Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.3971
Peer-review started: December 21, 2020
First decision: February 11, 2021
Revised: February 26, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: June 6, 2021
Processing time: 143 Days and 12.9 Hours
Gastric pull-up after esophagectomy is still a demanding surgical procedure and associated with considerable morbidity such as anastomotic leaks, fistulas or stenoses. These complications are usually managed by endoscopy, but in extreme cases multidisciplinary management including reoperations may be necessary. Here, we report managing therapy-refractory pseudoachalasia after Ivor Lewis esophagectomy by bypassing colonic pull-up.
A 70-year-old male with dysphagia and regurgitation after esophagectomy with gastric pull-up reconstruction was transferred to our tertiary hospital. Since endoscopic approaches including balloon dilatation and stenting failed, retrosternal colonic pull-up with Roux-en-Y reconstruction was performed with no subsequent adverse events.
Secondary colonic pull-up is a demanding but successful surgical procedure in patients suffering from therapy-refractory complaints after esophagectomy with gastric pull-up reconstruction.
Core Tip: Esophageal surgery with gastric pull-up reconstruction remains the only curative therapy for malignant tumors of the esophagus. Despite significant progress in minimally invasive techniques and improvements in perioperative care/complication management, it is still associated with high rates of morbidity and mortality such as anastomotic leak, fistula and stenosis. One of the most frequent long-term functional complications of esophageal surgery with gastric pull-up is delayed gastric emptying which can be usually treated successfully by endoscopic approaches. However, there are cases with a therapy-refractory complaints where salvage operations are required. Here, we present such a case where an operation with secondary colonic pull-up after oncological esophagectomy with gastric tube reconstruction was performed due to pseudoachalsia caused by an anastomotic stenosis that was refractory to endoscopic interventions.
- Citation: Flemming S, Lock JF, Hankir M, Reimer S, Petritsch B, Germer CT, Seyfried F. Successful management of therapy-refractory pseudoachalasia after Ivor Lewis esophagectomy by bypassing colonic pull-up: A case report. World J Clin Cases 2021; 9(16): 3971-3978
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/3971.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.3971
Esophageal surgery remains the only curative therapy for malignant tumors of the esophagus/esophagogastric junction. Despite significant progress in minimally invasive techniques and improvements in perioperative care/complication management, it is still associated with high rates of morbidity and mortality. Usually, gastric pull-up procedures are used to restore continuity of the alimentary tract[1]. Furthermore, colonic or jejunal pull-up are usually not performed minimal invasive and necessitate at least three anastomoses to restore gastrointestinal continuity which may enhance the risk for additional postoperative complications[2].
One of the most frequent long-term functional complications of esophageal surgery with gastric pull-up is delayed gastric emptying (DGE) which occurs in up to 50% of patients and is a cause of dysphagia, regurgitation, vomiting and feelings of abdominal fullness[3,4]. The primary treatment options for DGE are endoscopic interventions and prokinetic medication, secondary surgical options include a pyloroplasty and gastrojejunostomy[5]. If DGE persists due to more complex structural and functional damage of the stomach and remnant esophagus colonic or jejunal interposition may become a valuable option as salvage operations.
Here, we present such a case study where a salvage operation with secondary colonic pull-up after oncological esophagectomy with gastric tube reconstruction was performed due to pseudoachalsia caused by an anastomotic stenosis that was refractory to endoscopic interventions.
The patient reported unintentional weight loss of 8 kg during the previous 5 mo.
A 70-year old male patient originally contacted our outpatient clinic in the Section of Gastroenterology at the Department of Internal Medicine in 2015 complaining of dysphagia along with regurgitation and vomiting. Anamnestic information revealed that the patient had an abdominal-thoracic esophagus resection (Ivor-Lewis) with gastric tube reconstruction due to cancer of the esophagogastric junction 3 years prior in the Ukraine.
Despite reduced nutritional status, the patient’s general health status was good. Physical examination did not show any further abnormalities.
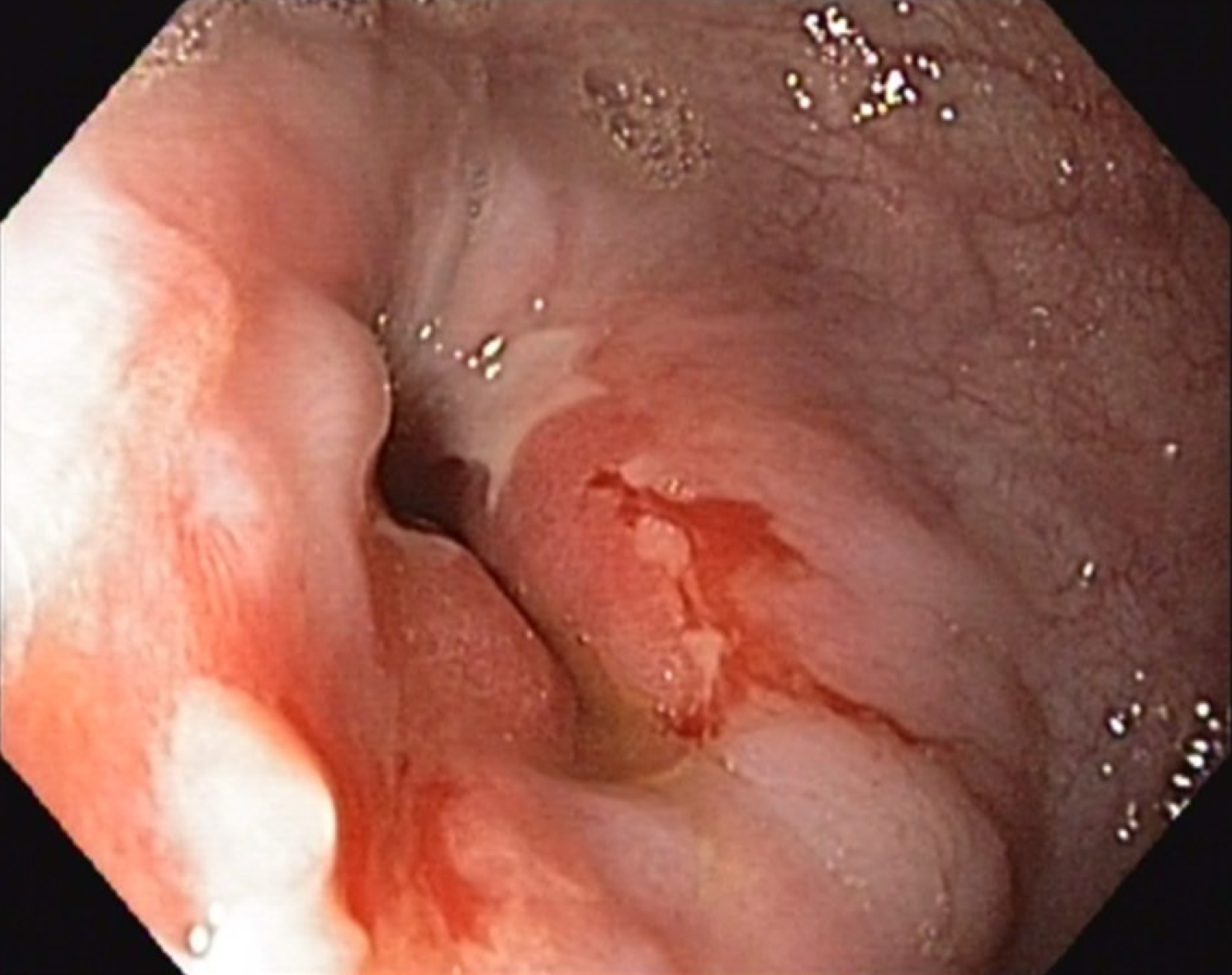
A computed tomography (CT) scan of the thorax and abdomen was initiated to exclude tumor recurrence. In parallel, endoscopic examination was performed which revealed a stenosis of the end-to-end esophago-gastrostomy with a residual lumen of 15 mm (Figure 1). The pre-stenotic esophagus was dilated and exhibited impaired peristalsis along with food retention and stasis esophagitis. The gastric tube also exhibited impaired peristalsis and food retention while the pylorus was tight and only passable when considerable pressure was applied to the endoscope.
In summary, the patient suffered from anastomotic stenosis resulting in dysphagia but also from DGE due to pylorospasm and hypomotility of gastric pull-up.
Based on the endoscopic findings and patient´s symptoms, multiple endoscopic balloon dilatations of the anastomosis and the pylorus were performed resulting in temporary improvement of complaints. However, due to the need for repetition of endoscopic interventions and only temporary relief of symptoms, endoscopic stent insertion was performed but failed due to recurrent stent dislocations. After stent removal, balloon dilatation therapy was reinitiated every 8 wk for almost 3 years. Adjunctive pro-kinetic medical therapy did not confer any clinically relevant improvements.
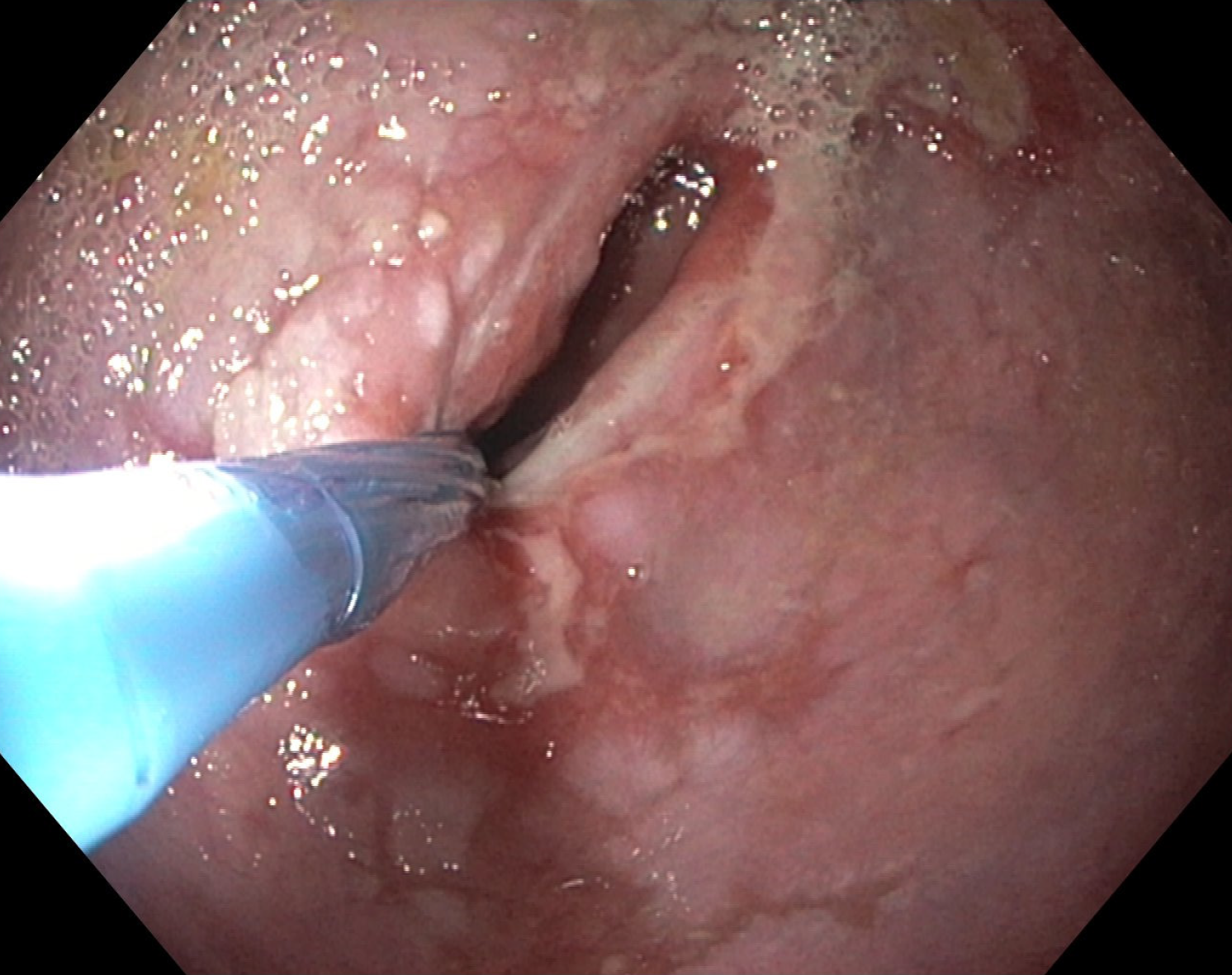
Over the time, the patient reported persistent and even progressively worsening dysphagia. Another CT scan did not reveal tumor recurrence. However, endoscopic examination revealed progressive esophagus dilatation with pre-stenotic bulging and residual lumen of the esophago-gastrostomy with 10mm (Figure 2). The esophagus and gastric tube still exhibited reduced motility and retention of food although the pylorus was now easily passable endoscopically. These endoscopic findings were in line with imaging findings, the progressive complaints and symptoms of the patient and their further weight loss. As a minimally invasive procedure, placement of a jejunal feeding tube for enteral nutrition was considered, but ultimately refused by the patient.
The case was presented to and discussed by the Interdisciplinary Board of Diseases of the Upper Gastrointestinal Tract who unanimously recommended a salvage operation with reconstruction of gastrointestinal continuity by secondary colonic pull-up without resection of the gastric pull-up to avoid thoracotomy. Gastric pyloroplasty or gastro-jejunostomy as therapeutic alternatives were excluded since these two approaches would not address the problem of dysphagia due to anastomotic stenosis of the esophago-gastrostomy.
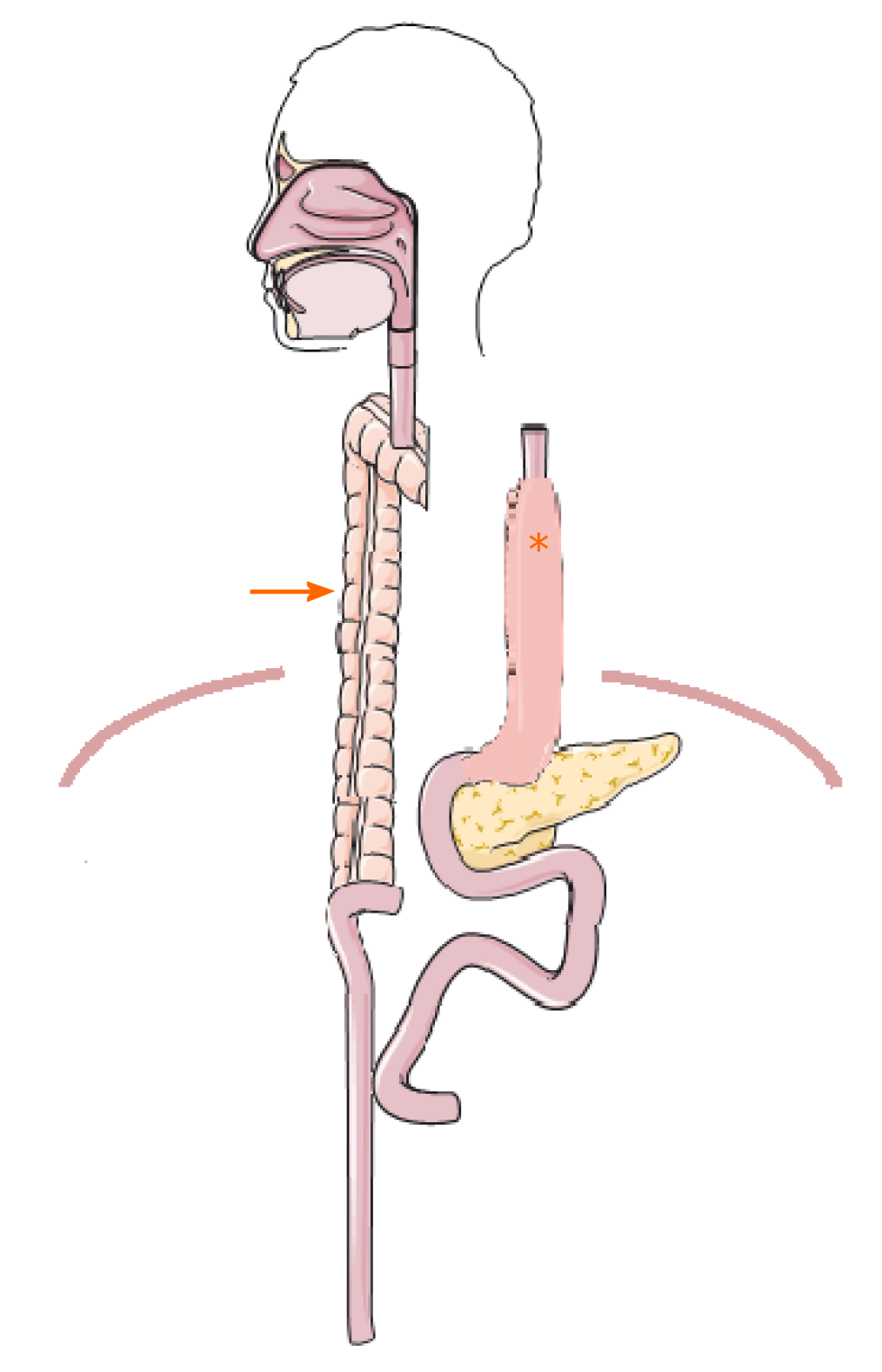
After a pre-habilitation period with parenteral nutrition, the operation was performed. First, a laparotomy was performed in order to mobilize and transect the transverse and left colon. A sufficient blood supply of the pedicled colon graft was ensured after mobilization. Then, the cervical area was explored and the esophagus was then transected and sealed at the aboral end. The colon could be pulled up to the neck retrosternally in a tension-free manner to create an end-to-side hand-sewn esophago-colostomy (Figure 3). By choosing this approach, an additional thoracotomy was omitted and the gastric tube remained untouched. The gastrointestinal passage was restored by an end-to-side colo-jejunostomy (25 mm circular GIA) and a side-to-side jejuno-jejunostomy (Roux-en-Y reconstruction, 60 mm Endo-GIA) as well as a side-to-side colo-colostomy (60 mm Endo-GIA).
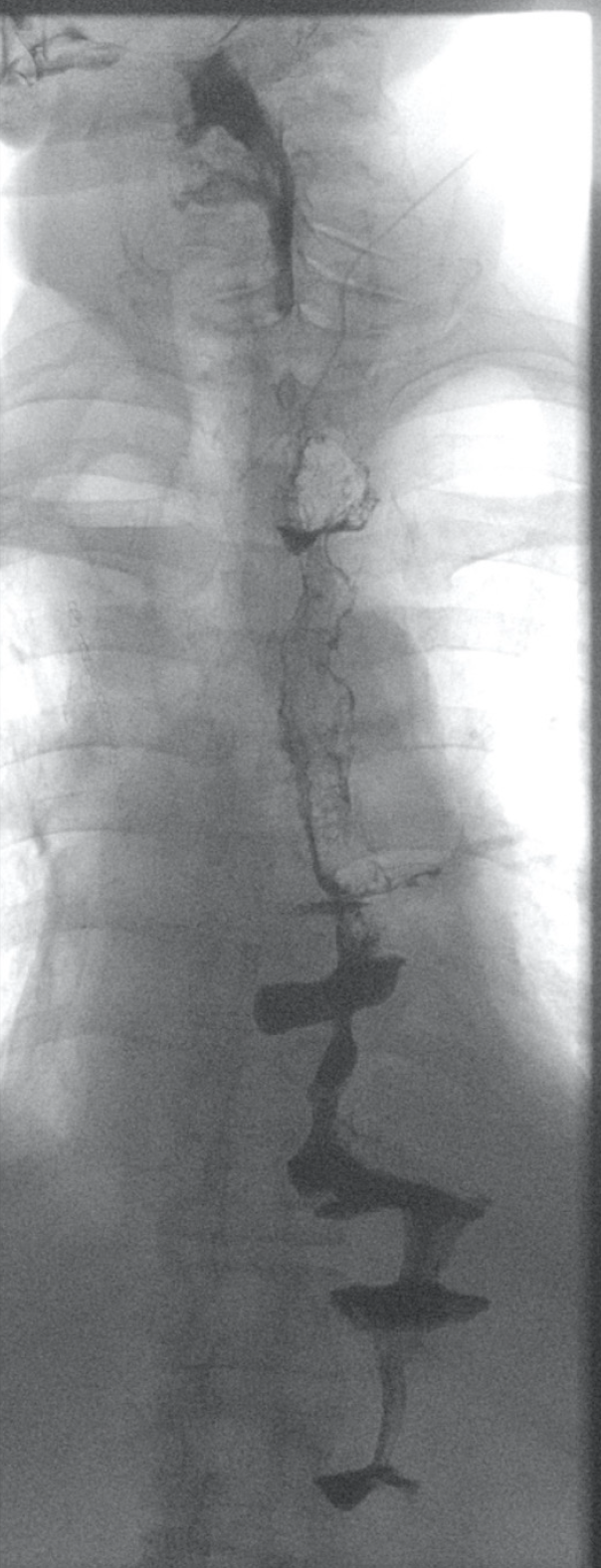
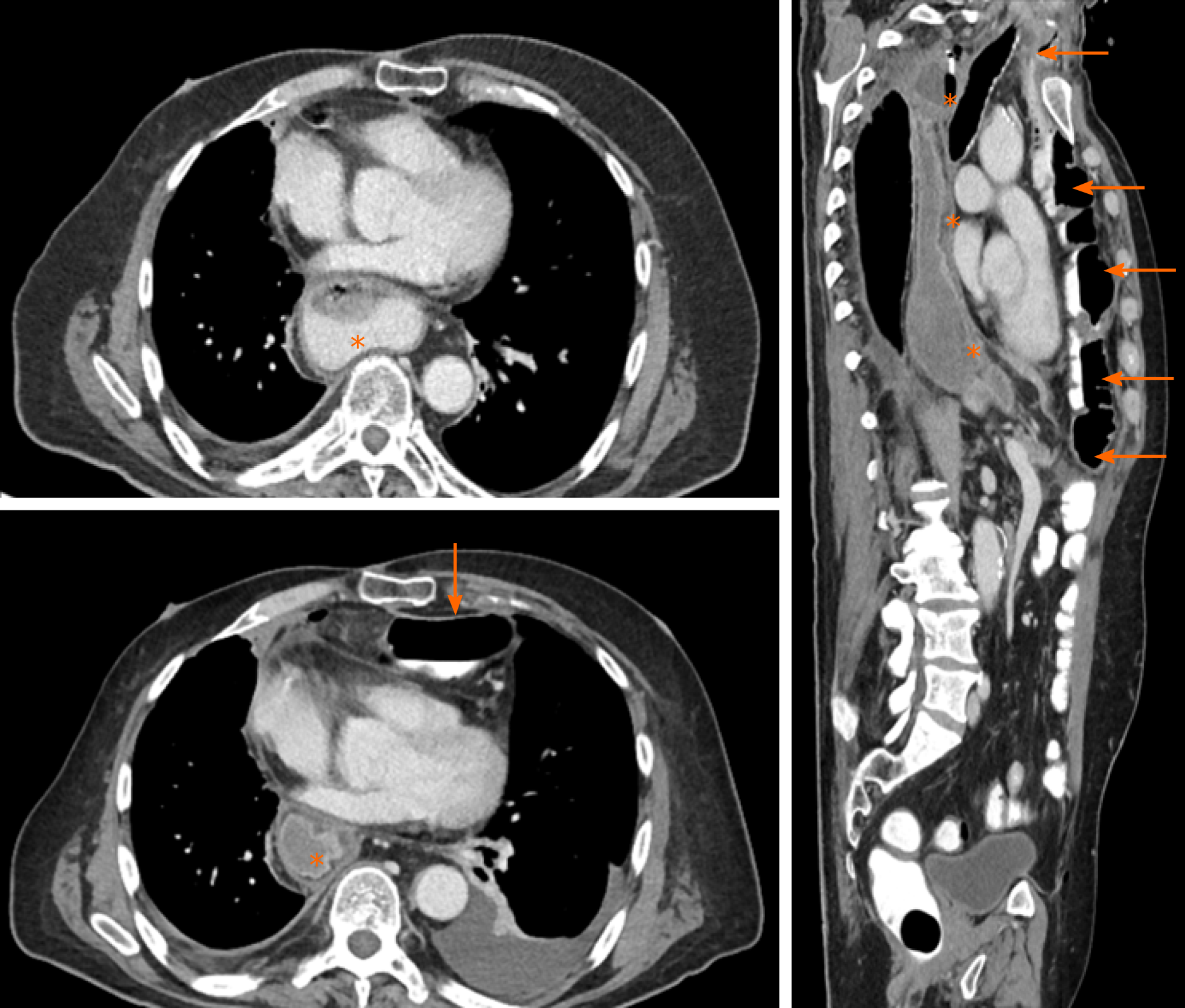
There were no postoperative complications apart from a pneumonia that required antibiotic therapy for 5 d. Oral food intake could be increased gradually to a complete oral diet. Hence, the initial supportive enteral and parenteral nutrition could be stopped. Endoscopic and radiological re-examination after surgery revealed no abnormalities or signs of anastomotic leakage, retention of food or dilatation of the remnant esophagus (Figure 4). The patient reported no symptoms of dysphagia, regurgitation or vomiting at all time points during the postoperative follow-up. Notably, despite the presence of two abdominal organs in the thoracic region from gastric and colonic pull-up (Figure 5), postoperative cardio-pulmonary function of the patient was not compromised.
The continual development of surgical techniques as well as perioperative management of short-term complications has significantly improved the outcome of patients diagnosed with esophageal cancer[6,7]. However, there are still long-term complications such as DGE, anastomotic strictures and reflux resulting in clinical symptoms including dysphagia, regurgitation and vomiting. Patients suffering from long-term complications usually have a significantly impaired quality of life[6,8,9].
Functional dysphagia mostly occurs after cervical esophageal anastomosis due to insufficient esophageal peristalsis[3]. The main reason for severe postoperative dysphagia is anastomotic stricture. Endoscopic balloon dilatation is usually the therapy of choice[3,10] but tumor recurrence needs to be excluded. Endoscopic balloon angioplasty is also the primary therapeutic approach for DGE that is caused by pylorus dysfunction resulting in early satiety, regurgitation, dysphagia and vomiting. Furthermore, medication which stimulates gastric motility can be used to treat DGE[3,4,8,9].
In this particular case, the patient suffered from DGE due to pylorus dysfunction and reduced motility of the gastric tube. The main problem, however, was an anastomotic stricture caused by scar contracture resulting in dysphagia and a progressive inability to consume food orally. Repetitive endoscopic balloon dilatation, stenting and medication were unsuccessful in the long-term. Stenting of the anastomotic stricture was exceedingly difficult to perform due to the lack of long-segment anchoring of the stent caused by pre-stenotic esophageal dilatation, which resulted in stent dislocation. Given the therapy-refractory progression of disease, a “re-do” operation was indicated upon insistence of the patient and following appropriate interdisciplinary discussion.
If gastric pull-up has failed, colon interposition is a potential substitute for esophageal replacement[11]. Thus, colon interposition is not only indicated for oncological but also for benign diseases and in situations when salvage surgery becomes mandatory[12-15]. Since pedicled jejunum is limited with respect to its extension length because of poor connection of marginal vessels, super charged pedicled jejunum or free segment interposition are only suitable for full-length esophageal reconstruction requiring microvascular anastomosis and microsurgical techniques[16-18]. Furthermore, jejunal interposition is also associated with short- as well as long-term morbidity[4,16,17,19]. As recommended by Bakshi et al[19], we decided to use a colon interposition for the operation due to the characteristics of the colon defined by sufficient length, robust blood supply, and non-dependence of a microvascular anastomosis.
Further questions include which part of the colon and which route for digestive reconstruction should be used. A recent review suggests that the left colon placed retrosternally is the safest technique for colon interposition[20]. These results are supported by a national survey of specialist surgeons in the United Kingdom revealing that the left colon and retrosternal placement was the favored surgical approach[21,22]. An additional alternative of reconstruction would have been local resection of stenotic anastomosis re-anastomosis between the cervical esophagus and the gastric conduit but was withdrawn in this case due to the additional DGE[23].
Since we wanted to leave the gastric pull-up in a position that avoids re-exploration of the posterior mediastinum, we used a retrosternal route for the left colonic interposition as recommended by the most recent evidence. For restoring gastrointestinal continuity, we performed an end-to-side colo-jejunostomy and a side-to-side jejuno-jejunostomy (Roux-en-Y reconstruction) to avoid biliary reflux[24]. As a result of our reconstruction of the gastrointestinal passage, 2 abdominal organs were placed intrathoracically (Figures 3-5). This, however, did not compromise the patient’s cardiopulmonary status or physical performance.
Long-term complications after esophagectomy with gastric pull-up can usually be treated by non-surgical approaches such as endoscopic intervention, pro-kinetic medication and gradual oral diet under supervision. However, some patients with therapy-refractory complaints require surgical salvage treatment. In these cases, the retrosternal left colon interposition is one of the preferred reconstruction methods to improve alimentary satisfaction and quality of life. Since the operation of colon interposition after esophagectomy and patient´s follow up are demanding, these procedures should be performed in high volume centers which provide an interdisciplinary team of specialists ensuring the best possible outcome for the patient.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kobayashi S S-Editor: Zhang H L-Editor: A P-Editor: Li JH
| 1. | Butskiy O, Rahmanian R, White RA, Durham S, Anderson DW, Prisman E. Revisiting the gastric pull-up for pharyngoesophageal reconstruction: A systematic review and meta-analysis of mortality and morbidity. J Surg Oncol. 2016;114:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Oki M, Asato H, Suzuki Y, Umekawa K, Takushima A, Okazaki M, Harii K. Salvage reconstruction of the oesophagus: a retrospective study of 15 cases. J Plast Reconstr Aesthet Surg. 2010;63:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Chen KN. Managing complications I: leaks, strictures, emptying, reflux, chylothorax. J Thorac Dis. 2014;6 Suppl 3:S355-S363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 4. | Irino T, Tsekrekos A, Coppola A, Scandavini CM, Shetye A, Lundell L, Rouvelas I. Long-term functional outcomes after replacement of the esophagus with gastric, colonic, or jejunal conduits: a systematic literature review. Dis Esophagus. 2017;30:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Petrov RV, Bakhos CT, Abbas AE, Malik Z, Parkman HP. Endoscopic and Surgical Treatments for Gastroparesis: What to Do and Whom to Treat? Gastroenterol Clin North Am. 2020;49:539-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Pu S, Chen H, Zhou C, Yu S, Liao X, Zhu L, He J, Wang B. Major Postoperative Complications in Esophageal Cancer After Minimally Invasive Esophagectomy Compared With Open Esophagectomy: An Updated Meta-analysis. J Surg Res. 2021;257:554-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, D'Journo XB, Brigand C, Perniceni T, Carrère N, Mabrut JY, Msika S, Peschaud F, Prudhomme M, Bonnetain F, Piessen G; Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Group. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med. 2019;380:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 475] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 8. | Mboumi IW, Reddy S, Lidor AO. Complications After Esophagectomy. Surg Clin North Am. 2019;99:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Xu QL, Li H, Zhu YJ, Xu G. The treatments and postoperative complications of esophageal cancer: a review. J Cardiothorac Surg. 2020;15:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 10. | Briel JW, Tamhankar AP, Hagen JA, DeMeester SR, Johansson J, Choustoulakis E, Peters JH, Bremner CG, DeMeester TR. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg. 2004;198:536-41; discussion 541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Gust L, Ouattara M, Coosemans W, Nafteux P, Thomas PA, D'Journo XB. European perspective in Thoracic surgery-eso-coloplasty: when and how? J Thorac Dis. 2016;8:S387-S398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Reslinger V, Tranchart H, D'Annunzio E, Poghosyan T, Quero L, Munoz-Bongrand N, Corte H, Sarfati E, Cattan P, Chirica M. Esophageal reconstruction by colon interposition after esophagectomy for cancer analysis of current indications, operative outcomes, and long-term survival. J Surg Oncol. 2016;113:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Kitajima T, Momose K, Lee S, Haruta S, Shinohara H, Ueno M, Fujii T, Udagawa H. Benign esophageal stricture after thermal injury treated with esophagectomy and ileocolon interposition. World J Gastroenterol. 2014;20:9205-9209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Greene CL, DeMeester SR, Augustin F, Worrell SG, Oh DS, Hagen JA, DeMeester TR. Long-term quality of life and alimentary satisfaction after esophagectomy with colon interposition. Ann Thorac Surg. 2014;98:1713-9; discussion 1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Chirica M, Veyrie N, Munoz-Bongrand N, Zohar S, Halimi B, Celerier M, Cattan P, Sarfati E. Late morbidity after colon interposition for corrosive esophageal injury: risk factors, management, and outcome. A 20-years experience. Ann Surg. 2010;252:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Gaur P, Blackmon SH. Jejunal graft conduits after esophagectomy. J Thorac Dis. 2014;6 Suppl 3:S333-S340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 17. | Zhang Z, Guo Y, Liang C, Feng H, Liu D. Free jejunum interposition as salvage surgery after cervical esophagus injury. J Thorac Dis. 2016;8:E513-E516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Poh M, Selber JC, Skoracki R, Walsh GL, Yu P. Technical challenges of total esophageal reconstruction using a supercharged jejunal flap. Ann Surg. 2011;253:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Bakshi A, Sugarbaker DJ, Burt BM. Alternative conduits for esophageal replacement. Ann Cardiothorac Surg. 2017;6:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Brown J, Lewis WG, Foliaki A, Clark GWB, Blackshaw GRJC, Chan DSY. Colonic Interposition After Adult Oesophagectomy: Systematic Review and Meta-analysis of Conduit Choice and Outcome. J Gastrointest Surg. 2018;22:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Fisher RA, Griffiths EA, Evison F, Mason RC, Zylstra J, Davies AR, Alderson D, Gossage JA. A national audit of colonic interposition for esophageal replacement. Dis Esophagus. 2017;30:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Fisher RA, Gossage J, Griffiths E. Response to: "Colonic Interposition After Adult Oesophagectomy: Systematic Review and Meta-analysis of Conduit Choice and Outcome". J Gastrointest Surg. 2018;22:2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Kinoshita Y, Udagawa H, Tsutsumi K, Ueno M, Mine S, Ehara K. Surgical repair of refractory strictures of esophagogastric anastomoses caused by leakage following esophagectomy. Dis Esophagus. 2009;22:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Chang A. Colon Interposition for Staged Esophageal Reconstruction. Oper Tech Thorac Cardiothorac Surg. 2010;3:231-242. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |