Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.3960
Peer-review started: December 2, 2020
First decision: January 29, 2021
Revised: February 16, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: June 6, 2021
Processing time: 163 Days and 3.3 Hours
Since 1923, only a few hundred cases of pulmonary arterial sarcoma (PAS) have been reported. It is easy for PAS to be misdiagnosed as pulmonary thromboembolism, which makes treatment difficult. The median survival time without surgical treatment for PAS is only 1.5-3 mo. Echocardiography is widely used in screening for pulmonary artery space-occupying lesions in patients with chest pain, dyspnea, and cough; furthermore, it is typically considered the first imaging examination for patients with PAS.
In May 2017, a 39-year-old male patient experienced chest pain with no particular obvious cause. At that time, the cause was thought to be pulmonary embolism. In July 2017, positron emission tomography–computed tomography revealed space-occupying lesions in the right lung and multiple metastases in both lungs. The lesions of the right lung were biopsied, and pathology revealed undifferentiated sarcoma. Chemotherapy had been performed since July 2017 in another hospital. In December 2019, the patient was admitted to our hospital for the sake of CyberKnife treatment. Echocardiography suggested: (1) A right ventricular outflow tract (RVOT) solid mass of the main pulmonary artery; and (2) mild pulmonary valve regurgitation. Ultrasonography showed the absence of a thrombus in the deep veins of either lower limb.
PAS is a single, central space-occupying lesion involving the RVOT and pulmonary valve. Echocardiography of PAS has its own characteristics.
Core Tip: Echocardiography has a high sensitivity in the diagnosis of pulmonary artery space-occupying lesions. The misdiagnosis of pulmonary arterial sarcoma (PAS) by echocardiography is attributed to insufficient knowledge of this disease. If the following four features are detected, a diagnosis of PAS should be considered: (1) A single pulmonary arterial mass involving the right ventricular outflow tract or pulmonary valve; (2) varying degrees of pulmonary stenosis detected based on ultrasonic measurements; (3) the mass can move slightly and blood supply can be seen inside; and (4) use of ultrasonography of the lower extremity and inferior vena cava to exclude thrombus.
- Citation: Li X, Hong L, Huo XY. Undifferentiated intimal sarcoma of the pulmonary artery: A case report. World J Clin Cases 2021; 9(16): 3960-3965
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/3960.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.3960
Malignant lesions of the pulmonary artery mainly include primary lung sarcoma and metastasis, and benign lesions mainly include pulmonary thrombus, benign granuloma, and myxoma. Takayasu’s arteritis and Behcet disease are rarely observed. Since 1923, only a few hundred cases of pulmonary arterial sarcoma (PAS) have been reported in the literature. Because of the small number of cases, limited knowledge of the disease, and the lack of unique PAS clinical manifestations, it is easy for PAS to be misdiagnosed as pulmonary thromboembolism (PTE) or pulmonary hypertension, which makes treatment difficult. It has been reported that the vast majority of PAS patients cannot be diagnosed before surgery or death, and they die within a few days to a month after diagnosis[1]. The median survival time without surgical treatment for PAS is only 1.5 mo to 3 mo, but the mean survival time after early diagnosis and surgical treatment can be extended to 17 mo.
The diagnosis of PAS relies on pathological findings, but there are risks in sourcing the material for these examinations. Imaging examination is the best method for screening for pulmonary artery space-occupying lesions in PAS patients with chest pain, dyspnea, and cough. Positron emission tomography (PET)–computed tomography (CT) images show a mass shadow in patients with PAS as a result of the abnormally increased fludeoxyglucose intake, and PAS displays progressive enhancement in dynamic enhanced magnetic resonance imaging. CT images show that the proximal part of the PAS tumor is convex toward the right ventricular outflow tract (RVOT) or toward the blood flow. These features are useful in the differential diagnosis of benign and malignant pulmonary arterial masses[2]. Transthoracic echocardiography (TTE) is widely used because it is radiation-free and low-cost and has good repeatability, and it is typically the first imaging examination for patients with PAS.
PAS was diagnosed two years ago.
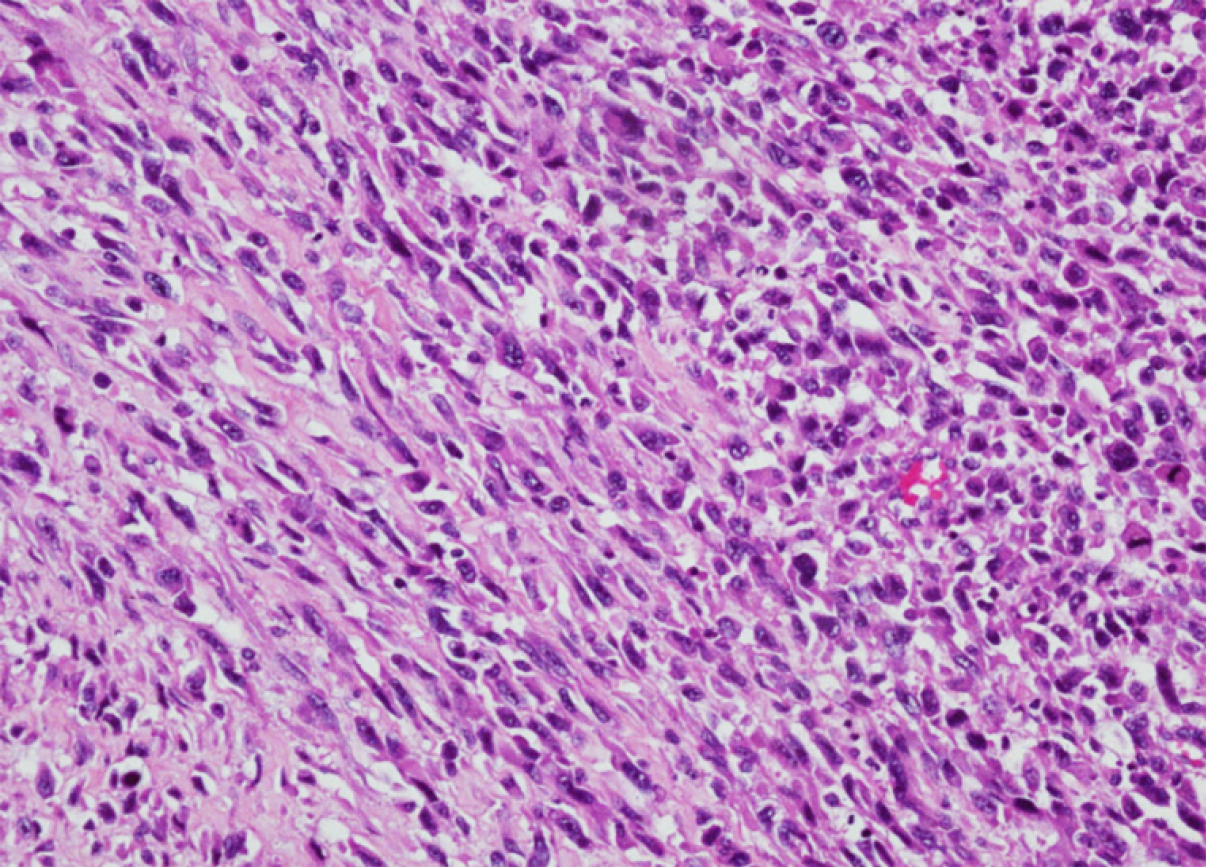
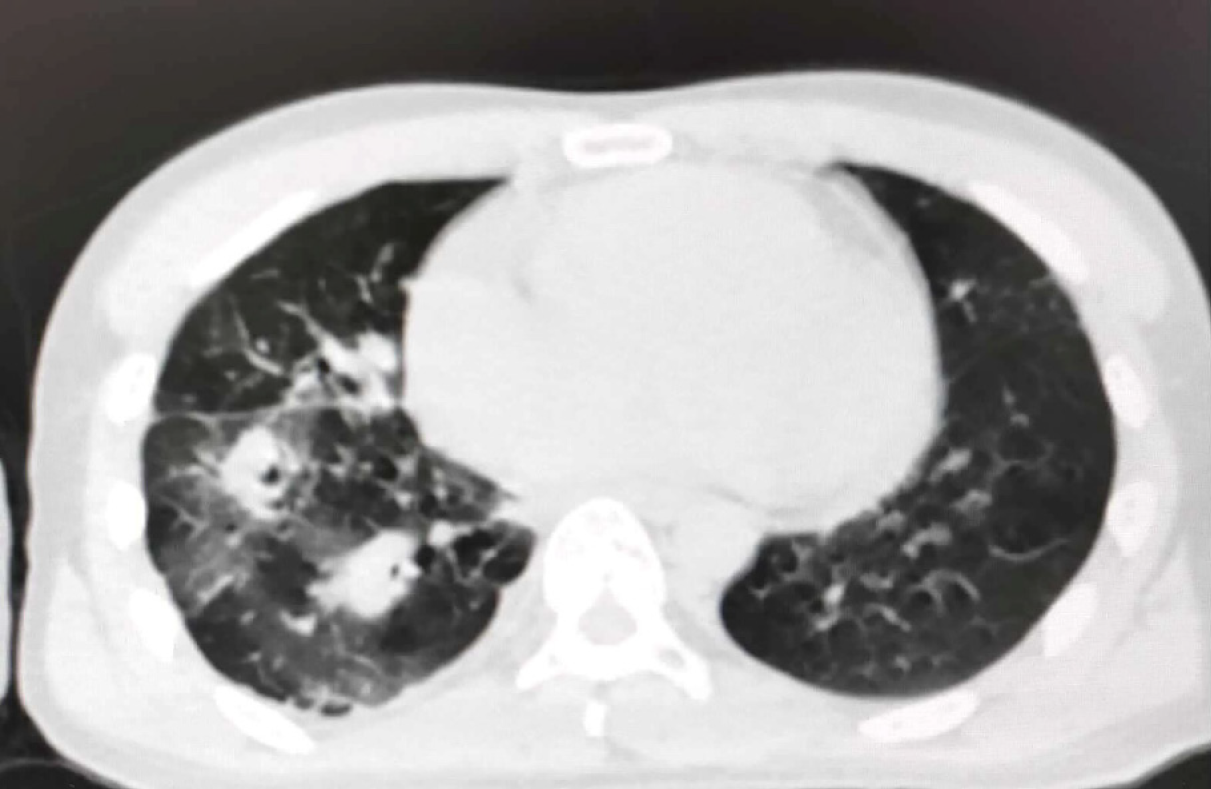
In May 2017, a 39-year-old patient experienced chest pain with no particular obvious cause. At that time, the cause was thought to be pulmonary embolism, but treatment for embolism was not effective. In July 2017, a PET-CT scan at another hospital showed space-occupying lesions in the right lung and multiple metastases in both lungs (images not available). The lesions of the right lung were biopsied, and pathology revealed undifferentiated sarcoma. The tumor was composed of spindle and pleomorphic cells with obvious atypia, arranged into fascicles (Figure 1). Chemo-therapy had been performed since July 2017 in another hospital. In November 2019, the PET-CT images showed that the tumor activity was inhibited after chemoradiotherapy in the hilum of the right lung. However, multiple nodules and patchy images of both lungs and nodules at the superior vena cava were found, indicating occurrence of metastasis. The patient was admitted to our hospital in December 2019, whose CT images acquired at our hospital showed multiple nodules and patchy images in the right lung (Figure 2). At that time, despite the absence of other symptoms, the patient reported that breathing difficulties may develop if he makes any slight movements.
The patient had a 25-year history of smoking. He had no history of exposure to chemical, radioactive, or toxic substances.
The patient was a businessman. There was no family history of genetic disease.
The patient is male, with a height of 168 cm and weight of 52 kg. The results of physical examination at our hospital suggested a heart rate of 82 beats per minute and normal rhythm, and no pathological murmur or pericardial friction rubs were found in all valve auscultation areas.
The patient's D-dimer value in another hospital was unknown.
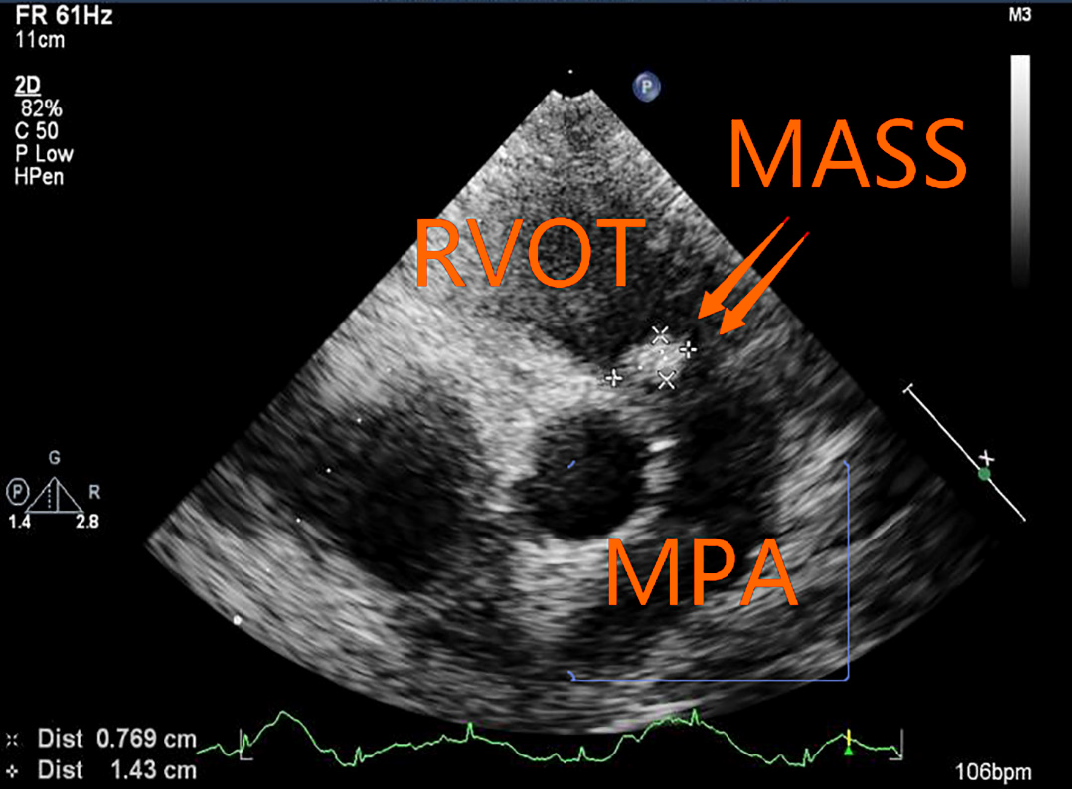
The patient's echocardiography performed at our hospital showed that the right ventricle was enlarged. The internal diameter of the right ventricular infundibulum was increased by about 30 mm, and the diameter of the main pulmonary artery (MPA) was about 29 mm. A solid mass of about 14 mm × 7 mm was detected below the pulmonary valve adjacent to the RVOT (Figure 3). During the cardiac cycle, the mass oscillated between the RVOT and MPA (Video 1). Mild pulmonary regurgitation was observed. A mosaic-like blood flow within the mass was detected. The velocity of pulmonary artery blood flow around the mass increased slightly and the MPA stenosis was estimated as approximately 50%. No space-occupying lesions were found in the branches of the right or left MPA. No thrombus was detected by ultrasound in the deep veins of either lower limb. These findings suggested: (1) an RVOT solid mass of the MPA; and (2) mild pulmonary valve regurgitation.
PAS with multiple metastases of both lungs.
Chemotherapy had been performed since July 2017. Doxorubicin and ifosfamide were administered for 20 courses of treatment, followed by radiotherapy for the right lung lesion in November 2019. In December 2019, the patient received CyberKnife treatment at our hospital.
The patient was discharged in January 2020 and died the following month.
PAS is a large arterial sarcoma with no recognized staging system. It can be categorized into two types: Intimal sarcoma and luminal sarcoma. The latter type is rarely seen, and PAS mainly refers to intimal sarcoma[3]. The World Health Organization classification (2013 version) of soft-tissue tumors includes PAS as a soft-tissue malignant tumor with uncertain differentiation, which may originate from multipotent mesenchymal cells in the intima, most of which are poorly differentiated spindle-cell sarcomas with the feature of fibroblast and/or myofibroblast differentiation. Cell atypia, mitosis, and necrosis vary among cases. Sebenik et al[4] classified tumors as “differentiated types” and “undifferentiated types.”
PAS is most likely to be misdiagnosed as PTE[5]. The basis for the differential diagnosis of PAS and PTE by ultrasound can be described as follows. First of all, there are no clear signs of PTE using TTE, and only an enlarged right heart and dilated pulmonary arteries can be detected, whereas TTE of PAS can clearly show space-occupying lesions of the pulmonary artery. Second, deep venous thrombosis is considered to be an indicator of PTE, and vascular ultrasound of PAS showed no thrombosis in the deep veins of the lower extremities or the inferior vena cava. Third, 85% of PAS occur in the MPA, which are a single, central space-occupying lesion involving the RVOT and pulmonary valve. PTE primarily occurs in the pulmonary lobar and segmental artery branches. The incidence of RVOT malignant tumors of the MPA is much greater than that of benign tumors, and angiosarcoma is often detected. When lesions of the MPA involve RVOT, the diagnosis is likely PAS. Additionally, the PAS is able to move slightly, and the blood flow signal is also seen in the tumor, whereas PTE can barely move without a blood flow signal inside. Lastly, different degrees of pulmonary artery stenosis can be detected in patients with PAS, whereas PTE is typically associated with different degrees of pulmonary hypertension. This is useful to distinguish PAS from PTE with the same right ventricular volume overloaded. In this case, a solid mass, detected in the RVOT-MPA, oscillated with cardiac cycle. The pulmonary valve was affected, and mild pulmonary valve regurgitation was detected. Blood flow signal was found in the tumor. The mass occupied approximately 50% of the internal diameter of the MPA. The local blood flow rate of the pulmonary artery was slightly increased, and ultrasound revealed no thrombosis in the deep veins. Ultrasonic findings were consistent with the PAS diagnosis.
The clinical manifestations of PAS and PTE are also different[6]. First, the course of PAS is progressive, and thrombolytic therapy is less effective. However, the course of acute PTE is rapid, and 11% of patients with PTE die within one hour. If the active thrombus enters the central pulmonary artery, the rate of early mortality ranges from 80% to 100%. Second, the diagnostic sensitivity of positive D-dimer for acute PTE is as high as 92%-100%. Although an increase in D-dimer can result in a false-positive diagnosis of PTE, D-dimer can be used as the primary basis for identification, and a negative D-dimer is basically a reason to exclude PTE. Third, when PAS is identified, metastases occur in 50% of cases, with distant metastases occurring in 16% of cases[7]. In this case, the initial onset occurred two years ago, the efficacy of thrombolytic therapy was unsatisfactory, and the PET-CT images showed multiple metastases in both lungs only after two months. It was more likely to be PAS although the D-dimer value was unknown.
Transesophageal echocardiography (TEE) is more advantageous than TTE in identifying whether there is sessile tumor, the capsule is intact, the internal echo is homogeneous, there is blood supply inside, and there is filling defect of pulmonary artery or not[8]. Although intracardiac or pulmonary thrombosis, which is missed by TTE, can be detected through TEE in about 80% of patients with PTE[9], the thrombus volume was smaller than that of PAS malignant tumor. No sessile tumor and no blood flow signal can be measured.
Taking all of the findings together, echocardiography has a high sensitivity for the diagnosis of pulmonary artery space-occupying lesions. The misdiagnosis of PAS by echocardiography is attributed to the lack of knowledge of this disease. If the following four features are detected, a diagnosis of PAS should be considered: (1) A single pulmonary arterial mass involving RVOT or the pulmonary valve; (2) varying degrees of pulmonary stenosis based on ultrasonic detection; (3) the mass is able to move slightly and blood supply can be seen inside; and (4) ultrasound of the lower extremity and inferior vena cava can exclude thrombus. If PAS cannot be identified by TTE, TEE should be performed. If PAS is initially diagnosed by ultrasound, the D-dimer test is negative, and other imaging findings are associated with lung space-occupying lesions, a clinical pathological biopsy should be performed.
Manuscript source: Unsolicited manuscript
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goebel WS S-Editor: Liu M L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Wyler von Ballmoos MC, Chan EY, Reardon MJ. Imaging and Surgical Treatment of Primary Pulmonary Artery Sarcoma. Int J Cardiovasc Imaging. 2019;35:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Xi XY, Gao W, Gong JN, Guo XJ, Wu JY, Yang YH, Yang MF. Value of 18F-FDG PET/CT in differentiating malignancy of pulmonary artery from pulmonary thromboembolism: a cohort study and literature review. Int J Cardiovasc Imaging. 2019;35:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Harbhajanka A, Dahoud W, Michael CW, Elliot R. Cytohistological correlation, immunohistochemistry and Murine Double Minute Clone 2 amplification of pulmonary artery intimal sarcoma: A case report with review of literature. Diagn Cytopathol. 2019;47:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Sebenik M, Ricci A Jr, DiPasquale B, Mody K, Pytel P, Jee KJ, Knuutila S, Scholes J. Undifferentiated intimal sarcoma of large systemic blood vessels: report of 14 cases with immunohistochemical profile and review of the literature. Am J Surg Pathol. 2005;29:1184-1193. [PubMed] |
| 5. | David S, Hoeper MM, Vogel-Claussen J, Cebotari S. Pulmonary Arterial Sarcoma Presenting as Acute Pulmonary Embolism. Am J Respir Crit Care Med. 2017;196:523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Deng L, Zhu J, Xu J, Guo S, Liu S, Song Y. Clinical presentation and surgical treatment of primary pulmonary artery sarcoma. Interact Cardiovasc Thorac Surg. 2018;26:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Todaro MC, Orlassino R, Fornengo C, Ravera A, Macchia G, Soffiantino F, Lovato RL, Senatore G. [A case of pulmonary artery sarcoma and review of the literature]. G Ital Cardiol (Rome). 2017;18:792-795. [PubMed] |
| 8. | Rudkovskaia AA, Bandyopadhyay D. Intraluminal Arterial Filling Defects Misdiagnosed as Pulmonary Emboli: What Else Could They Be? Clin Chest Med. 2018;39:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Fyfe DA, Kline CH, Sade RM, Gillette PC. Transesophageal echocardiography detects thrombus formation not identified by transthoracic echocardiography after the Fontan operation. J Am Coll Cardiol. 1991;18:1733-1737. [PubMed] |