Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.4007
Peer-review started: January 25, 2021
First decision: February 25, 2021
Revised: March 7, 2021
Accepted: March 23, 2021
Article in press: March 23, 2021
Published online: June 6, 2021
Processing time: 109 Days and 0 Hours
Tuberculosis (TB) is a widespread infectious disease, with an incidence that is increasing worldwide. Cutaneous TB (CTB) occurs rarely, accounting for less than 1% of all TB cases. Due to the clinical presentation and diagnostic difficulties, CTB is often clinically neglected and misdiagnosed.
A 32-year-old man underwent several debridement surgeries and skin flap transplantation after trauma. The wound remained unhealed, accompanied by sinus formation. According to empirical judgment, T-cell spot of TB test, and bacterial culture of pyogenic fluids, he was diagnosed with CTB due to infection with exogenous Mycobacterium tuberculosis. A comprehensive anti-TB regimen that included isoniazid, rifampicin, ethambutol, and pyrazinamide was applied. The sinus was filled with a hydrophilic fiber-containing silver dressing, and wound-protecting sponges were applied to part of the wound. The wound healed after 40 d. No ulceration was found within 2 mo after discharge; further follow-up will be conducted.
A non-healing wound may be caused by TB infection. Comprehensive treatment of CTB is effective.
Core Tip: Cutaneous tuberculosis (TB) is rare, and thus likely to be neglected and misdiagnosed. We report the case of a middle-aged man who suffered from repeated wound ulceration within 1 year after trauma. He was subsequently diagnosed with cutaneous TB (CTB) due to infection with exogenous Mycobacterium tuberculosis, which was confirmed by empirical judgment and a series of examinations. The patient received combination treatment with dressing change. The wound healed after 40 d. This case highlights the possibility of CTB infection in wounds that do not heal over a long period, and the importance of timely diagnosis and comprehensive treatment of this disease.
- Citation: Gao LJ, Huang ZH, Jin QY, Zhang GY, Gao MX, Qian JY, Zhu SX, Yu Y. Delayed diagnosis and comprehensive treatment of cutaneous tuberculosis: A case report. World J Clin Cases 2021; 9(16): 4007-4015
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/4007.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.4007
Cutaneous tuberculosis (TB) is a rare form of extra-pulmonary TB. It is characterized by the skin rash caused by Mycobacterium tuberculosis, the rare Mycobacterium bovis, and Calmette-Guerin vaccines. Due to the differences in the number of virulence factors, routes of transmission, pathogenesis, and immunity to Mycobacterium tuberculosis, the clinical manifestations in patients vary. According to its pathogenesis, clinical manifestations, and histological features, cutaneous TB (CTB) is mainly divided into three categories: Exogenous, endogenous, and bloodborne[1]. This report focuses on a patient who suffered from an ulcerated wound on the thigh due to trauma. After wound debridement, vacuum sealing drainage (VSD), and skin grafting, the skin still had a large area of rupture that remained unhealed for a long time. The patient was diagnosed with exogenous TB by laboratory tests. He recovered after wound dressing change combined with a comprehensive anti-TB regimen.
A 32-year-old unmarried man was admitted to our hospital due to repeated rupture of a wound on the right thigh after a skin graft.
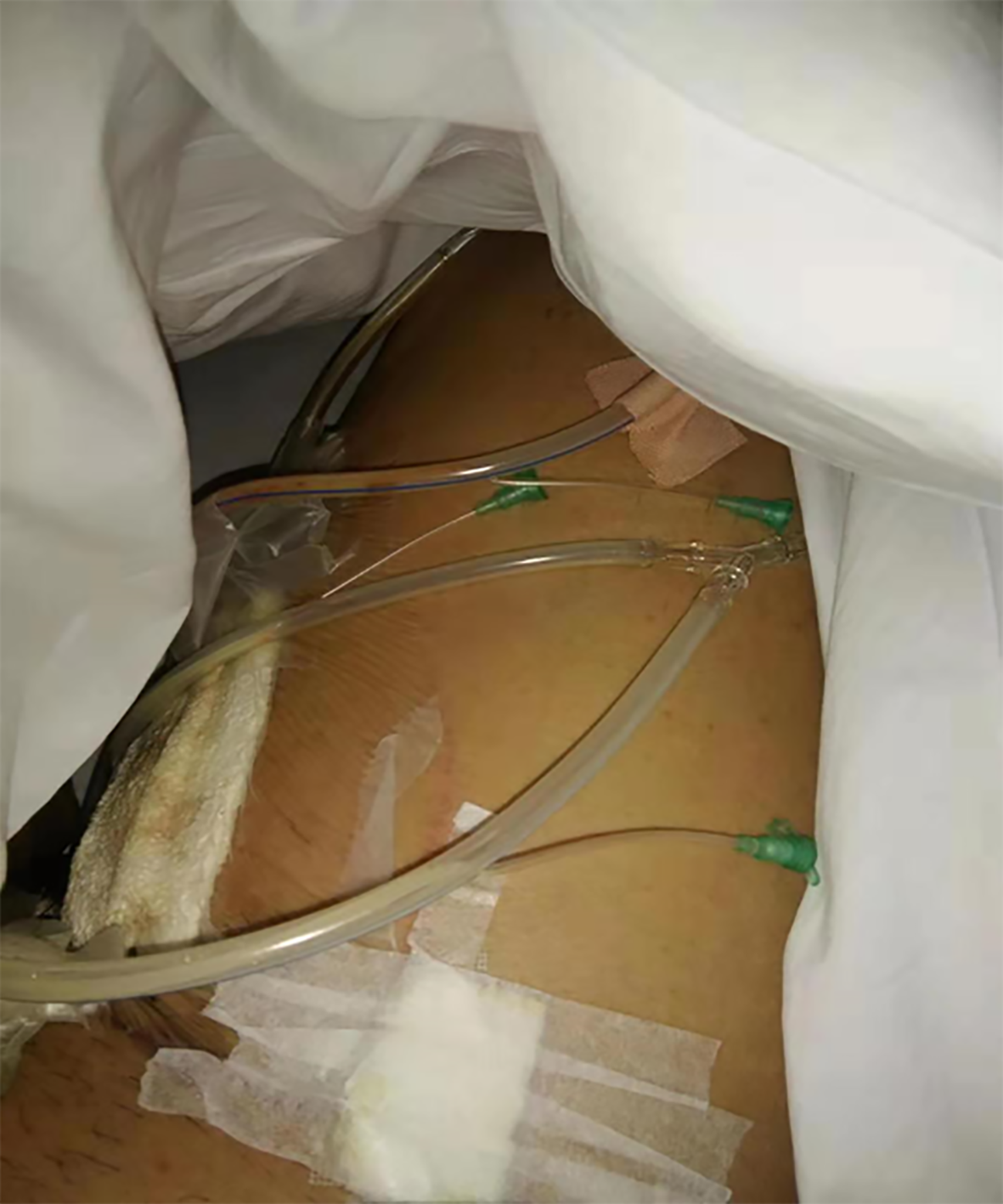
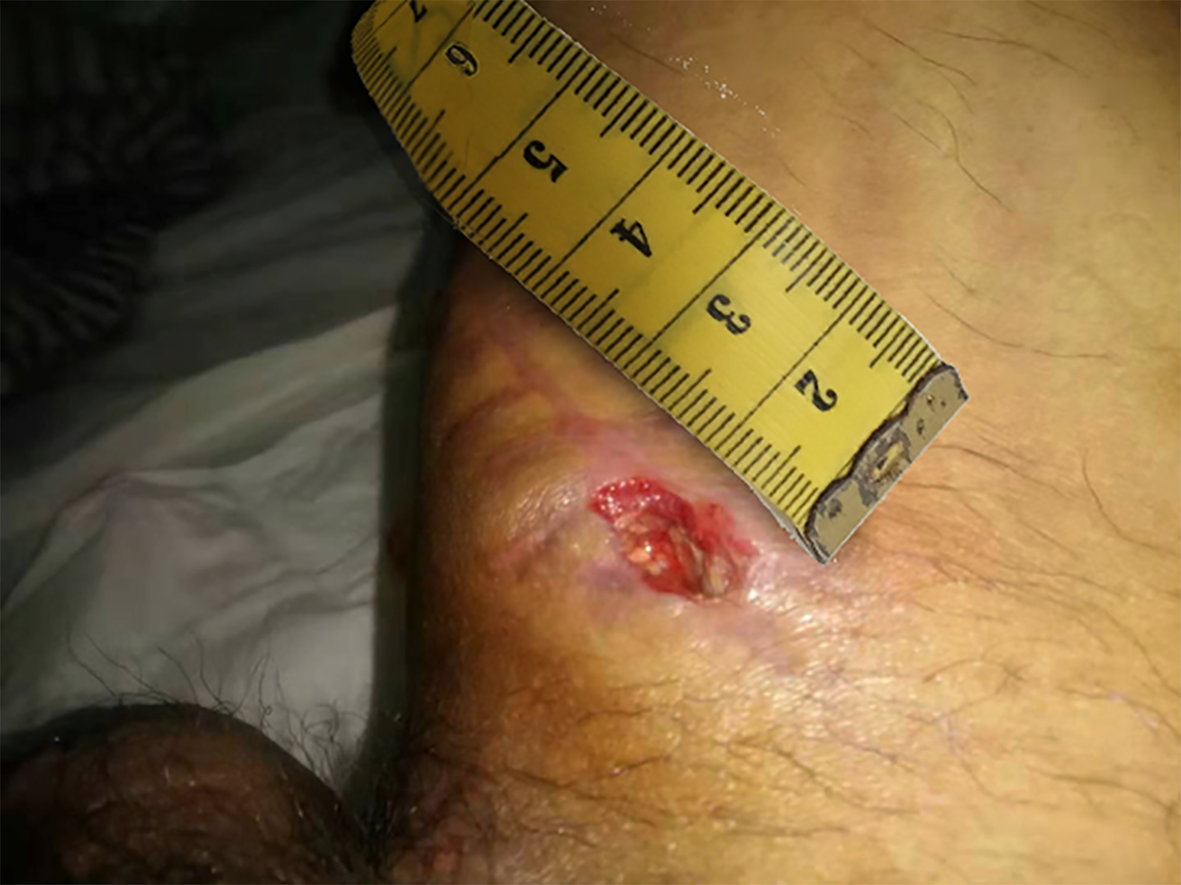
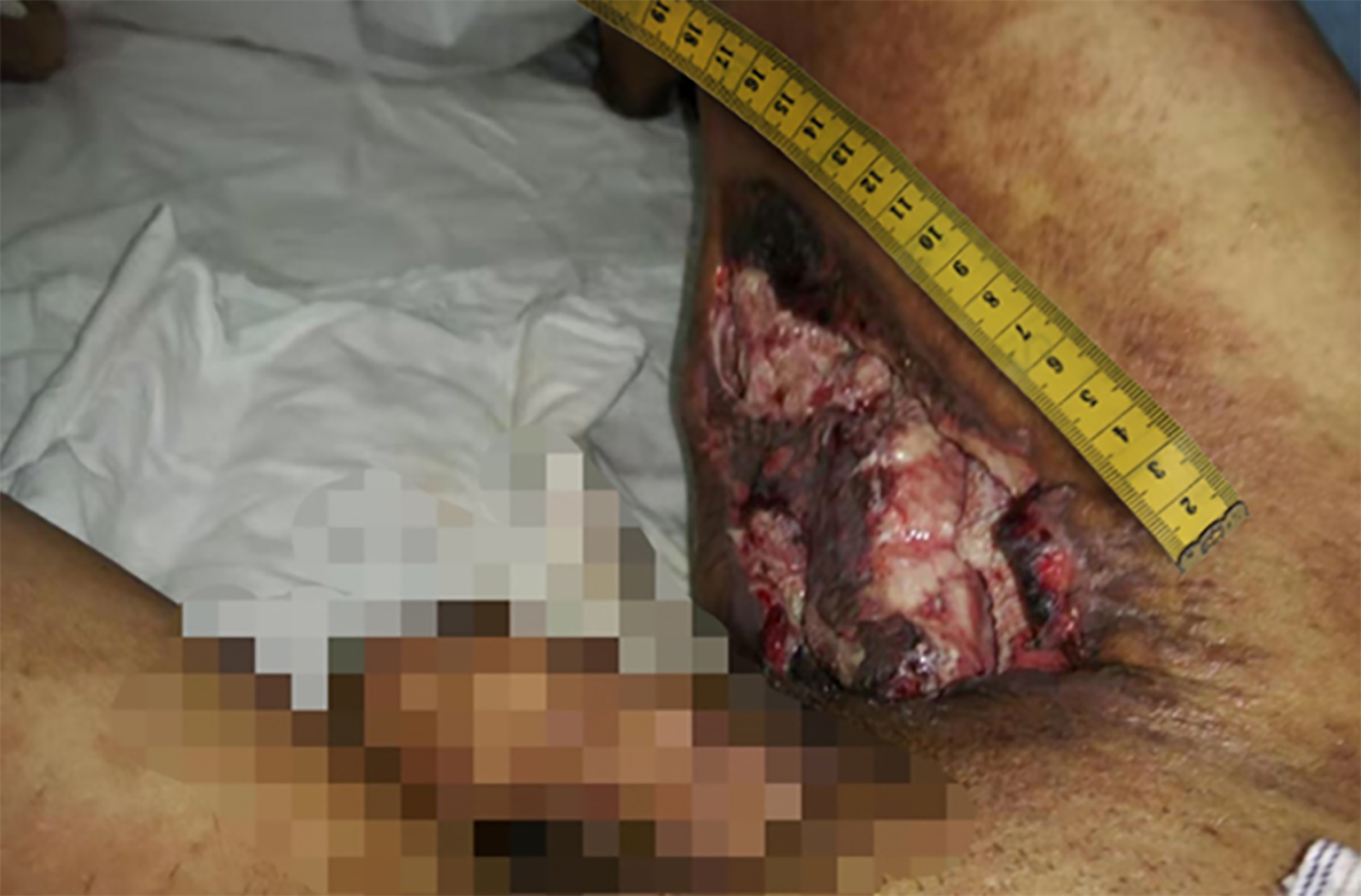
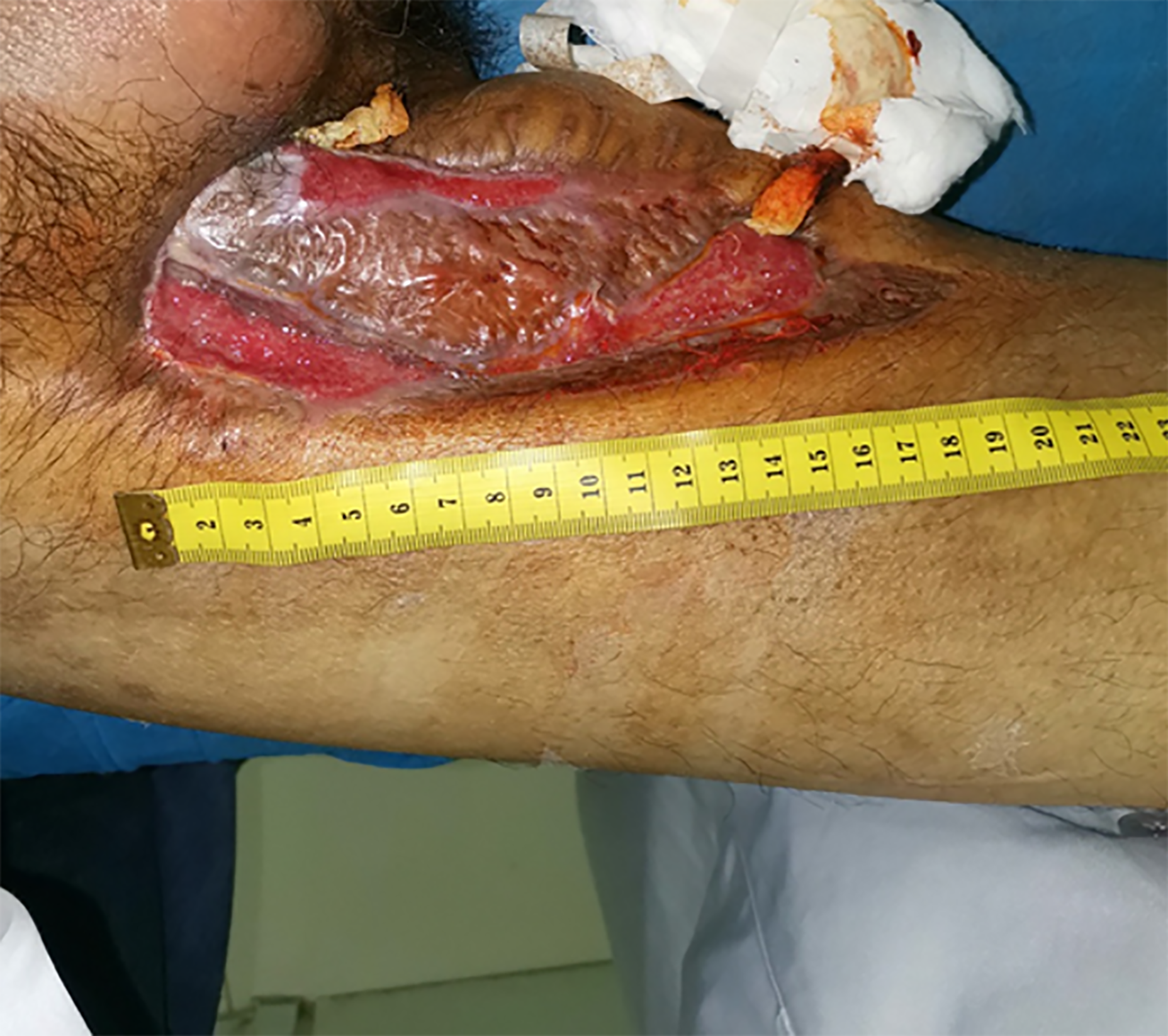
In October 2018, the patient’s inner right thigh was accidentally scratched by sharp objects, causing wound rupture and liquid discharge. After routine wound dressing and antibiotic treatment, a sinus appeared in the wound after 2 mo. In January 2019, he successively underwent debridement of the right thigh wound + VSD and debridement of the right thigh suture + VSD (Figure 1). At 1 mo after the operation, the surgical incision was ulcerated and a wound surface area of 1.5 × 1 cm2 appeared, with granulation tissue, edema, and pale yellow liquid discharge. There was swelling, redness, high skin temperature, and tenderness at the wound site (Figure 2). In March 2019, debridement of the right thigh wound was performed. The wound dressing was changed after surgery. After 2 wk, the wound surface area was about 15 × 8 cm2. The granulation tissue was pale, with a large amount of purulent discharge (Figure 3). Pathological examination of the wound indicated inflammatory granulation. Debridement was performed again, and VSD was conducted. After granulation tissue formed, autogenous skin grafting was performed (Figure 4). At 1 mo after skin grafting, the skin graft ruptured and multiple lesions appeared with sinus formation; the upper sinus was located at the root of the thigh, with a depth of about 6 cm. There was a little necrotic granulation tissue after curette scraping, and the lower sinus was about 2 cm. The granulated tissue in the wound was still fresh, with a large amount of exudate (Figure 5). The patient did not experience symptoms of low fever, night sweats, or weight loss.
The patient was physically healthy without a history of hypertension, heart disease, diabetes, drug or food allergy, or infectious diseases such as hepatitis, TB, and typhoid fever. He had appendicitis and abdominal wall fistula 6 years prior.
The patient had no family history of TB.
Physical examination showed the following: Body temperature, 36.6 °C; pulse, 98 beats/min; heart rate, 20 beats/min; blood pressure, 108/68 mmHg. Neither dry nor moist rales were heard from the lungs. The skin grafting area ruptured and multiple lesions appeared with sinus formation; the upper sinus was located at the root of the thigh, with a depth of about 6 cm. There was a little necrotic granulation tissue after curette scraping, and the lower sinus was about 2 cm. The granulated tissue in the wound was fresh, with a large amount of exudate. No swollen lymph nodes were found in the groin area.
Laboratory testing showed T-cell spot of TB test (T-SPOT) > 400 pg/mL; culture of pus from the wound, purified protein derivative (PPD), blood routine, liver and kidney function, procalcitonin, erythrocyte sedimentation rate, and immune indicators were all normal.
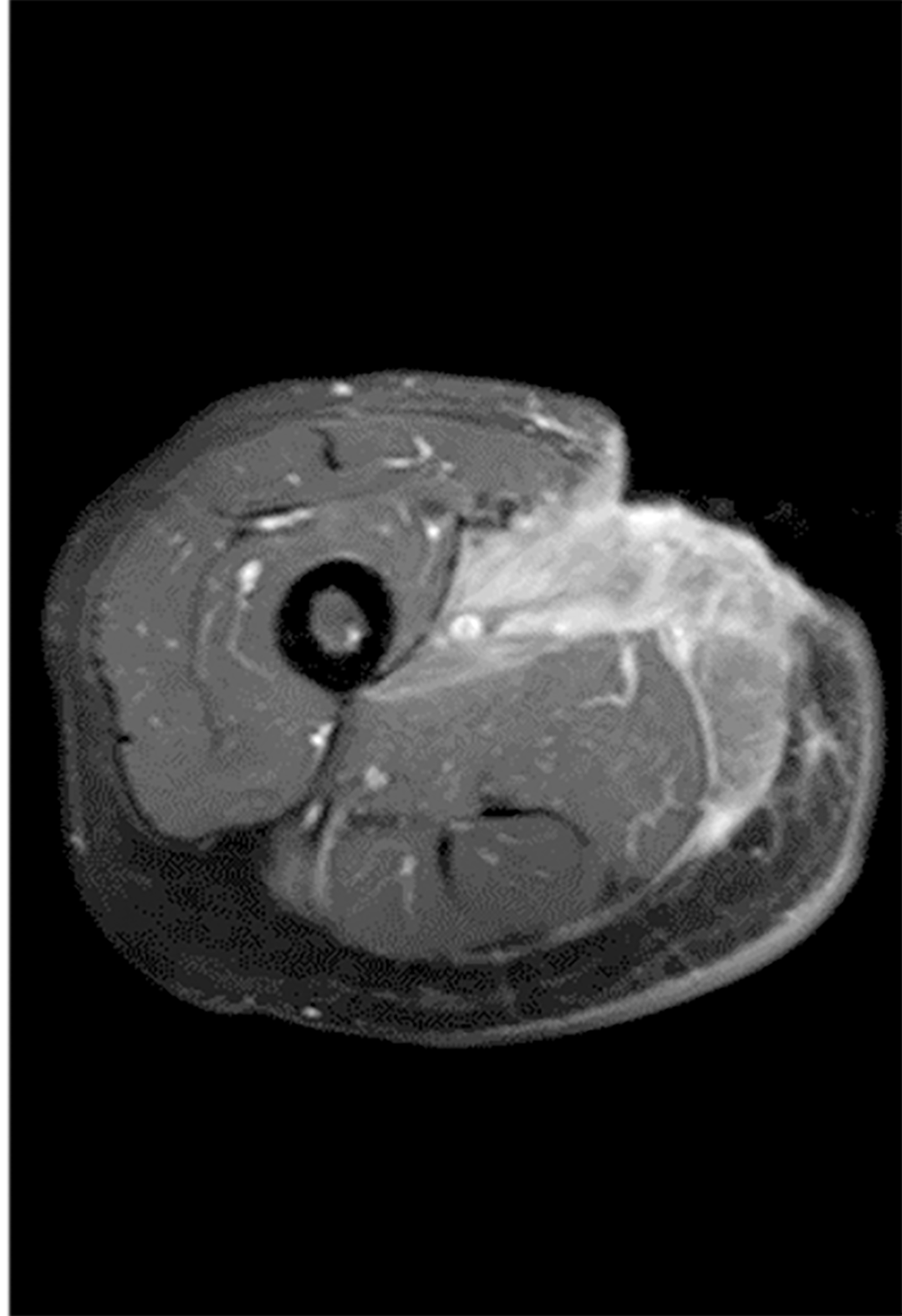
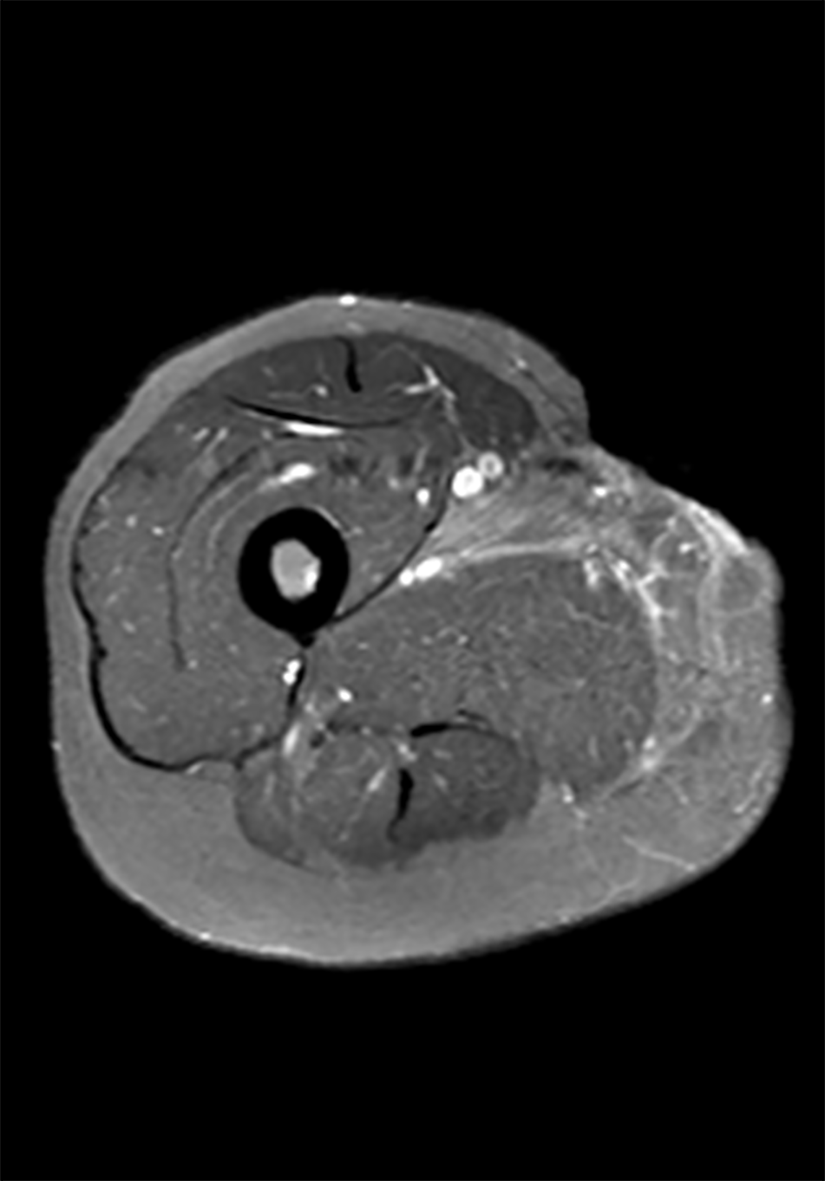
Chest computed tomography showed no obvious abnormalities in both the lungs and mediastinum. Magnetic resonance imaging (MRI) of the soft tissue of the right inner thigh and right groin area showed abnormal signals involving the right internal and external muscles of the pelvic floor. Combined with the medical history, the changes caused by surgery were considered. TB focus and sinus formation could be seen around the obturator muscle (Figure 6).
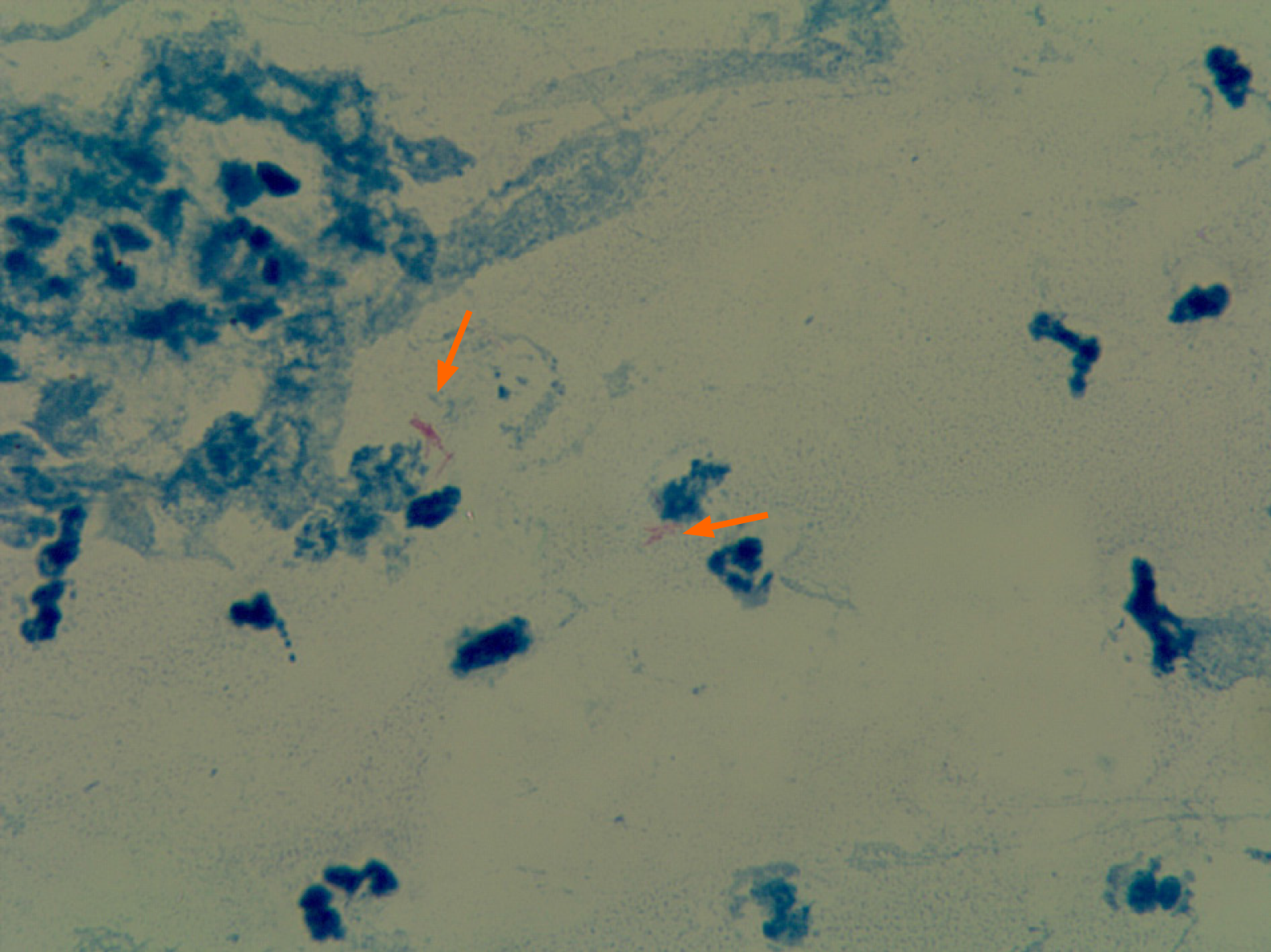
The final bacterial culture of pyogenic fluids suggested that Mycobacterium cultured for 45 d was positive for 2+ GeneXpert Mycobacterium tuberculosis (Figure 7) and was sensitive to rifampicin. The patient was diagnosed with exogenous Mycobacterium tuberculosis infection caused by trauma.
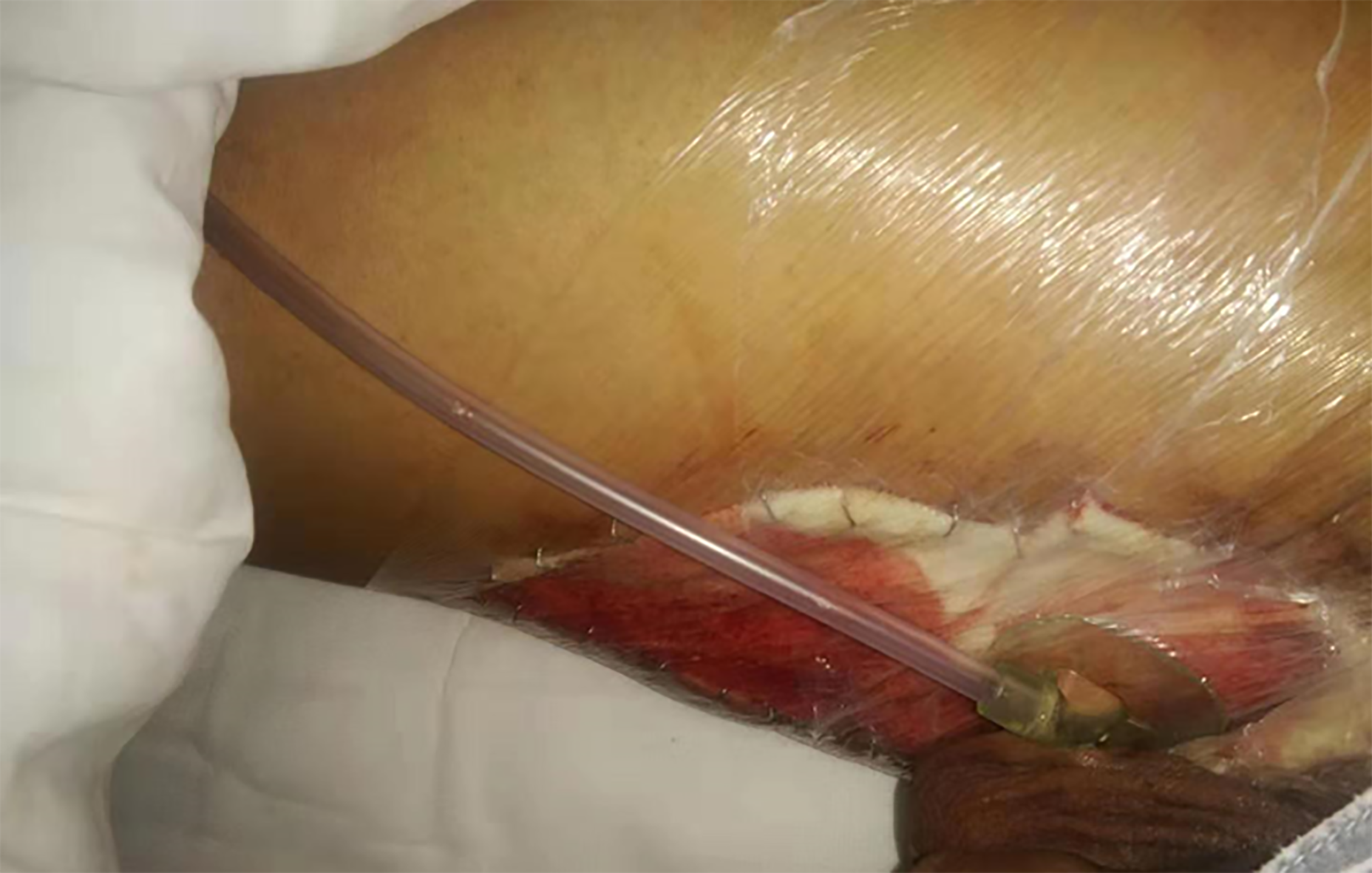
In May 2019, the HRZE quadruple anti-TB treatment was continued. Hydrophilic fiber-containing silver dressings were used to fill the sinus, and part of the wound was protected by applying a sponge externally and changing the dressing once a day.
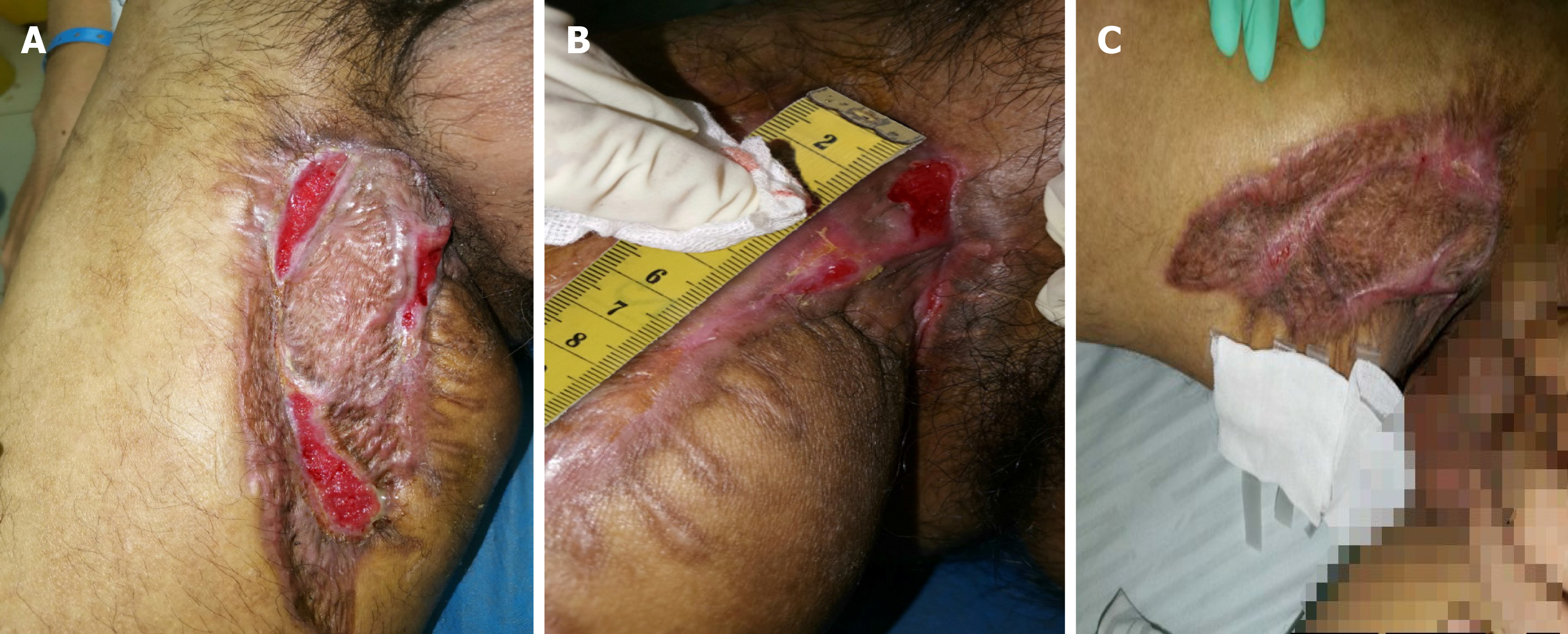
After 2 wk of treatment, the wound surface shrank, skin margins appeared, fresh granulation tissue developed, and no necrotic matter was seen (Figure 8A). However, the liver and kidney functions were abnormal, with uric acid of 673 mmol/L, alanine aminotransferase of 68 IU/L, and aspartate aminotransferase of 75 IU/L. The above-mentioned treatment was continued, with bicyclol tablets 25 mg tid and benbro
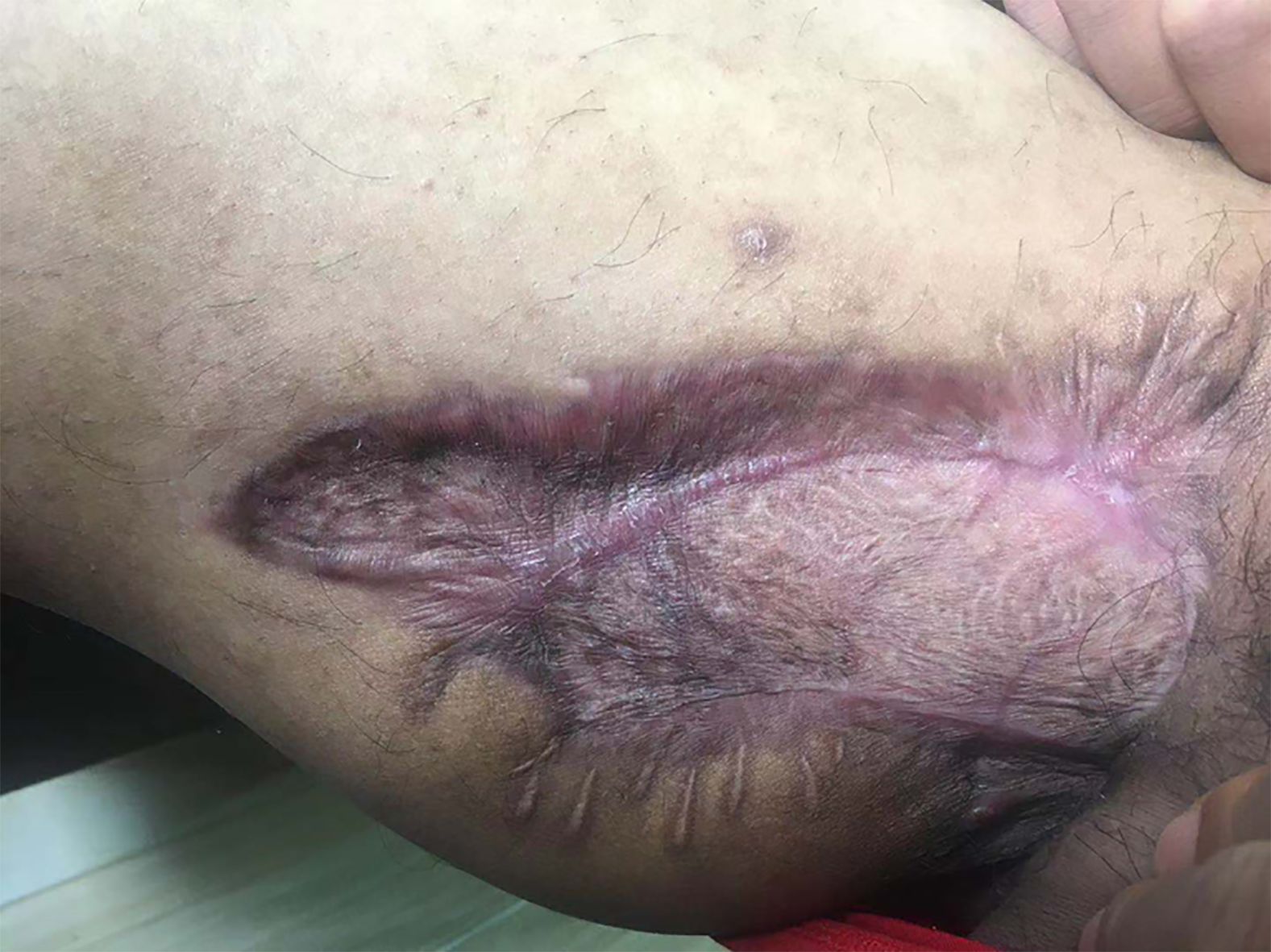
The wound healed after 40 d of treatment (Figure 8C). Re-examination of the right leg MRI showed that the lesions around the obturator external muscles and sinus of the anterior radiograph improved (Figure 9). The liver and kidney functions returned to normal. The patient was discharged. He was instructed to continue comprehensive anti-TB treatment and timely monitoring of blood and liver and kidney functions. A follow-up was performed 2 mo after discharge, by which time the wound had healed well (Figure 10).
We performed a retrospective study of patients with TB abscess in the PubMed database, using the key words “CTB” and “case report.” Only 11 records were obtained from 1958 to 2017. The patients tended to have primary diseases, which disguised the TB lesions. Because PPD, sputum culture, and X-rays can yield negative results and pus culture often takes a long time, the clinical diagnosis of skin TB is difficult. Additionally, according to the literature, more than half of the patients have abnormal immune functions, such as congenital hypothyroidism, peripheral vascular disease, rheumatoid arthritis, rheumatoid arthritis, and human immunodeficiency virus, suggesting a relationship between the use of immune modulators and the incidence of TB. In clinical practice, if a patient has unhealed wounds and the immune function of this patient is abnormal, the possibility of TB should be considered. Although these 11 records all emphasized the long course of CTB, difficulty of diagnosis, association with immune deficiency, and internal treatment of anti-TB, only two mentioned external treatment.
According to the World Health Organization (WHO), about 20% to 40% of the world's population is infected with Mycobacterium tuberculosis, with about 8-9 million new cases each year, posing a serious threat to human health. CTB is often overlooked due to its rare clinical manifestations and difficult diagnosis[2]. Among the three types, bloodborne and endogenous CTB generally spread from the TB lesions, such as lymph node TB, bone and joint TB, breast TB, and TB of the epididymis, to nearby skin tissues. The lesions can also spread to the skin via the blood vessels and lymphatic system, causing ulcerative skin damage, which is often well documented and relatively easy to be diagnosed. Exogenous CTB is often missed and misdiagnosed due to the lack of early symptoms. Although the patient’s age and sex in this case were consistent with those mentioned in the literature, his immune indicators were normal and there were no inducing factors for TB, which made early diagnosis more difficult. Therefore, when a wound remains unhealed for a long time, even if there is no inducement or typical signs of TB, the possibility of CTB should be taken into consideration.
At present, common clinical laboratory methods for the diagnosis of TB include histopathology, bacterial culture, anti-TB antibody detection, and acid-fast staining. However, these methods have the disadvantages of poor specificity, low detection rate, and long detection duration. Among them, histopathology and bacterial culture of pyogenic fluids are the gold standards for laboratory testing of Mycobacterium tuberculosis. However, in the case of a non-healing wound and many false negatives, repeated sampling is required for histopathological tests. Bacterial culture of pyogenic fluids takes a long time, which delays the diagnosis and treatment of the disease[3]. In this case report, the bacterial culture of pyogenic fluids from the patient developed Mycobacterium tuberculosis after 45 d and multiple histopathology tests were also negative, which is another reason for delayed diagnosis. T-SPOT is an emerging method for diagnosing TB, and only takes 24 h. The sensitivity and specificity in CTB diagnosis are as high as 91.6% and 75.8%, respectively[4], and are not influenced by the lesion site and the body’s immunity[5-8]. In this case, the patient’s T-SPOT > 400 pg/mL showed a high sensitivity. When clinical experience points to TB, T-SPOT is a very good diagnostic reference index. The development of novel methods for the diagnosis of TB is part of the WHO’s Global Plan to eradicate TB (2006-2015)[9]. New detection technologies established in the fields of molecular biology and immunology in recent years offer new hope for the early diagnosis and treatment of patients with suspected TB.
CTB features special infectious chronic wounds with persistent inflammation. Increased protease in tissues limits angiogenesis, which in turn leads to hypoxia and damage to the surrounding tissues, delays epithelialization and collagen deposition, and causes exudation and sinus, which is difficult to heal. It can be treated according to the concept of "wound bed preparation"[10], which is a systemic treatment method that promotes wound healing by avoiding systemic and local factors that may delay wound healing.
CTB should be treated according to the WHO’s recommended protocol, including a 2-mo intensive treatment phase and a 4-mo maintenance phase. The intensive treatment phase aims to quickly reduce the overall burden of Mycobacterium tuberculosis, whereas the maintenance phase aims to treat the remaining bacterial infection from the initial intensive or bactericidal phase. After 8 wk of treatment, the patient is no longer considered contagious, but long-term treatment is still needed to eradicate the disease[3,11]. In the literature review, although the risks of hepatotoxicity and nephrotoxicity of anti-TB drugs are not noted, their toxic effects are apparent and often become an important factor that interferes with the continuation of chemotherapy. Some inexperienced clinicians discontinue the course and the drug once adverse reactions occur, which leads to unnecessary side effects and drug resistance, thus aggravating the disease. In this case, 2 wk after the patient received anti-TB treatment, hepatic and renal function abnormalities occurred. However, hepatic and renal function returned to normal after intervention with enzyme-lowering and liver-protecting drugs and uric acid-lowering drugs. Adherence to a comprehensive anti-TB regimen without stopping or changing drugs blindly was also key to the successful treatment of this patient. Local treatment needs to focus on removing necrotic tissue, draining exudate, and restoring the balance of flora. Therefore, the use of corresponding excipients to create a relatively suitable wound microenvironment to ensure the formation of high-quality granulation tissue and to promote growth in the treatment of CTB is particularly critical. In the literature review, we learned the necessity for anti-TB chemotherapy. However, when the area of CTB lesion is too large, anti-TB treatment alone may not be effective. We chose a hydrophilic fiber silver-containing dressing to fill the sinus. When the wound exudate was absorbed by the dressing, it came into contact with the silver ions in the dressing, and the silver ions were released into the percolate, killing and inhibiting the bacterial growth. The silver ions can strengthen the epithelialization process of the wound while reducing the infection, promoting epithelialization and mitosis of the wound, and accelerating sinus healing[12]. Microdynamic negative pressure wound dressing is composed of special polyvinyl alcohol medical material with a high water absorption rate and medical transparent adhesive film (semi-permeable membrane). When the wound is covered topically, the wound fluid leaks into the sponge in real time. The sponge can quickly absorb wound exudate and bleeding, achieving a wound drainage effect, avoiding the residue of solid components such as cellulose or protein in the exudate between the wound and the dressing, and eliminating bacteria and other microorganisms in the wound medium to reduce the chance of wound infection and promote wound healing[13].
CTB is rare in clinical practice, and exogenous CTB is often missed and misdiagnosed. If the wound does not heal for a long time after trauma, the possibility of TB should be considered. T-SPOT has a high degree of sensitivity and specificity for the diagnosis of TB, with good diagnostic efficacy. Dressing change for part of the wound combined with a comprehensive anti-TB regimen has significant effects on CTB.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang ZX S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Hill MK, Sanders CV. Cutaneous Tuberculosis. Microbiol Spectr. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | van Zyl L, du Plessis J, Viljoen J. Cutaneous tuberculosis overview and current treatment regimens. Tuberculosis (Edinb). 2015;95:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27. [PubMed] |
| 4. | Lai CC, Tan CK, Lin SH, Liu WL, Liao CH, Huang YT, Hsueh PR. Diagnostic value of an enzyme-linked immunospot assay for interferon-γ in cutaneous tuberculosis. Diagn Microbiol Infect Dis. 2011;70:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | He M, Xun AY, Lai GS, Wu HH, Zou B, Wu ZG. T-SPOT.TB in the differential diagnosis of Crohn's disease and intestinal tuberculosis. Yixue Zongshu. 2014;20:4364-4366. [DOI] [Full Text] |
| 6. | Zeng J, Xiao NC, Peng HY. Comparison of diagnostic value of TSPOT.TB and tuberculosis antibodies in patients with HIV/AIDS combined with bacterial negative pulmonary tuberculosis. Shiyan Yu Jianyan Yixue. 2018;36:199-201. [DOI] [Full Text] |
| 7. | Liao B, Ding XP. T-SPOT.TB and ADA in the diagnosis of tuberculous pleurisy. Guoji Jianyan Yixue Zazhi. 2014;35:2323-2325. [DOI] [Full Text] |
| 8. | Wan R, Wang YL, Qi YW, Bai JS, Su JH, Zhao Q. Application of TB infection T cell spot test in the diagnosis of HIV/AIDS complicated with TB infection. Kunming Yike Daxue Xuebao. 2011;32:138-141. [DOI] [Full Text] |
| 9. | Zhang QH, Li GX, Bai J, Zhang XX, Cui L, Jie YS. Comparative Study of Laboratory Tests for Skin Tuberculosis. Xibu Yixue. 2016;28:1009-1012. [DOI] [Full Text] |
| 10. | Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11 Suppl 1:S1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 745] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 11. | Blomberg B, Fourie B. Fixed-dose combination drugs for tuberculosis: application in standardised treatment regimens. Drugs. 2003;63:535-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Jørgensen B, Price P, Andersen KE, Gottrup F, Bech-Thomsen N, Scanlon E, Kirsner R, Rheinen H, Roed-Petersen J, Romanelli M, Jemec G, Leaper DJ, Neumann MH, Veraart J, Coerper S, Agerslev RH, Bendz SH, Larsen JR, Sibbald RG. The silver-releasing foam dressing, Contreet Foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J. 2005;2:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Huang LL, Zou YR. Observation on the effect of 29 cases of pressure ulcer treated with wound wound sponge dressing. Xiandai Yiyao Weisheng. 2015;31:3157-3159. [DOI] [Full Text] |