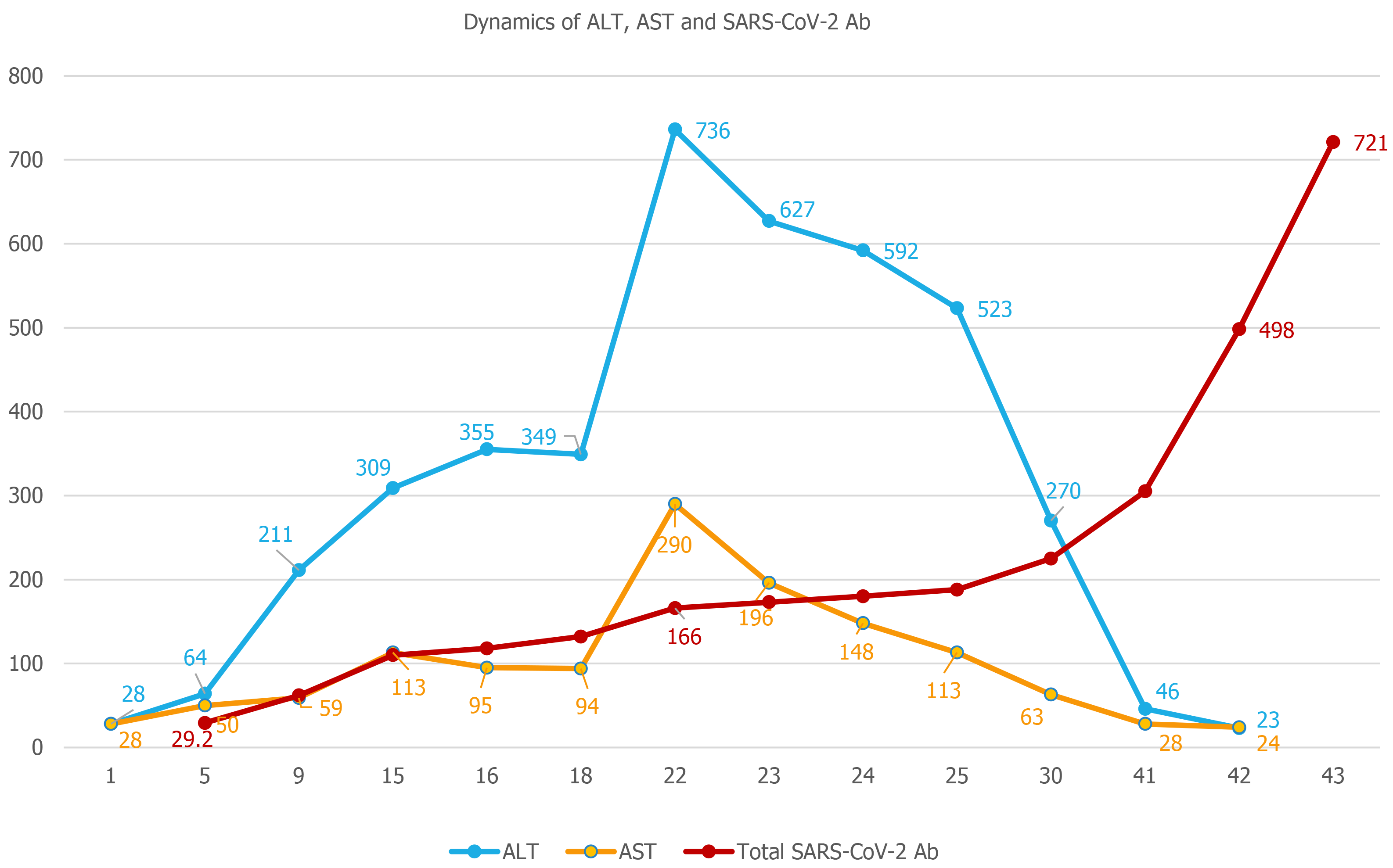
The remaining hypothesis, and the final diagnosis in our case, was post-COVID immune hepatitis, which occurred 20 days after the complete clinical remission of COVID-19 and paralleled a rapidly increasing titer of total anti-SARS-CoV-2 antibodies (determined through amplified chemiluminescence on VITROS 3600, Ortho Clinical Diagnosis, United States). Total antibodies appeared as early as day 6 (29.2 S/CO) and rapidly increased to a titer of 166 S/CO on day 23 (Figure 2).
We continued to monitor the patient closely over the next few days, until the ALT and AST levels started to decrease. Following this decrease, the patient was discharged on day 27, since the clinical remission and RT-PCR negative conversion criteria had been already met, and he continued to be followed-up as outpatient. A normalization of ALT levels was seen 20 d after the ALT peak. However, the titer of SARS-CoV-2 antibodies continued to increase up to 5 mo after the initial infection, reaching a peak of 865 S/CO at day 145 and then gradually decreasing to 657 S/CO at day 193 (6 mo after the initial infection).
This case has a set of particularities. Transmission of disease occurred from an asymptomatic SARS-CoV-2 carrier, which highlights the importance of preventive measures such as continued mask wearing, including in the presence of apparently asymptomatic persons. A recent meta-analysis has reported that the risk of transmission of SARS-CoV-2 infection from asymptomatic carriers is 18.8% with close contact, and that the overall percentage of asymptomatic patients among all those testing positive for SARS-CoV-2 can vary in quite a large range, from 20% to 75%, depending on the assayed population[1].
Our current case also led to secondary transmission to another member of the household, who was a healthcare worker, working at a COVID-19 unit. At the time when our current case was confirmed by RT-PCR the household member was negative for SARS-CoV-2 by RT-PCR, and approximately 5 d after symptom onset in our case, the household member became symptomatic and also tested positive for SARS-CoV-2 by RT-PCR. In our experience, when wearing appropriate personal protective equipment in the COVID ward, the overall risk of acquiring infection at work might theoretically be lower than that of acquiring infection elsewhere, particularly in the household, where permanent mask wearing is most often not possible.
Furthermore, while elderly patients with associated comorbidities have clearly been characterized as one of the most important groups at risk of severe or complicated COVID-19, our reported case highlights the fact that close attention should also be paid to young patients with mild forms of disease, and a high index of suspicion should be maintained for post-COVID complications, particularly since young patients may develop a strong immune response, potentially associating immune complica
Hepatocytolysis can be a relatively common feature of COVID-19, but it generally occurs during the acute phase of the infection, with a French cohort study reporting that up to 36.3% of patients had abnormal liver function tests[2], similar to the rates of 31.6%[3] and 37.2%[4] reported from China, with even higher rates (62%) reported from the United States[5]. However, most of these clinical observations come from patients with moderate, severe or critical forms of disease, since this is the patient population that most often requires management in the hospital. Liver cytolysis has also been associated with more extensive lung lesions during the acute phase of COVID-19[3]. However, while the overall prevalence of liver cytolysis in patients with COVID-19 appears to be quite high, reported at 46.9% in a pooled meta-analysis[6], relatively fewer cases display high-grade cytolysis, i.e., only 6.4% of the patients from the French cohort had ALT levels above 5 times the upper normal limit[2]. Because of the fact that in Romania at the time when our case occurred, hospital isolation was mandatory for all patients testing positive for SARS-CoV-2 infection, we were able to detect this biochemistry finding, an ALT level 15 times the upper normal limit in a patient who had had mild COVID. ALT levels above 5 times the upper normal limit in COVID-19 are associated with a poor prognosis, specifically: significantly higher risk of severe lung involvement, intensive care admission, and death[2,6]. This was not the case in our patient, where an even higher ALT level was not associated with worsening of COVID, and it occurred after remission of all clinical signs and symptoms for 20 d.
Residual inflammation has been documented by (18F) fluorodeoxyglucose positron emission tomography/CT in patients convalescing post-severe COVID, in organs such as the lungs, mediastinal lymph nodes, liver and spleen, immediately after RT-PCR negative conversion[7]. This residual inflammation could theoretically explain to some extent the pattern of hepatocytolysis seen in our patient, however, our case was a mild form of disease, not severe, and displayed a much higher ALT increase, while in the cited study most patients (6/7) had normal ALT levels at the time when positron emission tomography/computed tomography detected liver inflammation, and only one of the patients had low-grade cytolysis (ALT 1.5 times the upper normal limit)[7].
In the case we have reported, this finding was mostly inconsequential, as it did not associate liver failure, or long-term consequences. However, it is not implausible to speculate that had this type of ALT flare occurred in a patient with pre-existing liver damage, or pre-existing autoimmunity, it could have associated important clinical implications. Therefore, we consider that it is essential to maintain a high index of suspicion for post-COVID complications in all patients, not only those who have had a severe or complicated form of disease.
We have reported an interesting case of transient post-COVID immune hepatitis, in a young male patient with no pre-existing comorbidities. Close attention should also be paid to young patients with mild forms of disease, and a high index of suspicion should be maintained for post-COVID complications.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Society for Clinical Microbiology and Infectious Diseases, No. 107847.
Specialty type: Infectious diseases
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jin SY, Lipiński P, Vieira A S-Editor: Zhang H L-Editor: A P-Editor: Li JH
| 1. | Yanes-Lane M, Winters N, Fregonese F, Bastos M, Perlman-Arrow S, Campbell JR, Menzies D. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: A systematic review and meta-analysis. PLoS One. 2020;15:e0241536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 2. | Chaibi S, Boussier J, Hajj WE, Abitbol Y, Taieb S, Horaist C, Jouannaud V, Wang P, Piquet J, Maurer C, Lahmek P, Nahon S. Liver function test abnormalities are associated with a poorer prognosis in Covid-19 patients: Results of a French cohort. Clin Res Hepatol Gastroenterol. 2020;101556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 4. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 5. | Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, Sharaiha RZ; WCM-GI research group*. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology 2020; 159: 1137-1140. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 6. | Del Zompo F, De Siena M, Ianiro G, Gasbarrini A, Pompili M, Ponziani FR. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:13072-13088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 7. | Bai Y, Xu J, Chen L, Fu C, Kang Y, Zhang W, Fakhri GE, Gu J, Shao F, Wang M. Inflammatory response in lungs and extrapulmonary sites detected by [18F] fluorodeoxyglucose PET/CT in convalescing COVID-19 patients tested negative for coronavirus. Eur J Nucl Med Mol Imaging. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |